A Look to the Future: Potential Theranostic Applications in Head and Neck Tumors
Simple Summary
Abstract
1. Introduction
2. 2-Deoxy-2-[18F]fluoro-D-glucose (FDG)
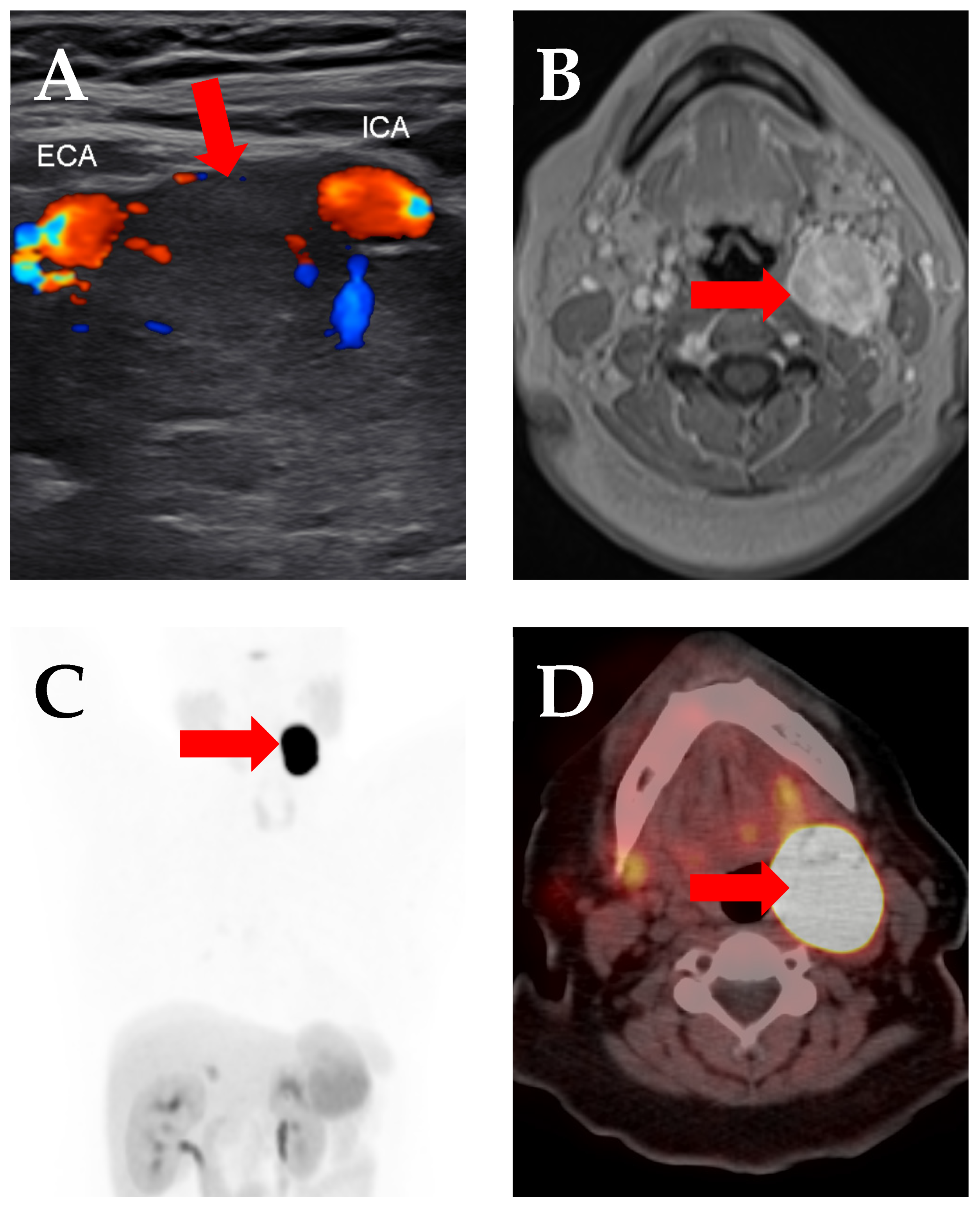
3. Prostate-Specific Membrane Antigen (PSMA)
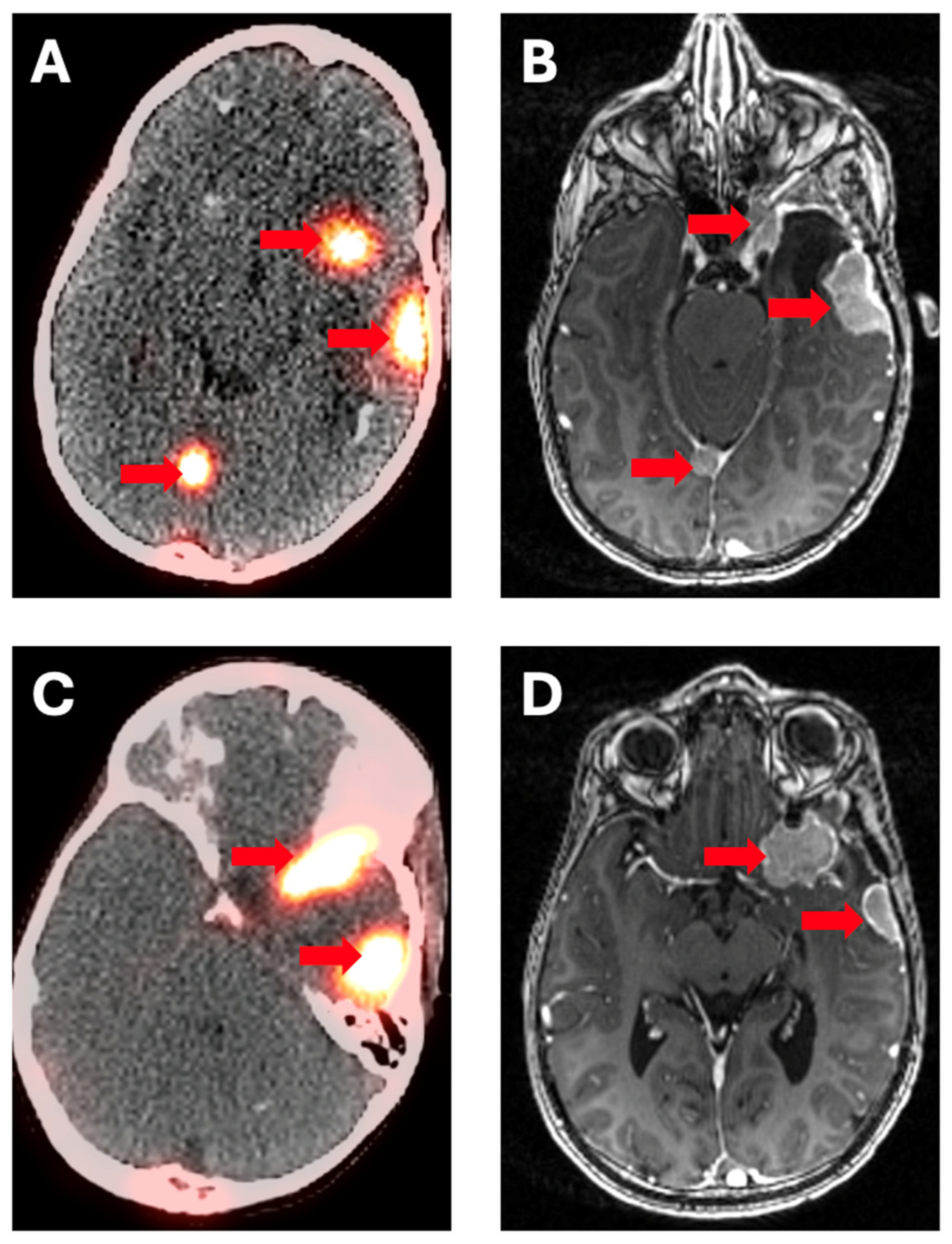
4. Somatostatin Receptor Inhibitors (DOTATATE)
5. Fibroblast-Activating Protein (FAP)
6. Carbonic Anhydrase IX (CAIX) Inhibitors
7. CXCR4
8. meta-I-benzylguanidine (MIBG) Derivatives
9. Discussion
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Modoni, S.; Frangos, S.; Iakovou, I.; Boero, M.; Mansi, L. Theragnostics before we found its name. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Oldan, J.D.; Pomper, M.G.; Werner, R.A.; Higuchi, T.; Rowe, S.P. The cutting edge: Promising oncology radiotracers in clinical development. Diagn. Interv. Imaging 2024, 105, 400–406. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, M.; Zang, J.; Jiang, Y.; Chen, X.; Zhu, Z.; Chen, X. A pilot study of (68) Ga-PSMA-617 PET/CT imaging and (177)Lu-EB-PSMA-617 radioligand therapy in patients with adenoid cystic carcinoma. EJNMMI Res. 2022, 12, 52. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with (177)Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Terroir, M.; Lamesa, C.; Krim, M.; Vija, L.; Texier, J.S.; Cassou-Mounat, T.; Delord, J.P.; Vallot, D.; Courbon, F. RadioLigand Therapy with [(177)Lu]Lu-PSMA-617 for Salivary Gland Cancers: Literature Review and First Compassionate Use in France. Pharmaceuticals 2023, 16, 754. [Google Scholar] [CrossRef] [PubMed]
- Has Simsek, D.; Kuyumcu, S.; Agaoglu, F.Y.; Unal, S.N. Radionuclide Therapy with 177Lu-PSMA in a Case of Metastatic Adenoid Cystic Carcinoma of the Parotid. Clin. Nucl. Med. 2019, 44, 764–766. [Google Scholar] [CrossRef]
- Civan, C.; Kasper, S.; Berliner, C.; Fragoso-Costa, P.; Grunwald, V.; Pogorzelski, M.; Schaarschmidt, B.M.; Lang, S.; Kersting, D.; Nader, M.; et al. PSMA-Directed Imaging and Therapy of Salivary Gland Tumors: A Single-Center Retrospective Study. J. Nucl. Med. 2023, 64, 372–378. [Google Scholar] [CrossRef]
- Van Herpen, C.M.L.; Uijen, M.; van Ruitenbeek, N.; Driessen, C.M.L.; Gotthardt, M.; Nagarajah, J. 177Lu-PSMA radioligand therapy for patients with recurrent/metastatic (R/M) salivary gland cancer (SGC): A phase II pilot study. J. Clin. Oncol. 2023, 41, 6099. [Google Scholar] [CrossRef]
- de Vries, L.H.; Lodewijk, L.; Braat, A.; Krijger, G.C.; Valk, G.D.; Lam, M.; Borel Rinkes, I.H.M.; Vriens, M.R.; de Keizer, B. (68)Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with (177)Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef]
- Assadi, M.; Ahmadzadehfar, H. (177)Lu-DOTATATE and (177)Lu-prostate-specific membrane antigen therapy in a patient with advanced metastatic radioiodine-refractory differentiated thyroid cancer after failure of tyrosine kinase inhibitors treatment. World J. Nucl. Med. 2019, 18, 406–408. [Google Scholar] [CrossRef]
- Rubino, M.; Di Stasio, G.D.; Bodei, L.; Papi, S.; Rocca, P.A.; Ferrari, M.E.; Fodor, C.I.; Bagnardi, V.; Frassoni, S.; Mei, R.; et al. Peptide receptor radionuclide therapy with 177Lu- or 90Y-SSTR peptides in malignant pheochromocytomas (PCCs) and paragangliomas (PGLs): Results from a single institutional retrospective analysis. Endocrine 2024, 84, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Estevao, R.; Duarte, H.; Lopes, F.; Fernandes, J.; Monteiro, E. Peptide receptor radionuclide therapy in head and neck paragangliomas—Report of 14 cases. Rev. Laryngol. Otol. Rhinol. 2015, 136, 155–158. [Google Scholar]
- Puranik, A.D.; Kulkarni, H.R.; Singh, A.; Baum, R.P. Peptide receptor radionuclide therapy with (90)Y/ (177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours). Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1223–1230. [Google Scholar] [CrossRef]
- Roll, W.; Muther, M.; Sporns, P.B.; Zinnhardt, B.; Suero Molina, E.; Seifert, R.; Schafers, M.; Weckesser, M.; Stegger, L.; Beule, A.G.; et al. Somatostatin Receptor-Targeted Radioligand Therapy in Head and Neck Paraganglioma. World Neurosurg. 2020, 143, e391–e399. [Google Scholar] [CrossRef]
- Zovato, S.; Kumanova, A.; Dematte, S.; Sansovini, M.; Bodei, L.; Di Sarra, D.; Casagranda, E.; Severi, S.; Ambrosetti, A.; Schiavi, F.; et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm. Metab. Res. 2012, 44, 411–414. [Google Scholar] [CrossRef]
- Yadav, M.P.; Raju, S.; Ballal, S.; Bal, C. Complete Response to 177 Lu-DOTATATE PRRT in a 9-Year-Old Child with Metastatic Carotid Body Paraganglioma. Clin. Nucl. Med. 2024, 49, e33–e34. [Google Scholar] [CrossRef]
- Lon, I.; Kunikowska, J.; Jedrusik, P.; Gora, J.; Toutounchi, S.; Placha, G.; Gaciong, Z. Familial SDHB gene mutation in disseminated non-hypoxia-related malignant paraganglioma treated with [(90)Y]Y/[(177)Lu]Lu-DOTATATE. Intractable Rare Dis. Res. 2021, 10, 207–213. [Google Scholar] [CrossRef]
- Zahid, A.; Johnson, D.R.; Kizilbash, S.H. Efficacy of (177)Lu-Dotatate Therapy in the Treatment of Recurrent Meningioma. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 236–240. [Google Scholar] [CrossRef]
- Pirisino, R.; Filippi, L.; D’Agostini, A.; Bagni, O. Management of a Patient with Metastatic Gastrointestinal Neuroendocrine Tumor and Meningioma Submitted to Peptide Receptor Radionuclide Therapy with 177 Lu-DOTATATE. Clin. Nucl. Med. 2022, 47, e692–e695. [Google Scholar] [CrossRef]
- Wrange, E.K.M.; Harders, S.M.W. A rare case of metastatic atypical meningioma that highlights the shortcomings of treatment options at present. Acta Radiol. Open 2022, 11, 20584601221109919. [Google Scholar] [CrossRef]
- Seystahl, K.; Stoecklein, V.; Schuller, U.; Rushing, E.; Nicolas, G.; Schafer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.C.; et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016, 18, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Muther, M.; Roll, W.; Brokinkel, B.; Zinnhardt, B.; Sporns, P.B.; Seifert, R.; Schafers, M.; Weckesser, M.; Stegger, L.; Stummer, W.; et al. Response assessment of somatostatin receptor targeted radioligand therapies for progressive intracranial meningioma. Nuklearmedizin 2020, 59, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kurz, S.C.; Zan, E.; Cordova, C.; Troxel, A.B.; Barbaro, M.; Silverman, J.S.; Snuderl, M.; Zagzag, D.; Kondziolka, D.; Golfinos, J.G.; et al. Evaluation of the SSTR2-targeted Radiopharmaceutical 177Lu-DOTATATE and SSTR2-specific 68Ga-DOTATATE PET as Imaging Biomarker in Patients with Intracranial Meningioma. Clin. Cancer Res. 2024, 30, 680–686. [Google Scholar] [CrossRef]
- Severi, S.; Grassi, I.; Bongiovanni, A.; Nicolini, S.; Marini, I.; Arpa, D.; Ranallo, N.; Azzali, I.; Di Iorio, V.; Sarnelli, A.; et al. Peptide Receptor Radionuclide Therapy in Advanced Refractory Meningiomas: Efficacy and Toxicity in a Long Follow-up. J. Nucl. Med. 2024, 65, 1409–1415. [Google Scholar] [CrossRef]
- Braat, A.; Snijders, T.J.; Seute, T.; Vonken, E.P.A. Will (177)Lu-DOTATATE Treatment Become More Effective in Salvage Meningioma Patients, When Boosting Somatostatin Receptor Saturation? A Promising Case on Intra-arterial Administration. Cardiovasc. Intervent. Radiol. 2019, 42, 1649–1652. [Google Scholar] [CrossRef]
- Puranik, A.D.; Dev, I.D.; Rangarajan, V.; Kulkarni, S.; Shetty, N.; Gala, K.; Sahu, A.; Bhattacharya, K.; Dasgupta, A.; Chatterjee, A.; et al. PRRT with Lu-177 DOTATATE in Treatment-Refractory Progressive Meningioma: Initial Experience from a Tertiary-Care Neuro-Oncology Center. Neurol. India 2024, 72, 278–284. [Google Scholar] [CrossRef]
- Amerein, A.; Maurer, C.; Kircher, M.; Gable, A.; Krebold, A.; Rinscheid, A.; Viering, O.; Pfob, C.H.; Bundschuh, R.A.; Behrens, L.; et al. Intraarterial Administration of Peptide Receptor Radionuclide Therapy in Patients with Advanced Meningioma: Initial Safety and Efficacy. J. Nucl. Med. 2024, 65, 1911–1916. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2022, 32, 65–77. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Rosch, F.; ArunRaj, S.T.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Bal, C. First-in-Human Experience with 177Lu-DOTAGA.(SA.FAPi)2 Therapy in an Uncommon Case of Aggressive Medullary Thyroid Carcinoma Clinically Mimicking as Anaplastic Thyroid Cancer. Clin. Nucl. Med. 2022, 47, e444–e445. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Zhao, T.; Wang, H.; Chen, Y.; Xu, W.; Pang, Y.; Guo, W.; Sun, L.; Wu, H.; et al. Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study. Clin. Cancer Res. 2023, 29, 4740–4750. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Sun, L.; Wu, H.; Chen, H. FAP-Targeted Radionuclide Therapy of Advanced Radioiodine-Refractory Differentiated Thyroid Cancer with Multiple Cycles of 177 Lu-FAPI-46. Clin. Nucl. Med. 2022, 47, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients with Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Pang, Y.; Zhao, L.; Lin, L.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. FAP-targeted radionuclide therapy with [(177)Lu]Lu-FAPI-46 in metastatic nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Barashki, S.; Divband, G.; Askari, E.; Amini, H.; Aryana, K. Fibroblast Activation Protein Inhibitor Imaging and Therapy in a Patient with Multiple Endocrine Neoplasia Type 2A Syndrome. Clin. Nucl. Med. 2022, 47, e284–e286. [Google Scholar] [CrossRef]
- Rowe, S.P.; Drzezga, A.; Neumaier, B.; Dietlein, M.; Gorin, M.A.; Zalutsky, M.R.; Pomper, M.G. Prostate-Specific Membrane Antigen-Targeted Radiohalogenated PET and Therapeutic Agents for Prostate Cancer. J. Nucl. Med. 2016, 57, 90S–96S. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, K.C. Molecular theranostics: A primer for the imaging professional. AJR Am. J. Roentgenol. 2011, 197, 318–324. [Google Scholar] [CrossRef]
- Quintos-Meneses, H.A.; Aranda-Lara, L.; Morales-Avila, E.; Torres-Garcia, E.; Camacho-Lopez, M.A.; Sanchez-Holguin, M.; Luna-Gutierrez, M.A.; Ramirez-Duran, N.; Isaac-Olive, K. In vitro irradiation of doxorubicin with (18)F-FDG Cerenkov radiation and its potential application as a theragnostic system. J. Photochem. Photobiol. B 2020, 210, 111961. [Google Scholar] [CrossRef]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef]
- Wang, J.H.; Kiess, A.P. PSMA-targeted therapy for non-prostate cancers. Front. Oncol. 2023, 13, 1220586. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; et al. Overall survival with [(177)Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Racca, M.; Dall’Armellina, S.; Delgado Bolton, R.C.; Albano, D.; Dondi, F.; Bertagna, F.; Annunziata, S.; Treglia, G. Potential Role of PSMA-Targeted PET in Thyroid Malignant Disease: A Systematic Review. Diagnostics 2023, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Russell, J.; Rooper, L.M.; Ladenson, P.W.; Pomper, M.G.; Rowe, S.P. The prostate-specific membrane antigen (PSMA)-targeted radiotracer (18)F-DCFPyL detects tumor neovasculature in metastatic, advanced, radioiodine-refractory, differentiated thyroid cancer. Med. Oncol. 2020, 37, 98. [Google Scholar] [CrossRef]
- Brada, M.D.; Rushing, E.J.; Bachinger, D.; Zoller, L.; Burger, I.A.; Hullner, M.W.; Moch, H.; Huber, A.; Eckhard, A.H.; Rupp, N.J. Immunohistochemical Expression Pattern of Theragnostic Targets SSTR2 and PSMA in Endolymphatic Sac Tumors: A Single Institution Case Series. Head Neck Pathol. 2022, 16, 1012–1018. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- van Essen, M.; Krenning, E.P.; Kooij, P.P.; Bakker, W.H.; Feelders, R.A.; de Herder, W.W.; Wolbers, J.G.; Kwekkeboom, D.J. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J. Nucl. Med. 2006, 47, 1599–1606. [Google Scholar]
- Hanscheid, H.; Sweeney, R.A.; Flentje, M.; Buck, A.K.; Lohr, M.; Samnick, S.; Kreissl, M.; Verburg, F.A. PET SUV correlates with radionuclide uptake in peptide receptor therapy in meningioma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1284–1288. [Google Scholar] [CrossRef]
- Dressen, M.S.; Muthukrishnan, A.; Tragon, T.R.; Lieberman, F.S.; Mountz, J.M. Complementary Molecular and Metabolic Characterization of Meningiomas with DOTATATE and FDG-PET: Advancing Treatment Planning and Prognostication. Clin. Nucl. Med. 2019, 44, e26–e27. [Google Scholar] [CrossRef]
- Backhaus, P.; Huss, S.; Kosek, V.; Weckesser, M.; Rahbar, K. Lung Metastases of Intracranial Atypical Meningioma Diagnosed on Posttherapeutic Imaging After 177Lu-DOTATATE Therapy. Clin. Nucl. Med. 2018, 43, e184–e185. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jager, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jager, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Kumar, V.; Kline, B.; Escobar, D.; Aslam, M.; Cooperberg, M.R.; Aggarwal, R.R.; de Kouchkovsky, I.; Chou, J.; Meng, M.V.; et al. Initial Experience with (68)Ga-FAP-2286 PET Imaging in Patients with Urothelial Cancer. J. Nucl. Med. 2024, 65, 199–205. [Google Scholar] [CrossRef] [PubMed]
- van den Hoven, A.F.; Keijsers, R.G.M.; Lam, M.; Glaudemans, A.; Verburg, F.A.; Vogel, W.V.; Lavalaye, J. Current research topics in FAPI theranostics: A bibliometric analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1014–1027. [Google Scholar] [CrossRef]
- Guglielmo, P.; Alongi, P.; Baratto, L.; Conte, M.; Abenavoli, E.M.; Buschiazzo, A.; Celesti, G.; Dondi, F.; Filice, R.; Gorica, J.; et al. FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature. Cancers 2024, 16, 839. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, C.; Huang, C.; Li, D. Head-to-head comparison of [(68)Ga]Ga-FAPI-04 PET and [(18)F]FDG PET in the detection of cancer recurrence: A systematic review and meta-analysis. Transl. Cancer Res. 2024, 13, 2779–2789. [Google Scholar] [CrossRef]
- Slania, S.L.; Das, D.; Lisok, A.; Du, Y.; Jiang, Z.; Mease, R.C.; Rowe, S.P.; Nimmagadda, S.; Yang, X.; Pomper, M.G. Imaging of Fibroblast Activation Protein in Cancer Xenografts Using Novel (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine-Based Small Molecules. J. Med. Chem. 2021, 64, 4059–4070. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Kovan, B.; Sanli, Y.; Buyukkaya, F.; Has Simsek, D.; Ozkan, Z.G.; Isik, E.G.; Ekenel, M.; Turkmen, C. Safety of Fibroblast Activation Protein-Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021, 46, 641–646. [Google Scholar] [CrossRef]
- Lin, J.; Wang, D.; Liu, J.; Yang, L.; Liu, J. Carbonic anhydrase IX-based tumor imaging and therapy: A review. Sheng Wu Gong Cheng Xue Bao 2023, 39, 116–131. [Google Scholar] [CrossRef]
- Gieling, R.G.; Williams, K.J. Carbonic anhydrase IX as a target for metastatic disease. Bioorg. Med. Chem. 2013, 21, 1470–1476. [Google Scholar] [CrossRef]
- Brockton, N.; Dort, J.; Lau, H.; Hao, D.; Brar, S.; Klimowicz, A.; Petrillo, S.; Diaz, R.; Doll, C.; Magliocco, A. High stromal carbonic anhydrase IX expression is associated with decreased survival in P16-negative head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 249–257. [Google Scholar] [CrossRef]
- Huizing, F.J.; Hoeben, B.A.W.; Lok, J.; Boerman, O.C.; Heskamp, S.; Bussink, J. Imaging carbonic anhydrase IX as a method for monitoring hypoxia-related radioresistance in preclinical head and neck cancer models. Phys. Imaging Radiat. Oncol. 2021, 19, 145–150. [Google Scholar] [CrossRef]
- Huizing, F.J.; Garousi, J.; Lok, J.; Franssen, G.; Hoeben, B.A.W.; Frejd, F.Y.; Boerman, O.C.; Bussink, J.; Tolmachev, V.; Heskamp, S. CAIX-targeting radiotracers for hypoxia imaging in head and neck cancer models. Sci. Rep. 2019, 9, 18898. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.A.; Wichmann, C.W.; Osellame, L.D.; Cao, Z.; Guo, N.; Scott, A.M.; Donnelly, P.S. Tumor targeted alpha particle therapy with an actinium-225 labelled antibody for carbonic anhydrase IX. Chem. Sci. 2024, 15, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Iikuni, S.; Ono, M.; Watanabe, H.; Shimizu, Y.; Sano, K.; Saji, H. Cancer radiotheranostics targeting carbonic anhydrase-IX with (111)In- and (90)Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics 2018, 8, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.; Haug, A.; Buske, C.; Heidegger, S.; Illert, A.L.; Bassermann, F.; Herhaus, P.; Buck, A.; Duell, J.; Topp, M.S.; et al. CXCR4-targeted Theranostics in Hematooncology: Opportunities and Challenges. Nuklearmedizin 2024, 63, 57–61. [Google Scholar] [CrossRef]
- Lindenberg, L.; Ahlman, M.; Lin, F.; Mena, E.; Choyke, P. Advances in PET Imaging of the CXCR4 Receptor: [(68)Ga]Ga-PentixaFor. Semin. Nucl. Med. 2024, 54, 163–170. [Google Scholar] [CrossRef]
- Roustaei, H.; Norouzbeigi, N.; Vosoughi, H.; Aryana, K. A dataset of [(68)Ga]Ga-Pentixafor PET/CT images of patients with high-grade Glioma. Data Brief 2023, 48, 109236. [Google Scholar] [CrossRef]
- Waheed, A.; Singh, B.; Watts, A.; Kaur, H.; Singh, H.; Dhingra, K.; Ahuja, C.; Madan, R.; Singh, A.; Radotra, B.D. 68 Ga-Pentixafor PET/CT for In Vivo Imaging of CXCR4 Receptors in Glioma Demonstrating a Potential for Response Assessment to Radiochemotherapy: Preliminary Results. Clin. Nucl. Med. 2024, 49, e141–e148. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, F. Research Progress of CXCR4-Targeting Radioligands for Oncologic Imaging. Korean J. Radiol. 2023, 24, 871–889. [Google Scholar] [CrossRef]
- Waked, A.; Crabbe, M.; Neirinckx, V.; Perez, S.R.; Wellens, J.; Rogister, B.; Benotmane, M.A.; Vermeulen, K. Preclinical evaluation of CXCR4 peptides for targeted radionuclide therapy in glioblastoma. EJNMMI Radiopharm. Chem. 2024, 9, 52. [Google Scholar] [CrossRef]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Taieb, D.; Hicks, R.J.; Hindie, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kumar, R.; Passah, A.; Tripathi, M.; Agarwala, S.; Khadgawat, R.; Bal, C. Prospective evaluation of 68Ga-DOTANOC positron emission tomography/computed tomography and 131I-meta-iodobenzylguanidine single-photon emission computed tomography/computed tomography in extra-adrenal paragangliomas, including uncommon primary sites and to define their diagnostic roles in current scenario. Nucl. Med. Commun. 2019, 40, 1230–1242. [Google Scholar] [CrossRef]
- Al-Ward, R.; Brondani, V.B.; Sawani, S.; Potter, C.L.; Xu, G.; Waguespack, S.G.; Varghese, J.; Habra, M.A.; Lu, Y.; Jimenez, C. High-Specific-Activity 131 I-MIBG for the Treatment of Advanced Pheochromocytoma and Paraganglioma. Clin. Nucl. Med. 2024, 49, 610–620. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Solnes, L.B.; Werner, R.A.; Jones, K.M.; Sadaghiani, M.S.; Bailey, C.R.; Lapa, C.; Pomper, M.G.; Rowe, S.P. Theranostics: Leveraging Molecular Imaging and Therapy to Impact Patient Management and Secure the Future of Nuclear Medicine. J. Nucl. Med. 2020, 61, 311–318. [Google Scholar] [CrossRef]
- Xu, X.; Jané, P.; Taelman, V.; Jané, E.; Dumont, R.A.; Garama, Y.; Kim, F.; Del Val Gómez, M.; Gariani, K.; Walter, M.A. The Theranostic Genome. Nat. Commun. 2024, 15, 10904. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, D.; Yu, Y.; Zhang, L.; Wang, B.; Karges, J.; Xiao, H. Theranostic imaging and multimodal photodynamic therapy and immunotherapy using the mTOR signaling pathway. Nat. Commun. 2023, 14, 5350. [Google Scholar] [CrossRef]
| Target | Diagnostic Agent | Therapeutic Agent | Targets |
|---|---|---|---|
| PSMA | 18F-flotufolastat *, 18F-piflufolastat *, 68Ga-PSMA-gozetotide * | 177Lu-PSMA-617 * (vipivotide tetraxetan) | Prostate cancer *, adenoid cystic carcinoma, iodine-refractory well-differentiated thyroid cancer, endolymphatic sac tumor (under study) |
| DOTATATE | 68Ga-DOTATATE (gozetotide) *, 68Ga-DOTATOC (edotreotide) *, 64Cu-DOTATATE * | 177Lu-DOTATATE *, 90Y-DOTATATE | SSTR-positive neuroendocrine tumors (incl. gastroentericopancreatic, paraganglioma/pheochromocytoma) *, meningioma |
| FAP | 68Ga-FAPI-04, 68Ga-FAPI-46, others | 177Lu-DOTAGA.(SA.FAPi)2, 177Lu-EB-FAPI, | |
| CAIX | 89Zr-girentuximab | None yet | Renal cell cancer |
| CXCR4 | 68Ga-pentixafor | 177Lu-DOTAT-POL3026 | Glioblastoma |
| Alpha2 adreno-ceptor | 123I-MIBG * | 131I-MIBG * (no longer made) | Neuroblastoma |
| Therapeutic Agent | Targets | n | Efficacy | Ref. |
|---|---|---|---|---|
| 177Lu-PSMA-617 * | ACC | 4 | 2 PR, 2 mixed | [3] |
| 177Lu-PSMA-617 * | Salivary gland cancer (mixed) | 6 | 1 SD, 1 PR; 4 pain red | [4] |
| 177Lu-PSMA-617 * | ACC | 1 | SD, Pain reduction | [5] |
| 177Lu-PSMA-617 * | ACC | 1 | PD, Pain reduction | [6] |
| 177Lu-PSMA-617 * | Salivary gland cancer (mixed) | 5 | 4 PD, 1 SD | [7] |
| 177Lu-PSMA-617 * | ACC, salivary duct carcinoma | 15 | 4 PD, 3 SD (5 discontinued tx) | [8] |
| 177Lu-PSMA-617 * | Iodine-refractory DTC | 2 | 1 PD, 1 PR | [9] |
| 177Lu-PSMA-617 * | Iodine-refractory DTC | 1 | 1 PD | [10] |
| 177Lu-DOTATATE *, 90Y-DOTATATE, 90Y-DOTATOC | Paraganglioma/pheochromocytoma | 30 | 4 PD, 19 SD, 7 PR | [11] |
| 177Lu-DOTATATE * | HN Paraganglioma | 14 | 10 PR (by SUV) | [12] |
| 177Lu-DOTATATE *, 90Y-DOTATATE | HN paraganglioma | 9 | 4 PR, 5 SD | [13] |
| 177Lu-DOTATATE * | HN paraganglioma | 7 | 4 PR, 3 SD | [14] |
| 177Lu-DOTATATE * | HN/mediastinal paraganglioma | 4 | 2 PR, 2 SD | [15] |
| 177Lu-DOTATATE * | Carotid body paraganglioma | 1 | PR | [16] |
| 177Lu-DOTATATE *, 90Y-DOTATATE | Carotid body paraganglioma | 1 | SD | [17] |
| 177Lu-DOTATATE * | Meningioma (recurrent) | 1 | SD | [18] |
| 177Lu-DOTATATE * | Meningioma (metastatic) | 1 | SD | [19] |
| 177Lu-DOTATATE * | Meningioma (metastatic) | 1 | PD | [20] |
| 177Lu-DOTATATE * | Meningioma (refractory, prog.) | 20 | 10 PR, 10 SD | [21] |
| 177Lu-DOTATATE * | Meningioma (progressive) | 4 | 2 PD, 2 SD | [22] |
| 177Lu-DOTATATE * | Meningioma (progressive) | 14 | 7 PD, 7 SD | [23] |
| 177Lu-DOTATATE *, 90Y-DOTATOC | Meningioma | 42 | 23 SD, 1 PR | [24] |
| 177Lu-DOTATATE * (IA) | Meningioma | 1 | 1 PR | [25] |
| 177Lu-DOTATATE * (IA) | Meningioma | 8 | 1 PD, 7 SD | [26] |
| 177Lu-DOTATATE * (IA) | Meningioma | 13 | 3 PD, 8 SD, 1 PR, 1 CR | [27] |
| 177Lu-DOTAGA.(SA.FAPi)2 | RAI/TKI-refractory DTC | 15 | 8 PD, 4 SD, 3 PR | [28] |
| 177Lu-DOTAGA.(SA.FAPi)2 | Medullary thyroid cancer | 1 | PR | [29] |
| 177Lu-EB-FAPI | DTC | 12 | 2 PD, 7 SD, 3 PR | [30] |
| 177Lu-FAPI-46 | DTC | 1 | SD | [31] |
| 177Lu-FAPI-46 | Nasopharyngeal cancer | 1 | SD | [32] |
| 177Lu-FAPI-46 | Nasopharyngeal cancer | 1 | Mixed response | [33] |
| 177Lu-FAPI-46 | MEN 2A (multiple cancers) | 1 | Symptomatic only | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldan, J.D.; Solnes, L.B.; Chin, B.B.; Rowe, S.P. A Look to the Future: Potential Theranostic Applications in Head and Neck Tumors. Cancers 2025, 17, 695. https://doi.org/10.3390/cancers17040695
Oldan JD, Solnes LB, Chin BB, Rowe SP. A Look to the Future: Potential Theranostic Applications in Head and Neck Tumors. Cancers. 2025; 17(4):695. https://doi.org/10.3390/cancers17040695
Chicago/Turabian StyleOldan, Jorge D., Lilja B. Solnes, Bennett B. Chin, and Steven P. Rowe. 2025. "A Look to the Future: Potential Theranostic Applications in Head and Neck Tumors" Cancers 17, no. 4: 695. https://doi.org/10.3390/cancers17040695
APA StyleOldan, J. D., Solnes, L. B., Chin, B. B., & Rowe, S. P. (2025). A Look to the Future: Potential Theranostic Applications in Head and Neck Tumors. Cancers, 17(4), 695. https://doi.org/10.3390/cancers17040695










