Post-COVID-19 Condition Prediction in Hospitalised Cancer Patients: A Machine Learning-Based Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Data Management
2.3. Definitions
2.4. Statistical Analysis
2.5. Model Implementation and Evaluation
3. Results
3.1. Descriptive Analysis of Population and Their Clinical Features
3.2. Predictive Model Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Wise, J. Long COVID: WHO calls on countries to offer patients more rehabilitation. BMJ 2021, 372, n405. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Lancet, T. Facing up to long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. RETRACTED: 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Kingstone, T.; Taylor, A.K.; O’Donnell, C.A.; Atherton, H.; Blane, D.N.; Chew-Graham, C.A. Finding the’right’GP: A qualitative study of the experiences of people with long-COVID. BJGP Open 2020, 4, bjgpopen20X101143. [Google Scholar] [CrossRef]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after COVID-19: Qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Devine, O.J.; Reese, H.E.; Silk, B.J.; Iuliano, A.D.; Threlkel, R.; Vu, Q.M.; Plumb, I.D.; Cadwell, B.L.; Rose, C.; et al. Point prevalence estimates of activity-limiting long-term symptoms among United States adults 1 month after reported severe acute respiratory syndrome coronavirus 2 infection, 1 November 2021. J. Infect. Dis. 2023, 227, 855–863. [Google Scholar] [CrossRef]
- Fankuchen, O.; Lau, J.; Rajan, M.; Swed, B.; Martin, P.; Hidalgo, M.; Yamshon, S.; Pinheiro, L.; Shah, M.A. Long Covid in Cancer: A Matched Cohort Study of 1-year Mortality and Long COVID Prevalence Among Patients With Cancer Who Survived an Initial Severe SARS-CoV-2 Infection. Am. J. Clin. Oncol. 2023, 46, 300–305. [Google Scholar] [CrossRef]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef]
- Fillmore, N.R.; La, J.; Szalat, R.E.; Tuck, D.P.; Nguyen, V.; Yildirim, C.; Do, N.V.; Brophy, M.T.; Munshi, N.C. Prevalence and outcome of COVID-19 infection in cancer patients: A national Veterans Affairs study. JNCI J. Natl. Cancer Inst. 2021, 113, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Finn, O. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, viii6–viii9. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Metabolic reprogramming of immune cells in cancer progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef]
- Pazukhina, E.; Andreeva, M.; Spiridonova, E.; Bobkova, P.; Shikhaleva, A.; El-Taravi, Y.; Rumyantsev, M.; Gamirova, A.; Bairashevskaia, A.; Petrova, P.; et al. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: A prospective, cohort study in Moscow (StopCOVID). BMC Med. 2022, 20, 244. [Google Scholar] [CrossRef]
- Xu, H.; Lu, T.; Liu, Y.; Yang, J.; Ren, S.; Han, B.; Lai, H.; Ge, L.; Liu, J. Prevalence and risk factors for long COVID among cancer patients: A systematic review and meta-analysis. Front. Oncol. 2025, 14, 1506366. [Google Scholar] [CrossRef]
- Debie, Y.; Palte, Z.; Salman, H.; Verbruggen, L.; Vanhoutte, G.; Chhajlani, S.; Raats, S.; Roelant, E.; Vandamme, T.; Peeters, M.; et al. Long-term effects of the COVID-19 pandemic for patients with cancer. Qual. Life Res 2024, 33, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Dagher, H.; Chaftari, A.M.; Subbiah, I.M.; Malek, A.E.; Jiang, Y.; Lamie, P.; Granwehr, B.; John, T.; Yepez, E.; Borjan, J.; et al. Long COVID in cancer patients: Preponderance of symptoms in majority of patients over long time period. Elife 2023, 7, e81182. [Google Scholar] [CrossRef]
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin. Exp. Allergy 2021, 51, 1107–1120. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2101341. [Google Scholar] [CrossRef] [PubMed]
- COVID; WHO. Core Case Report Form Acute Respiratory Infection Clinical Characterisation Data Tool. 19 April 2020. Available online: https://isaric.org/wp-content/uploads/2021/02/ISARIC-WHO-COVID-19-CORE-CRF_EN.pdf (accessed on 2 February 2021).
- Munblit, D.; Nekliudov, N.A.; Bugaeva, P.; Blyuss, O.; Kislova, M.; Listovskaya, E.; Gamirova, A.; Shikhaleva, A.; Belyaev, V.; Timashev, P.; et al. Stop COVID cohort: An observational study of 3480 patients admitted to the Sechenov University Hospital Network in Moscow City for suspected coronavirus disease 2019 (COVID-19) infection. Clin. Infect. Dis. 2021, 73, 1–11. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, V.; Singh, A. Stacking ensemble-based intelligent machine learning model for predicting post-COVID-19 complications. N. Gener. Comput. 2022, 40, 987–1007. [Google Scholar] [CrossRef]
- Shakhovska, N.; Yakovyna, V.; Chopyak, V. A new hybrid ensemble machine-learning model for severity risk assessment and post-COVID prediction system. Math. Biosci. Eng 2022, 19, 6102–6123. [Google Scholar] [CrossRef]
- Reme, B.A.; Gjesvik, J.; Magnusson, K. Predictors of the post-COVID condition following mild SARS-CoV-2 infection. Nat. Commun. 2023, 14, 5839. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shahbaz, S.; Osman, M.; Redmond, D.; Bozorgmehr, N.; Rosychuk, R.J.; Lam, G.; Sligl, W.; Cohen Tervaert, J.W.; Elahi, S. Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J. Autoimmun. 2024, 147, 103267. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Iglesias, M.J.; Tremble, K.; Russell, B.; Moss, C.; Dolly, S.; Sita-Lumsden, A.; Cortellini, A.; Pinato, D.J.; Rigg, A.; Karagiannis, S.N.; et al. Long-term effects of COVID-19 on cancer patients: The experience from Guy’s Cancer Centre. Future Oncol. 2022, 18, 3585–3594. [Google Scholar] [CrossRef]
| Variable | PCC | Non-PCC | p-Value |
|---|---|---|---|
| Continuous variables | |||
| Length of stay, median [IQR] | 15.0 [12.0–18.25] | 15.0 [13.0–21.0] | 0.299 |
| Age, median [IQR] | 67.5 [59.75–73.25] | 70.0 [63.0–78.0] | 0.096 |
| Categorical variables | |||
| Sex | |||
| Male | 57 (59.4%) | 43 (39.4%) | 0.05 |
| Female | 39 (40.6%) | 66 (60.6%) | |
| ICU | 95 (99%) | 108 (99.1%) | 1 |
| Antibodies found | 40 (41.7%) | 63 (57.8%) | 0.025 |
| Symptoms at hospital admission | |||
| Fever | 90 (93.8%) | 101 (92.7%) | 0.789 |
| Cough | 59 (61.5%) | 89 (81.7%) | 0.002 |
| Sore throat | 6 (6.2%) | 6 (5.5%) | 1 |
| Runny nose | 2 (2.1%) | 14 (12.8%) | 0.004 |
| Wheezing | 10 (10.4%) | 11 (10.1%) | 1 |
| Shortness of breath | 63 (65.6%) | 82 (75.2%) | 0.166 |
| Lower chest wall indrawing | 0 (0%) | 0 (0%) | |
| Chest pain | 8 (8.3%) | 12 (11%) | 0.639 |
| Conjunctivitis | 1 (1%) | 1 (0.9%) | 1 |
| Lymphadenopathy | 2 (2.1%) | 2 (1.8%) | 1 |
| Headache | 17 (17.7%) | 28 (25.7%) | 0.18 |
| Loss of smell | 16 (16.7%) | 23 (21.1%) | 0.478 |
| Loss of taste | 9 (9.4%) | 11 (10.1%) | 1 |
| Seizures | 0 (0%) | 0 (0%) | |
| Fatigue | 85 (88.5%) | 94 (86.2%) | 0.678 |
| Anorexia | 15 (15.6%) | 18 (16.5%) | 1 |
| Altered consciousness/confusion | 3 (3.1%) | 0 (0%) | 0.101 |
| Muscle aches | 10 (10.4%) | 20 (18.3%) | 0.118 |
| Joint pain | 4 (4.2%) | 5 (4.6%) | 1 |
| Inability to walk | 2 (2.1%) | 0 (0%) | 0.218 |
| Abdominal pain | 0 (0%) | 2 (1.8%) | 0.5 |
| Diarrhoea | 14 (14.6%) | 14 (12.8%) | 0.839 |
| Vomiting | 7 (7.3%) | 14 (12.8%) | 0.25 |
| Rash | 1 (1%) | 1 (0.9%) | 1 |
| Bleeding | 2 (2.1%) | 1 (0.9%) | 0.601 |
| Comorbidities | |||
| Chronic cardiac disease | 43 (44.8%) | 48 (44%) | 1 |
| Hypertension | 70 (72.9%) | 75 (68.8%) | 0.542 |
| History of revascularization | 8 (8.3%) | 12 (11%) | 0.639 |
| Chronic pulmonary disease | 8 (8.3%) | 14 (12.8%) | 0.368 |
| Asthma | 5 (5.2%) | 5 (4.6%) | 1 |
| Chronic kidney disease | 9 (9.4%) | 14 (12.8%) | 0.509 |
| Obesity | 20 (20.8%) | 27 (24.8%) | 0.618 |
| Moderate liver disease | 3 (3.1%) | 3 (2.8%) | 1 |
| Mild liver disease | 4 (4.2%) | 3 (2.8%) | 0.708 |
| Asplenia | 0 (0%) | 0 (0%) | |
| Chronic neurological disorder | 7 (7.3%) | 5 (4.6%) | 0.553 |
| Chronic hematologic disease | 8 (8.3%) | 6 (5.5%) | 0.581 |
| Diabetes Mellitus | 28 (29.2%) | 26 (23.9%) | 0.429 |
| Rheumatologic disorder | 3 (3.1%) | 6 (5.5%) | 0.506 |
| Dementia | 2 (2.1%) | 2 (1.8%) | 1 |
| Tuberculosis | 1 (1%) | 0 (0%) | 0.468 |
| Malnutrition | 2 (2.1%) | 2 (1.8%) | 1 |
| Smoking | 13 (13.5%) | 9 (8.3%) | 0.262 |
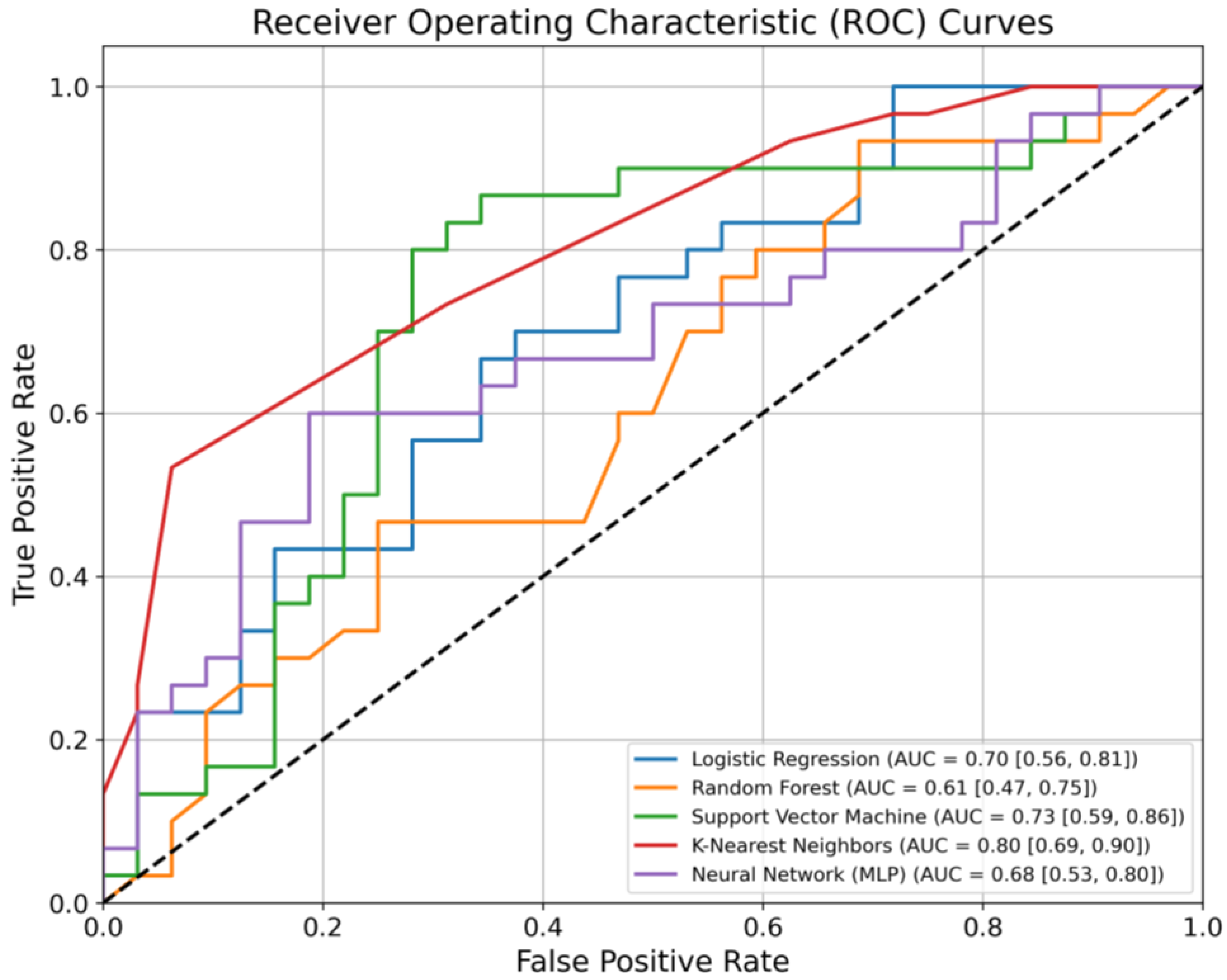
| Model | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|
| Logistic Regression | 0.70 (0.56–0.81) | 0.67 | 0.66 |
| Random Forest | 0.61 (0.47–0.75) | 0.7 | 0.47 |
| Support Vector Machine | 0.73 (0.59–0.86) | 0.87 | 0.63 |
| K-nearest Neighbours | 0.80 (0.69–0.90) | 0.73 | 0.69 |
| Multi-Layer Perceptron | 0.68 (0.53–0.80) | 0.67 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahvash Mohammadi, S.; Rumyantsev, M.; Abdeeva, E.; Baimukhambetova, D.; Bobkova, P.; El-Taravi, Y.; Pikuza, M.; Trefilova, A.; Zolotarev, A.; Andreeva, M.; et al. Post-COVID-19 Condition Prediction in Hospitalised Cancer Patients: A Machine Learning-Based Approach. Cancers 2025, 17, 687. https://doi.org/10.3390/cancers17040687
Mahvash Mohammadi S, Rumyantsev M, Abdeeva E, Baimukhambetova D, Bobkova P, El-Taravi Y, Pikuza M, Trefilova A, Zolotarev A, Andreeva M, et al. Post-COVID-19 Condition Prediction in Hospitalised Cancer Patients: A Machine Learning-Based Approach. Cancers. 2025; 17(4):687. https://doi.org/10.3390/cancers17040687
Chicago/Turabian StyleMahvash Mohammadi, Sara, Mikhail Rumyantsev, Elina Abdeeva, Dina Baimukhambetova, Polina Bobkova, Yasmin El-Taravi, Maria Pikuza, Anastasia Trefilova, Aleksandr Zolotarev, Margarita Andreeva, and et al. 2025. "Post-COVID-19 Condition Prediction in Hospitalised Cancer Patients: A Machine Learning-Based Approach" Cancers 17, no. 4: 687. https://doi.org/10.3390/cancers17040687
APA StyleMahvash Mohammadi, S., Rumyantsev, M., Abdeeva, E., Baimukhambetova, D., Bobkova, P., El-Taravi, Y., Pikuza, M., Trefilova, A., Zolotarev, A., Andreeva, M., Iakovleva, E., Bulanov, N., Avdeev, S., Pazukhina, E., Zaikin, A., Kapustina, V., Fomin, V., Svistunov, A. A., Timashev, P., ... Sechenov StopCOVID Research Team. (2025). Post-COVID-19 Condition Prediction in Hospitalised Cancer Patients: A Machine Learning-Based Approach. Cancers, 17(4), 687. https://doi.org/10.3390/cancers17040687





