Identifying Rural Hotspots for Head and Neck Cancer Using the Bayesian Mapping Approach
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Patient Cohort
3.2. Incidence Risk Mapping
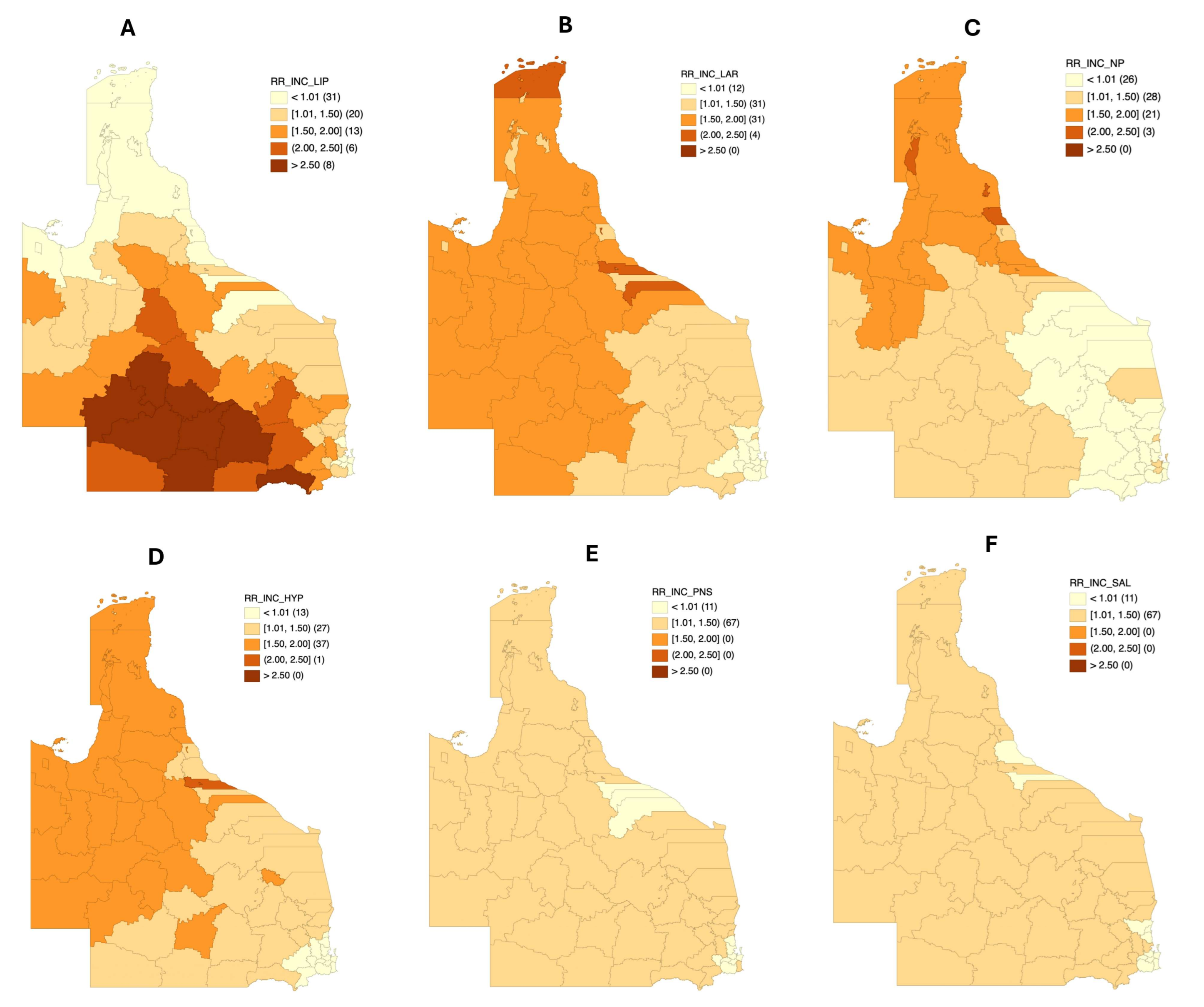
3.3. Incidence Risk Mapping by Different Anatomical Sites
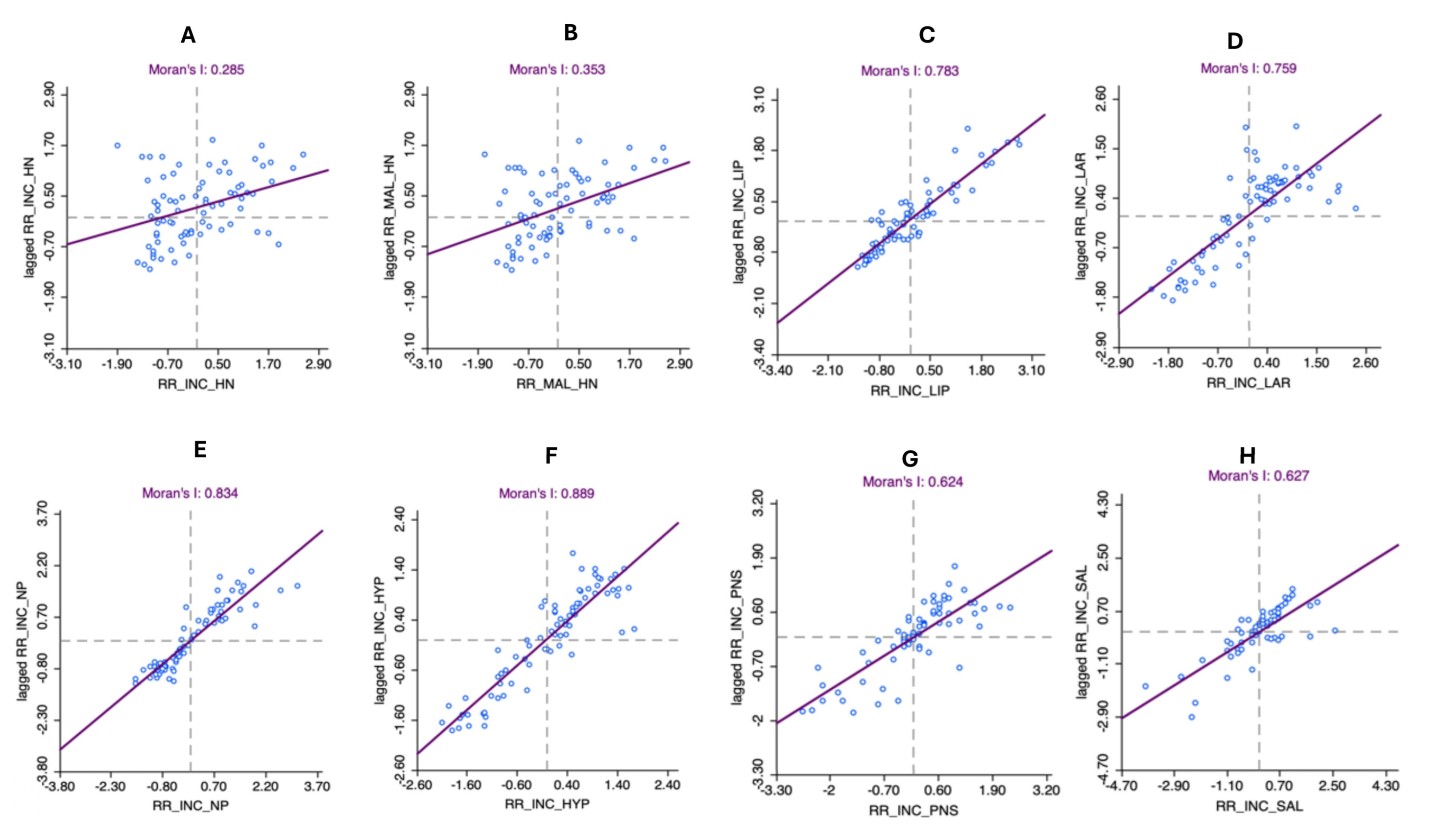
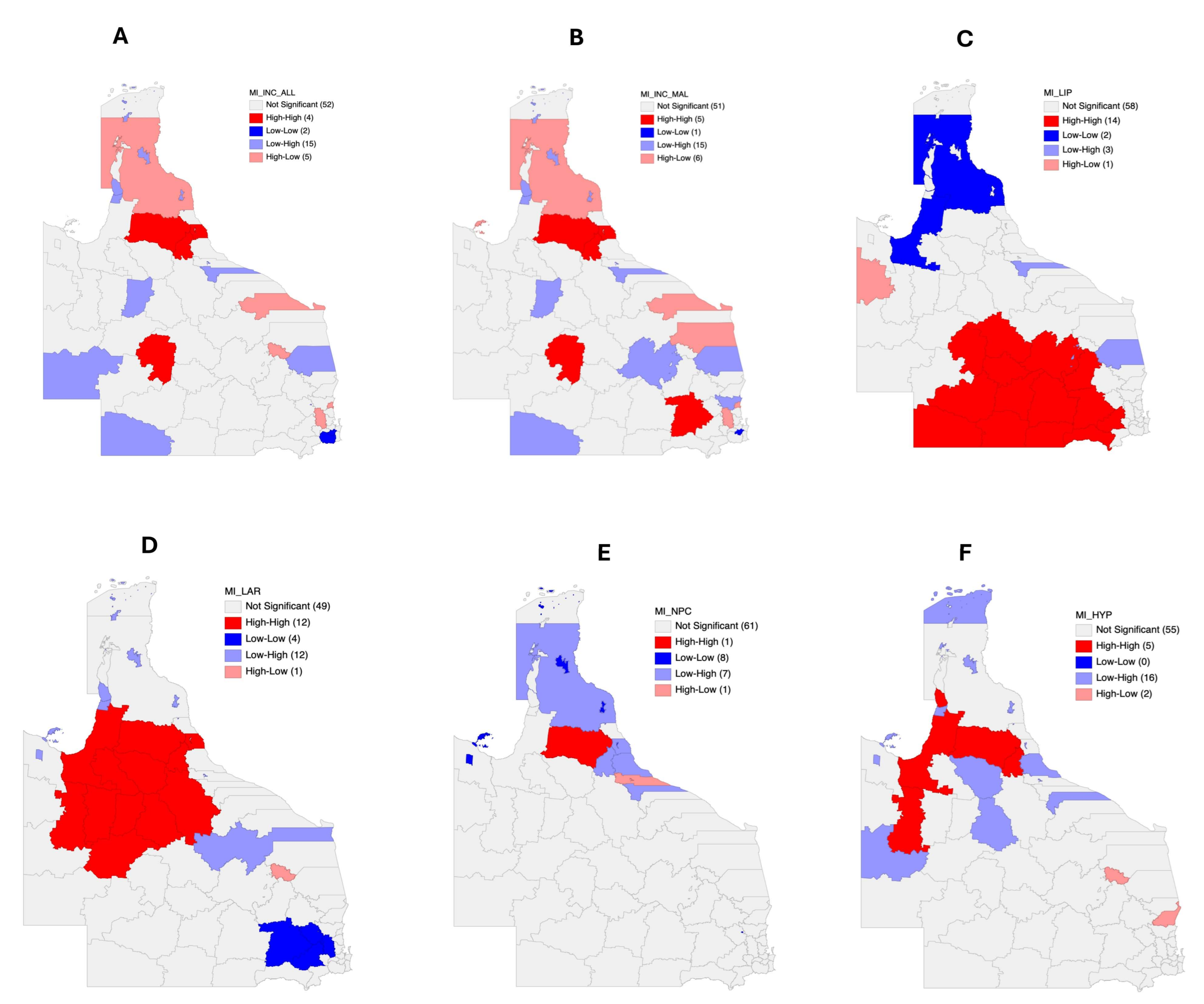
3.4. Autocorrelation Analysis for Head and Neck Cancer Incidence
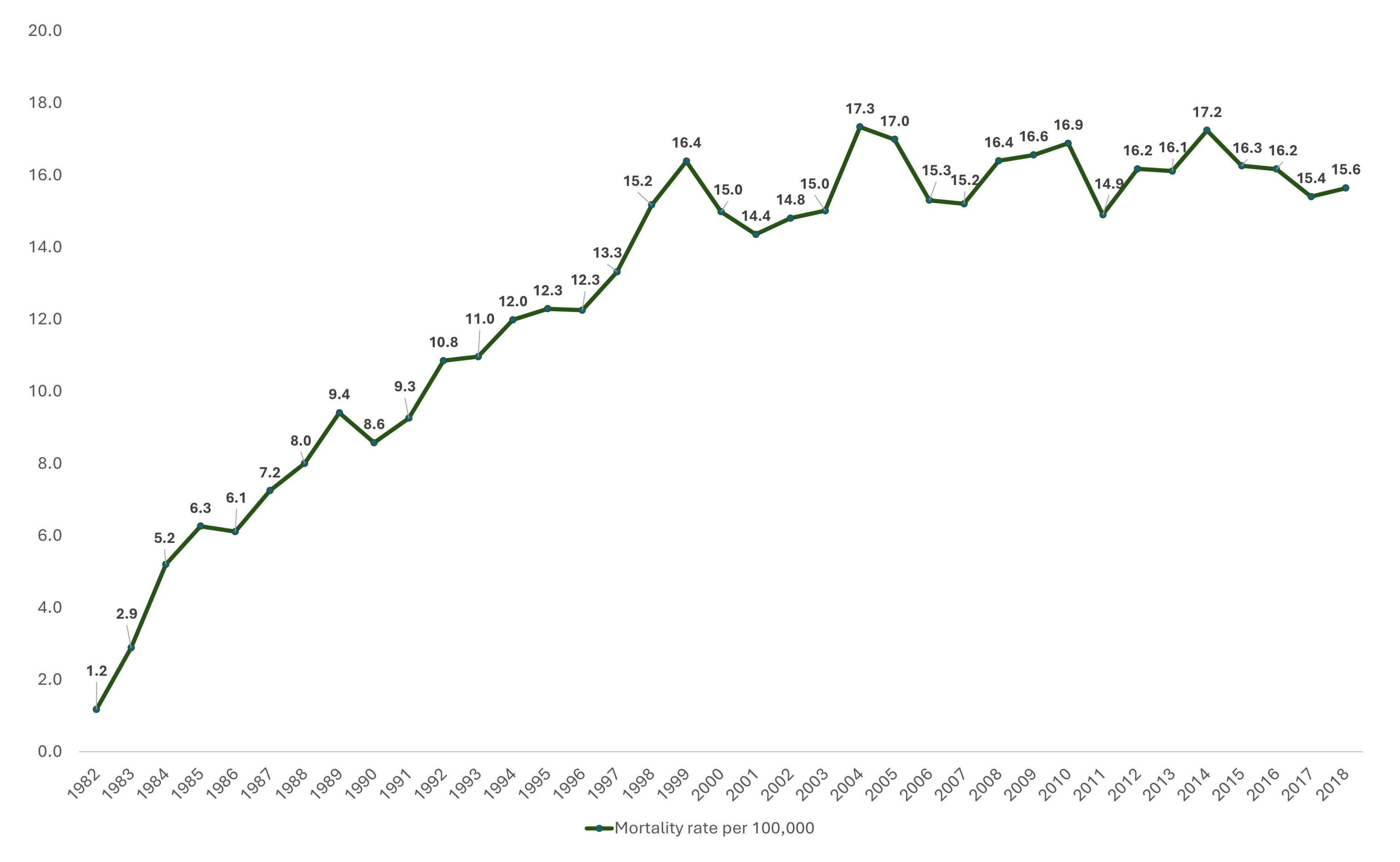
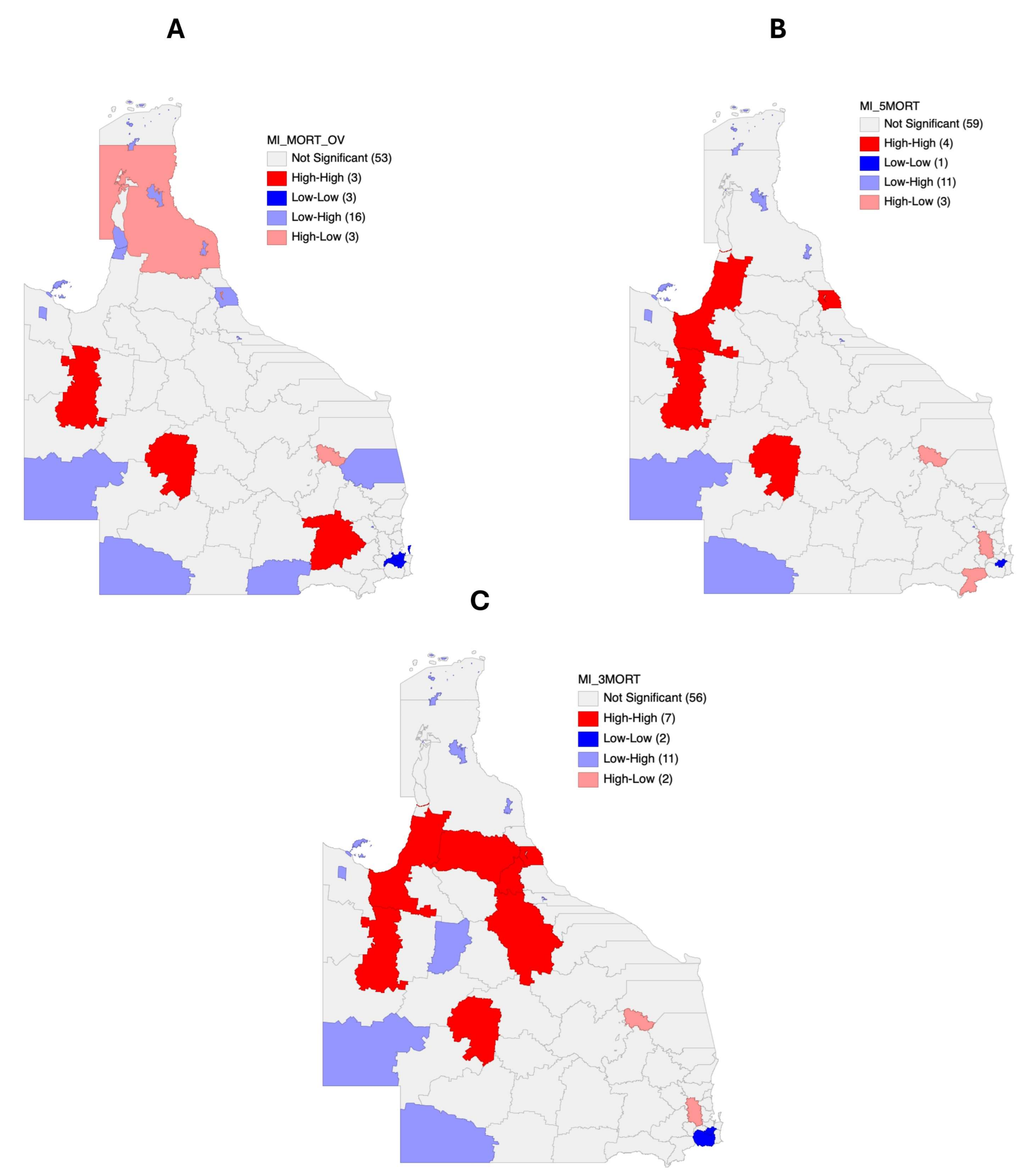
3.5. Mortality Risk Mapping
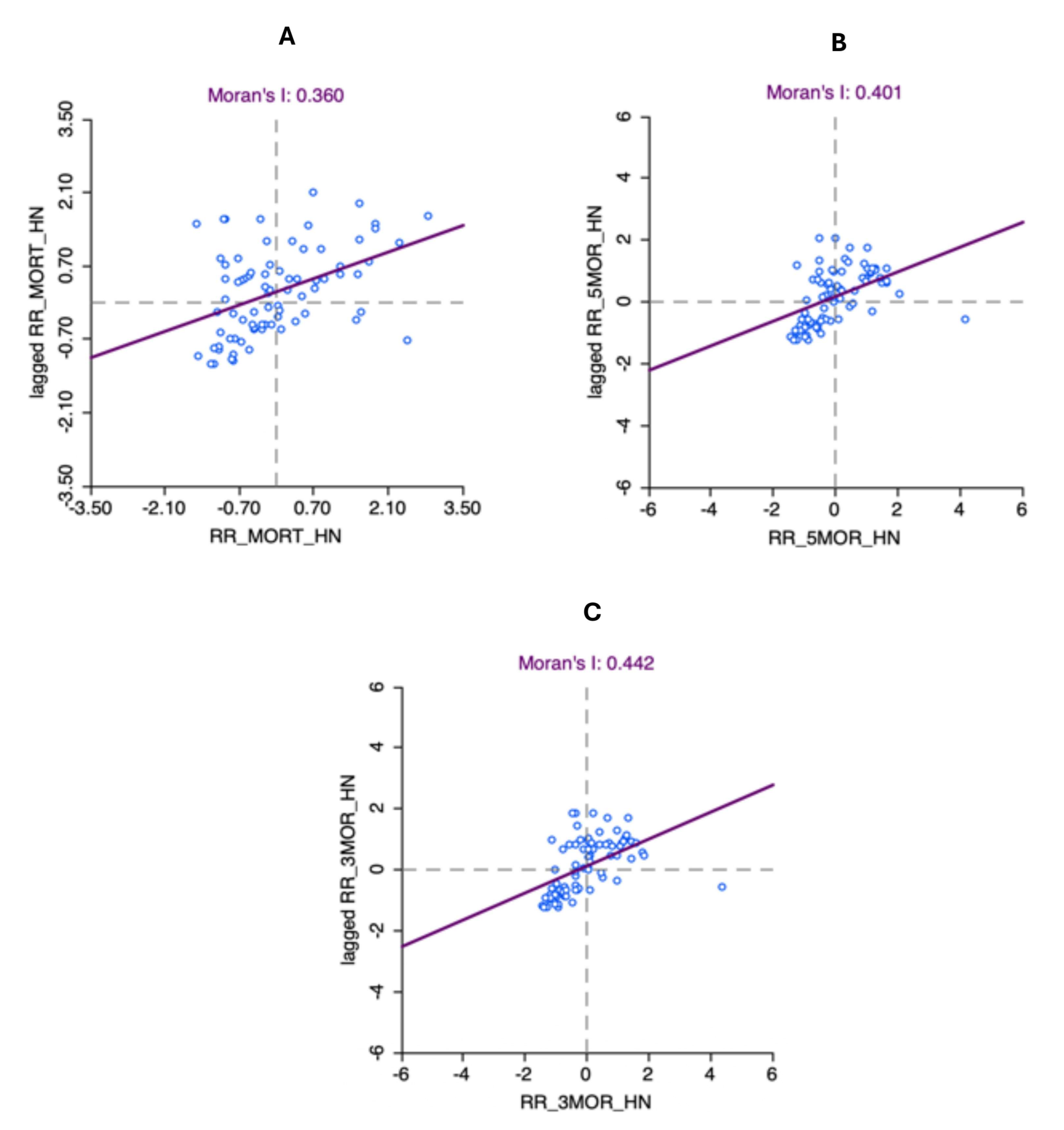
3.6. Autocorrelation Analysis for Head and Neck Cancer Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australian Bureau of Statistics. Census DataPacks. Available online: https://www.abs.gov.au/census/find-census-data/datapacks?release=2016&product=GCP&geography=LGA&header=S (accessed on 12 June 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Walsh, P.; D’Costa, I.; Bhatti, O. Head and neck squamous cell carcinoma survivorship care. Aust. J. Gen. Pract. 2019, 48, 846–848. [Google Scholar] [CrossRef] [PubMed]
- What Is Head and Neck Cancer? Available online: https://www.headandneckcancer.org.au/head-and-neck-cancer-definition/what-is-oral-cancer/ (accessed on 21 February 2025).
- Jayaraj, R.; Singh, J.; Baxi, S.; Ramamoorthi, R.; Thomas, M. Trends in incidence of head and neck cancer in the northern territory, Australia, between 2007 and 2010. Asian Pac. J. Cancer Prev. 2014, 15, 7753–7756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, A.; Sharma, D.; Choi, S.W.; Ramamurthy, P.; Thomson, P. Oral cancer in Australia: Rising incidence and worsening mortality. J. Oral Pathol. Med. 2023, 52, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Ramamoorthi, R.; Baxi, S.; Jayaraj, R.; Thomas, M. The risk factors of head and neck cancer and their general patterns in Australia: A descriptive review and update. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Pateman, K.A.; Cockburn, N.L.; Batstone, M.D.; Ford, P.J. Quality of life of head and neck cancer patients in urban and regional areas: An Australian perspective. Aust. J. Rural Health 2018, 26, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.R.; Zhang, X.; Dempsey, K.; Garling, L.; Guthridge, S. Trends in cancer incidence and survival for Indigenous and non-Indigenous people in the Northern Territory. Med. J. Aust. 2016, 205, 454–458. [Google Scholar] [CrossRef] [PubMed]
- 10-Year Relative Survival—National Cancer Control Indicators. 2022. Available online: https://ncci.canceraustralia.gov.au/outcomes/relative-survival-rate/10-year-relative-survival (accessed on 10 February 2025).
- National Rural Health Alliance. Cancer in Rural Australia—Fact Sheet July 2022; National Rural Health Alliance: Canberra, Australia, 2022; pp. 1–5.
- Shahid, S.; Teng, T.H.; Bessarab, D.; Aoun, S.; Baxi, S.; Thompson, S.C. Factors contributing to delayed diagnosis of cancer among Aboriginal people in Australia: A qualitative study. BMJ Open 2016, 6, e010909. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, J.; Choi, S.W.; Thomson, P. Bayesian disease mapping and the ‘High-Risk’ oral cancer population in Hong Kong. J. Oral Pathol. Med. 2020, 49, 907–913. [Google Scholar] [CrossRef]
- MacNab, Y.C. Bayesian disease mapping: Past, present, and future. Spat. Stat. 2022, 50, 100593. [Google Scholar] [CrossRef]
- Ramamurthy, P.; Sharma, D.; Adeoye, J.; Choi, S.W.; Thomson, P. Bayesian Disease Mapping to Identify High-Risk Population for Oral Cancer: A Retrospective Spatiotemporal Analysis. Int. J. Dent. 2023, 2023, 3243373. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.W.; Cramb, S.M.; Aitken, J.F.; Mengersen, K.L.; Baade, P.D. Development of the Australian Cancer Atlas: Spatial modelling, visualisation, and reporting of estimates. Int. J. Health Geogr. 2019, 18, 21. [Google Scholar] [CrossRef]
- Adeoye, J.A. New World vs Old World: Refining Oral Cancer Screening, Diagnosis, and Prediction. Ph.D. Thesis, The University of Hong Kong, Hong Kong, China, 2023. [Google Scholar]
- Queensland Government. Local Government Area Boundaries—Queensland. Available online: https://www.data.qld.gov.au/dataset/local-government-area-boundaries-queensland (accessed on 16 December 2023).
- Zhang, L.; Wan, X.; Shi, R.; Gong, P.; Si, Y. Comparing spatial patterns of 11 common cancers in Mainland China. BMC Public Health 2022, 22, 1551. [Google Scholar] [CrossRef] [PubMed]
- Bizuayehu, H.M.; Dadi, A.F.; Ahmed, K.Y.; Tegegne, T.K.; Hassen, T.A.; Kibret, G.D.; Ketema, D.B.; Bore, M.G.; Thapa, S.; Odo, D.B.; et al. Burden of 30 cancers among men: Global statistics in 2022 and projections for 2050 using population-based estimates. Cancer 2024, 130, 3708–3723. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jayaraj, R.; Baxi, S.; Ramamoorthi, R.; Thomas, M. Incidence and mortality from mucosal head and neck cancers amongst Australian states and territories: What it means for the northern territory. Asian Pac. J. Cancer Prev. 2013, 14, 5621–5624. [Google Scholar] [CrossRef]
- Foley, J.; Wishart, L.R.; Ward, E.C.; Burns, C.L.; Packer, R.L.; Philpot, S.; Kenny, L.M.; Stevens, M. Exploring the impact of remoteness on people with head and neck cancer: Utilisation of a state-wide dataset. Aust. J. Rural Health 2023, 31, 726–743. [Google Scholar] [CrossRef]
- Inverso, G.; Mahal, B.A.; Aizer, A.A.; Donoff, R.B.; Chau, N.G.; Haddad, R.I. Marital status and head and neck cancer outcomes. Cancer 2015, 121, 1273–1278. [Google Scholar] [CrossRef]
- Dasgupta, P.; Turrell, G.; Aitken, J.F.; Baade, P.D. Partner status and survival after cancer: A competing risks analysis. Cancer Epidemiol. 2016, 41, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Krajc, K.; Mirosevic, S.; Sajovic, J.; Klemenc Ketis, Z.; Spiegel, D.; Drevensek, G.; Drevensek, M. Marital status and survival in cancer patients: A systematic review and meta-analysis. Cancer Med. 2023, 12, 1685–1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ramamurthy, P.; Sharma, D.; Choi, S.-W.; Thomson, P. Oral cancer survival and marital status: Observations from an Australian population. Fac. Dent. J. 2023, 14, 90–96. [Google Scholar] [CrossRef]
- Jong, K.E.; Smith, D.P.; Yu, X.Q.; O’Connell, D.L.; Goldstein, D.; Armstrong, B.K. Remoteness of residence and survival from cancer in New South Wales. Med. J. Aust. 2004, 180, 618–622. [Google Scholar] [CrossRef]
- Hegney, D.; Pearce, S.; Rogers-Clark, C.; Martin-McDonald, K.; Buikstra, E. Close, but still too far. The experience of Australian people with cancer commuting from a regional to a capital city for radiotherapy treatment. Eur. J. Cancer Care 2005, 14, 75–82. [Google Scholar] [CrossRef]
- Afshar, N.; English, D.R.; Blakely, T.; Thursfield, V.; Farrugia, H.; Giles, G.G.; Milne, R.L. Differences in cancer survival by area-level socio-economic disadvantage: A population-based study using cancer registry data. PLoS ONE 2020, 15, e0228551. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Ambs, A.; Keating, N.L.; He, Y.; Doucette, W.R.; Tisnado, D.; Clauser, S.; Kahn, K.L. The role of primary care physicians in cancer care. J. Gen. Intern. Med. 2009, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Venchiarutti, R.L.; Tracy, M.; Clark, J.A.; Palme, C.E.; Young, J.M. Geographic variation in referral practices for patients with suspected head and neck cancer: A survey of general practitioners using a clinical vignette. Aust. J. Rural Health 2022, 30, 501–511. [Google Scholar] [CrossRef]
- Taberna, M.; Gil Moncayo, F.; Jane-Salas, E.; Antonio, M.; Arribas, L.; Vilajosana, E.; Peralvez Torres, E.; Mesia, R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front. Oncol. 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Maslin-Prothero, S. The role of the multidisciplinary team in recruiting to cancer clinical trials. Eur. J. Cancer Care 2006, 15, 146–154. [Google Scholar] [CrossRef]
- Murray, R.B.; Craig, H. A sufficient pipeline of doctors for rural communities is vital for Australia’s overall medical workforce. Med. J. Aust. 2023, 219 (Suppl. S3), S5–S7. [Google Scholar] [CrossRef]
- Cortie, C.H.; Garne, D.; Parker-Newlyn, L.; Ivers, R.G.; Mullan, J.; Mansfield, K.J.; Bonney, A. The Australian health workforce: Disproportionate shortfalls in small rural towns. Aust. J. Rural Health 2024, 32, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Schlichting, J.; Chioreso, C.; Ward, M.; Vikas, P. Challenges of Rural Cancer Care in the United States. Oncology 2015, 29, 633–640. [Google Scholar]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the Rural Cancer Care Gap: Three Institutional Approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Santos Salas, A.; Bassah, N.; Pujadas Botey, A.; Robson, P.; Beranek, J.; Iyiola, I.; Kennedy, M. Interventions to improve access to cancer care in underserved populations in high income countries: A systematic review. Oncol. Rev. 2024, 18, 1427441. [Google Scholar] [CrossRef]
- Balfe, M.; O’Brien, K.; Timmons, A.; Butow, P.; Sullivan, E.O.; Gooberman-Hill, R.; Sharp, L. The unmet supportive care needs of long-term head and neck cancer caregivers in the extended survivorship period. J. Clin. Nurs. 2016, 25, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Neadley, K.; Smith, A.; Martin, S.; Boyd, M.; Hocking, C.; Shoubridge, C. Health Navigator intervention to address the unmet social needs of populations living with cancer attending outpatient treatment at a major metropolitan hospital in Australia: Protocol for a mixed-methods feasibility trial. BMJ Open 2024, 14, e080403. [Google Scholar] [CrossRef] [PubMed]
- Stiller, A.; Goodwin, B.C.; Crawford-Williams, F.; March, S.; Ireland, M.; Aitken, J.F.; Dunn, J.; Chambers, S.K. The Supportive Care Needs of Regional and Remote Cancer Caregivers. Curr. Oncol. 2021, 28, 3041–3057. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Downes, N.; Mendiola, A.; Cordova, A.; Lukity, K.; Imani, J. Multidisciplinary Management of Breast Cancer and Role of the Patient Navigator. Obstet. Gynecol. Clin. N. Am. 2022, 49, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Domenico, E.B.L.; Kalinke, L.P. Nurse Navigator of Cancer Patients: Contributions to the discussion on the national stage. Rev. Bras. Enferm. 2024, 77, e770201. [Google Scholar] [CrossRef] [PubMed]
- Emfield Rowett, K.; Christensen, D. Oncology Nurse Navigation: Expansion of the Navigator Role Through Telehealth. Clin. J. Oncol. Nurs. 2020, 24, 24–31. [Google Scholar] [CrossRef]
- Thackrah, R.D.; Papertalk, L.P.; Taylor, K.; Taylor, E.V.; Greville, H.; Pilkington, L.G.; Thompson, S.C. Perspectives of Aboriginal People Affected by Cancer on the Need for an Aboriginal Navigator in Cancer Treatment and Support: A Qualitative Study. Healthcare 2022, 11, 114. [Google Scholar] [CrossRef]
- Bell, L.; Anderson, K.; Girgis, A.; Aoun, S.; Cunningham, J.; Wakefield, C.E.; Shahid, S.; Smith, A.B.; Diaz, A.; Lindsay, D.; et al. “We Have to Be Strong Ourselves”: Exploring the Support Needs of Informal Carers of Aboriginal and Torres Strait Islander People with Cancer. Int. J. Environ. Res. Public Health 2021, 18, 7281. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Bernardes, C.M.; Beesley, V.; Hawkes, A.L.; Baade, P.; Garvey, G. Unmet supportive care needs of Australian Aboriginal and Torres Strait Islanders with cancer: A prospective, longitudinal study. Support. Care Cancer 2017, 25, 869–877. [Google Scholar] [CrossRef]
- Das, M. Australia’s new cancer nursing and navigation plan. Lancet Oncol. 2024, 25, 18. [Google Scholar] [CrossRef] [PubMed]



| Variable | Category | N (%) |
|---|---|---|
| Age | <40 years | 1349 (5.7) |
| 40–64 years | 12,077 (50.6) | |
| ≥65 years | 10,427 (43.7) | |
| Gender | Female | 5362 (22.5) |
| Male | 18,491 (77.5) | |
| Tumor site | Lip | 6512 (27.3) |
| Oral cavity | 6166 (25.8) | |
| Oropharynx | 5118 (21.5) | |
| Nasopharynx | 334 (1.4) | |
| Hypopharynx | 1261 (5.3) | |
| Larynx | 4024 (16.9) | |
| Nasal cavity and paranasal sinuses | 374 (1.6) | |
| Salivary glands | 64 (0.3) | |
| Tumor grade | Well differentiated | 4878 (20.5) |
| Moderately differentiated | 10,985 (46.1) | |
| Poorly differentiated | 4305 (18.0) | |
| Undifferentiated | 84 (0.4) | |
| Unknown | 3601 (15.1) | |
| Status | Alive | 9721 (40.8) |
| Dead | 14,132 (59.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramamurthy, P.; Adeoye, J.; Choi, S.-W.; Thomson, P.; Sharma, D. Identifying Rural Hotspots for Head and Neck Cancer Using the Bayesian Mapping Approach. Cancers 2025, 17, 819. https://doi.org/10.3390/cancers17050819
Ramamurthy P, Adeoye J, Choi S-W, Thomson P, Sharma D. Identifying Rural Hotspots for Head and Neck Cancer Using the Bayesian Mapping Approach. Cancers. 2025; 17(5):819. https://doi.org/10.3390/cancers17050819
Chicago/Turabian StyleRamamurthy, Poornima, John Adeoye, Siu-Wai Choi, Peter Thomson, and Dileep Sharma. 2025. "Identifying Rural Hotspots for Head and Neck Cancer Using the Bayesian Mapping Approach" Cancers 17, no. 5: 819. https://doi.org/10.3390/cancers17050819
APA StyleRamamurthy, P., Adeoye, J., Choi, S.-W., Thomson, P., & Sharma, D. (2025). Identifying Rural Hotspots for Head and Neck Cancer Using the Bayesian Mapping Approach. Cancers, 17(5), 819. https://doi.org/10.3390/cancers17050819









