The Current Role of Circulating Cell-Free DNA in the Management of Hepatocellular Carcinoma
Simple Summary
Abstract
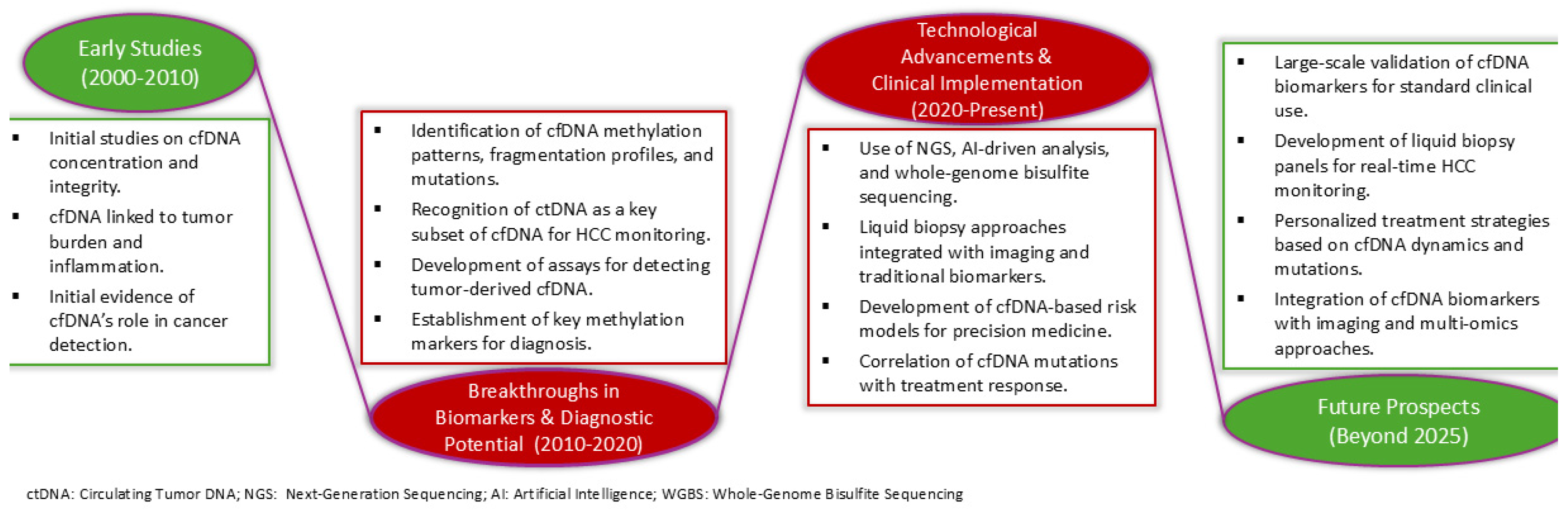
1. Introduction
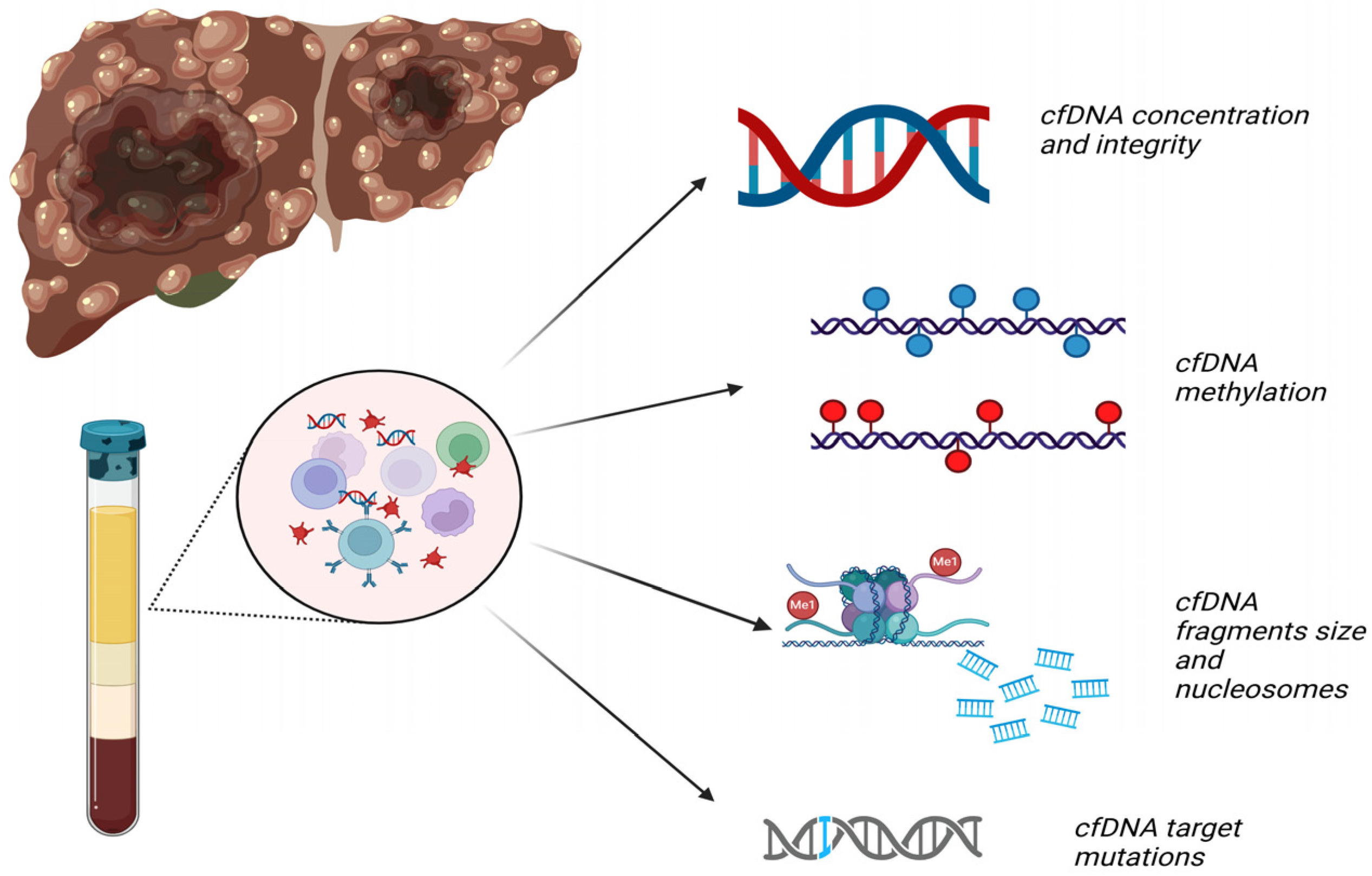
2. Circulating cfDNA
2.1. Types and Subtypes
2.2. Methods of Detection
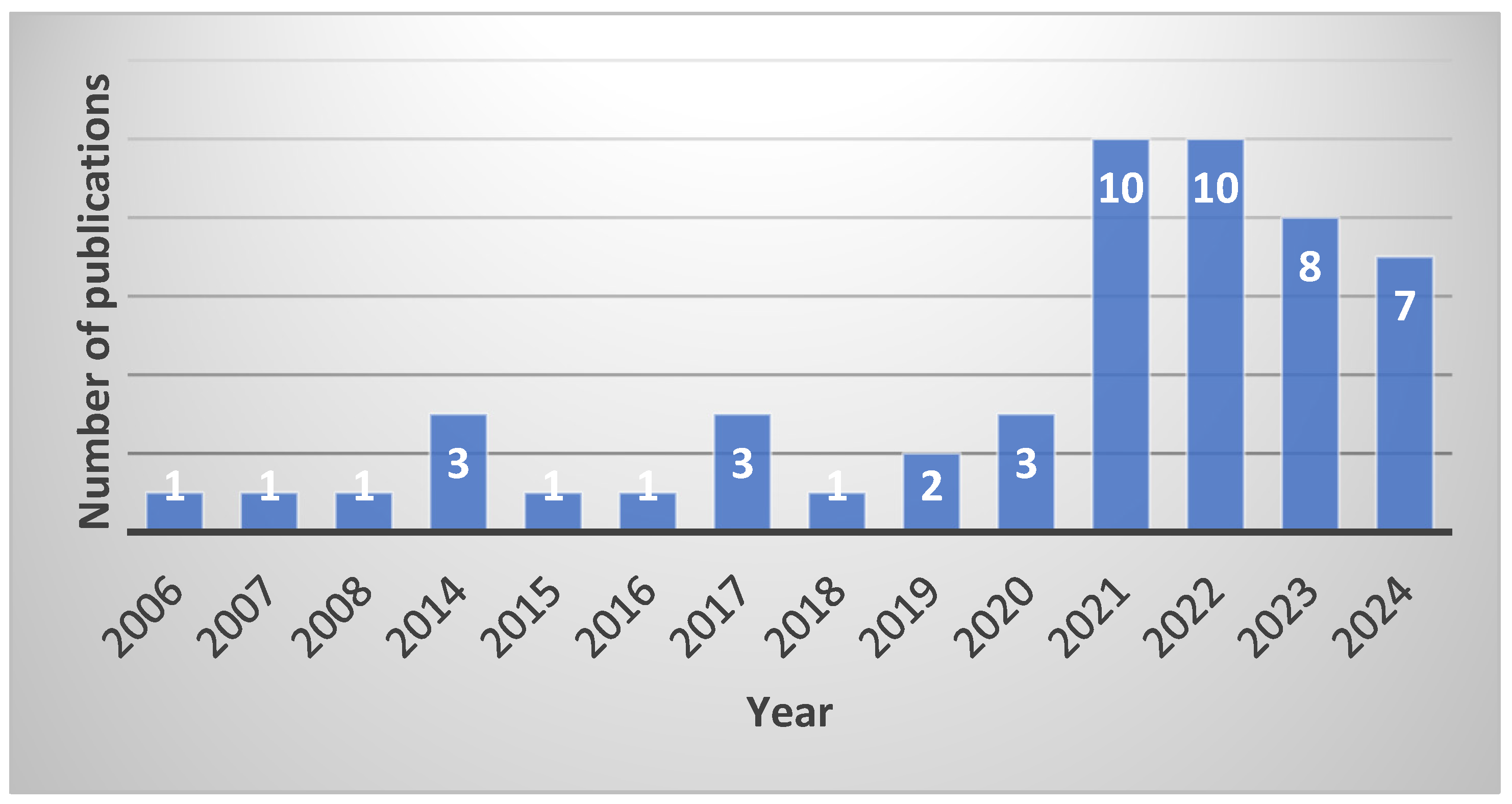
3. Search Strategy, Selection Criteria and Data Extraction
4. cfDNA for HCC Diagnosis
4.1. cfDNA Concentration and Integrity (Table 1)
4.2. cfDNA Methylation in HCC Diagnosis (Table 2)
| First Author, Year [Reference] | HCC Pts | Controls | Species of cfDNA | Sensitivity | Specificity | AUROC (95% CI) | Other Key Findings | |
|---|---|---|---|---|---|---|---|---|
| Xu, 2017 [27] | Training cohort | 715 | 560 | HCC-specific methylation marker Panel by targeted bisulfite sequencing | 86% | 94% | 0.97 (0.96–0.98) | Correlation with tumor burden, stage and treatment response; prediction of survival |
| Validation cohort | 383 | 275 | 83% | 90.5% | 0.94 (0.93−0.96) | |||
| Wang, 2020 [33] | 97 | 80 and 46 CHB/CHC | cfDNA methylation ratio [methylation cp/(methyla-tion + unmethylation cp)] | 79% | 89% | 0.81 (0.72–0.90) | ||
| Lewin, 2021 [34] | Training cohort | 41 | 46 LC | cfDNA methylation markers: HCCBloodTest (Epigenomics AG) and NGS panel | 77% and 57%; NGS and AFP: 68% | 64% and 97%; NGS and AFP: 97% | NGS: 0.85 (0.78–0.91) NGS and AFP: 0.90 (0.84–0.95) | |
| Testing cohort | 60 | 103 LC | ||||||
| Luo, 2022 [28] | Training cohort | 120 | 290 (65 HBsAg+) and 92 LC | cfDNA methylation profiles based on tissue methylation profiles from pts and controls | 86% | 98% | 0.98 (0.97–0.99) | For early-stage HCC diagnosis: AUROC 0.93 (95% CI: 0.90–0.96) |
| Validation cohort | 67 | 242 (56 HBsAg+) and 111 LC | 84% | 96% | 0.97 (0.95–0.99) | |||
| Lin, 2022 [35] | Phase 2 study | 122 | 125 CLD | HelioLiver Test: methylation, clinical and tumor markers | 85% | 91% | 0.94 (0.92–0.97) | HelioLiver Test superior sensitivity for HCC detection than AFP and GALAD score |
| Wang, 2022 [29] | Training cohorts | 30 and 60 | 30 and 60 | (Epi)Genetic alterations in cfDNA and genome-wide discovery of methylation markers | 93% | 95% | 0.96 (0.93–1.00) | |
| Independent cohort | 58 | 198 | 90% | 94% | 0.93 (0.90–0.97) | |||
| Phan, 2022 [30] | Testing cohort | 58 | 121 LC or CH | cfDNA methylation markers (450 target regions, 18,000 CpG sites) | 62% | 91% | 0.84 (0.82–0.90) | Plus GALAD score—AUROC: 0.87 (95% CI: 0.85–0.94) (sensitivity: 69%, specificity: 96%) |
| Validation cohort | 48 | 72 LC or CH | 60% | 96% | 0.84 (0.82–0.90) | |||
| Deng, 2023 [31] | 62 | 39 and 67 CLD | cfDNA methylation by whole-genome sequencing plus deep learning techniques | 94% (early stage HCC: 90%) | 98.5% (early stage HCC: 89.5%) | 0.99 (0.98–0.99) | Superior diagnostic accuracy than AFP | |
| Guo, 2023 [32] | 73 | 84 and 22 CLD | cfDNA methylation by enzymatic methyl sequencing | 90% | 97% | 0.96 (0.93–0.99) | ||
| Han, 2014 [36] | 160 HBV | 133 (88 CHB) | Methylation of TGR5 promoter | TGR5+ AFP: 65–81% | TGR5 + AFP: 85–39% | TGR5 without AFP: 0.67 (0.61–0.73.) | ||
| Li, 2014 [37] | 136 HBV | 35 and 46 CHB | Methylation at IGFBP7 promoter | 65% | 83% | 0.74 | HCC with vascular invasion: higher IGFBP7 methylation rates (84% vs. 60%, p = 0.010) | |
| Huang, 2014 [38] | 66 | 43 CLD | Methylation at INK4A promoter | 65/39/20% for 5/7/10% CpG | 87/96.5/99% for 5/7/10% CpG cut-off | 0.82 | INK4A methylation and AFP: sensitivity 80% (45.5% for AFP alone) | |
| Oussalah, 2018 [39] | Initial study | 51 | 135 LC | mSEPT9 test: SEPT9 promoter methylation in cfDNA | 94% | 84% | 0.94 (0.90–0.97) | |
| Replication | 47 | 56 LC | 85% | 91% | 0.93 (0.86–0.97) | |||
| Kim, 2023 [40] | 313 | 413 (211 high risk) | Methylation markers of RNF135 and LDHB | 57%; and AFP: 70% | 94%; and AFP: 93% | 0.80 (0.76–0.83) | Superior sensitivity than AFP alone (45%) | |
| Cai, 2019 [41] | 1204 | 958 and 392 CHB/LC | Genome-wide 5-hydro-xymethylcytosines: 32-gene diagnostic model | 83% | 76% | 0.88 (0.86–0.91) Early stage HCC: 0.85 (0.81–0.89) | Superior performance than AFP alone | |
| Cai, 2021 [42] | Training set | 103 | 167 | HCC score: 5-hydroxy-xymethylcytosine signatures and AFP and des-γ-carboxy-prothrombin | 79% | 91% | 0.92 (0.88−0.92) | Prediction of relapse and survival after resection in high HCC recurrence risk pts |
| Test set | 32 | 60 | 94% | 78% | 0.95 (0.89−0.95) | |||
| Guo, 2024 [43] | Training cohort | 293 | 266 (96 CHB/LC) | Differentially methylated regions (DMRs) by NGS and quantitative methylation-specific PCR HepaAiQ: 20 best DMRs | 86% | 92% | 0.94 (0.93–0.96) | High postoperative HepaAiQ score: higher HCC recurrence risk (Hazard Ratio: 3.33, p < 0.001) |
| Validation cohort | 205 | 318 (100 CHB/LC) | 84% | 90% | 0.94 (0.93–0.95) | |||
| Independent cohort | 65 | 124 CHB/LC | 71% | 90% | ||||
| Kim, 2024 [44] | 36 | 213 HIV | Inflammation-DNAm score (54 CpGs) | 0.94 (0.90–0.98) | ||||
4.3. cfDNA Fragments Size and Nucleosomes (Table 3)
| First Author, Year [Reference] | HCC Pts | Controls | Species of cfDNA | Sensitivity | Specificity | AUROC (95% CI) | Other Key Findings | |
|---|---|---|---|---|---|---|---|---|
| Jin, 2021 [46] | 197 HBV | 187 HBV | Fragment size, tumor fraction, copy number and 4-mer end motifs | NA | NA | NA | These markers can help in HCC detection | |
| Meng, 2021 [48] | 76 | 247 | Copy numbers and fragment size plus AFP | 75% | 98% | 0.95 | High score: shorter recurrence-free survival | |
| Chen, 2021 [51] | Training | 255 | 260 and 347 LC | HIFI score = 4 cfDNA genomic features: nucleosome footprint, motif, 5hmC, fragmentation profiles | ||||
| Validation | 95 | 100 and 100 LC | 96% | 95% | 0.995 (0.99–1.000) | |||
| Test | 131 | 116 and 1800 LC | 95% | 98% | 0.996 (0.992–0.999) | |||
| Sun, 2022 [13] | 110 HCC (105 HBV, 5 HCV) | 342 (100 HBV and 99 HBV LC) | Fragment size by whole-genome sequencing | 87% | 88% | NA | ||
| Zhang, 2022 [47] | Training cohort | 159 (and 26/7 ICC/mixed) | 170 (51 LC/HBV) | cfDNA fragmentomic profiles using whole-genome sequencings | 97% | 99% | 0.995 | |
| Test cohort | 157 (and 26/6 ICC/mixed) | 164 (51 LC/HBV) | NA | NA | NA | |||
| Fan, 2023 [52] | Training cohort | 47 | 1706 LC | aMAP2 Plus score, aMAP score and AFP and 3 cfDNA signatures (nucleosome, fragment and motif scores) | 70% | 92% | 0.89 (0.83–0.94) | |
| Validation cohort | 67 | 2520 LC | 67% | 88% | 0.85 (0.80–0.90) | |||
| Foda, 2023 [49] | Training cohort | 75 | 426 (133 CLD) | cfDNA fragmentation profiles by low-coverage whole-genome sequencing and machine learning program | Average/High risk: 88%/85% | Average/High risk: 98%/80% | Average/High risk: 0.98 (0.97–0.99), /0.90 (0.86–0.94) | |
| Validation cohort | 90 | 133 (101 LC/HBV) | NA | NA | High risk: 0.97 (0.95–0.99) | |||
| Nguyen, 2023 [50] | Test cohort | 55 | 55 | ctDNA fragmentomics, 13 HCC-related gene mutations | 89% | 82% | 0.88 | Incorporation of mutation fragment length enhances early HCC detection |
| Validation | 54 | 53 | 81% | 81% | 0.86 | |||
| Chen, 2024 [53] | Stage 1 | 510 | 4561 LC | PreCar Score = 5 cfDNA genomic features: nucleo-some footprint, motif, 5hmC, fragmentation profiles | 94% | 95% | NA | PreCar Score: higher sensitivity than US or AFP; PreCar Score plus US: improved sensitivity for early/very early HCC |
| Stage 2 | 76 | 2487 LC | 51% | 96% | 0.79 (0.73–0.85) | |||
4.4. cfDNA Target Mutations (Table 4)
| First Author, Year [Reference] | HCC Pts | Controls | Species of cfDNA | Sensitivity | Specificity | Other Key Findings | |
|---|---|---|---|---|---|---|---|
| Wu, 2023 [54] | Test cohort | 151 | 145 LC | Gene mutation signatures by cSMART and NGS: TERT, TP53 and CTNNB1 muta-tions plus serum markers | 89% | 81% | |
| Validation cohort | 112 | 88 LC | 81% | 82% | |||
| Li, 2020 [55] | 50 HBV | Virus–host chimera DNA (vh-DNA) | 98% (detection limit: 1.5 cm) | NA | Correlation between vh-DNA copy number and tumor size: r = 0.7955, p < 0.0001. | ||
| Campani, 2024 [56] | 173 | 56 CLD | ctDNA and cfDNA: mutations in TERT, TP53, CTNNB1, PIK3CA and NFE2L2 | ctDNA mutations correlated with active HCC (40.2%) vs. controls (1.8%). | |||
5. cfDNA for HCC Prognosis
6. cfDNA and HCC Therapy
6.1. Surgical or Locoregional Therapies (Table 6)
| First Author, Year [Reference] | Study Population | Main Objective | Marker Type | Methodology | Key Findings |
|---|---|---|---|---|---|
| Tokuhisa, 2007 [20] | 96 HCV-HCC patients (87 resection) and 100 HCV carriers | cfDNA levels for prediction of survival and distant metastasis | cfDNA concentration | Real-time PCR quantification of cfDNA | Prognostic cutoff—High cfDNA levels (>117.8 ng/mL): shorter OS (HR: 3.4, 95% CI: 1.5–7.6, p = 0.004) and greater risk of EHR (HR: 4.5, 95% CI: 1.3–14.9, p = 0.014). Tumor characteristics—cfDNA levels positively associated with tumor size and differentiation. |
| Long, 2020 [58] | 82 HCC patients after hepatectomy | Postoperative cfDNA levels as biomarker for recurrence and prognosis in HCC patients | Postoperative cfDNA concentra-tions | cfDNA postoperatively using a fluorometric dsDNA assay | Postoperative cfDNA cutoff for recurrence: 2.95 ng/μL (AUC: 0.68, sensitivity: 88%, specificity: 45%). Survival analysis—High postoperative cfDNA (>2.95 ng/μL): poorer RFS (median 14 vs. 19.5 mos, p = 0.02). Independent risk factors for recurrence: cfDNA (HR: 1.287, p = 0.023), tumor number (HR: 0.037, p = 0.004) and microvascular invasion (HR: 0.127, p = 0.005). |
| Wang, 2021 [59] | 117 HBV-related HCC patients receiving radical treatments | Multi-level cfDNA CNV indicators for prognosis after radical treatments | cfDNA CNVs (TFx, P-score, S-score) | Low-coverage whole-genome sequencing of plasma cfDNA, CNV profiling at genome-wide, chromosomal-arm and bin levels | Genome-wide CNVs—Three genome-wide indicators (TFx, P-score and S-score): associated with poorer RFS and OS; High TFx (≥0.02), P-score (≥0.74) and S-score (≥0.04): associated with worse prognosis. Chromosomal-arm CNVs—17p loss/8q gain: HR 4.31/3.20 for death (p < 0.001) and HR 2.74/2.49 for recurrence (p ≤ 0.003). Bin-level CNVs—A novel bin score (1 Mb resolution): outperformed genome-wide and chromosomal-arm indicators in prognosis (AUC: 0.820 for 1-year survival and 0.746 for 3-year survival). |
| Fu, 2022 [60] | 258 HCC patients undergoing curative liver resection | Preoperative ctDNA for early recurrence prediction | ctDNA | Blood samples collected preoperatively, ctDNA detection and mutation analysis, RNA sequencing for immune profiling | Early recurrence prediction—Number of ctDNA-mutant genes: associated with early HCC recurrence (HR: 2.2, p < 0.001). High-risk patients—Mutations in HRGs (APC, ARID1A, CDKN2A, FAT1, LRP1B, MAP3K1, PREX2, TERT, TP53): worse RFS (HR: 13, p < 0.001). Prognostic nomogram—Combination of ctDNA risk level and TNM stage predicted recurrence with high accuracy (C index: 0.76). Therapy response prediction—FAT1 or LRP1B but no TP53 mutations: worse PFS with lenvatinib plus ICIs after recurrence (HR: 17, p < 0.001). Immune profiling—ctDNA status correlates with tumor immune infiltration. |
| Dong, 2022 [61] | 64 HCC patients treated with TACE, 57 LC patients and 32 healthy controls | cfDNA copy number profiling and TFx as biomarkers for TACE efficacy | cfDNA, CNV, TFx | LD-WGS of cfDNA pre- and post-TACE; tumor fraction and CNV profiling | Pre-TACE—High TFx (≥0.1): correlation with tumor burden and prediction of shorter PFS (97 vs. 189 days) and OS (243 vs. 630 days). Post-TACE—Reductions in TFx (>0.03): better PFS (163 vs. 63 days, p = 0.007) and aligned with imaging-based assessments. Lipiodol deposition—Amplifications in chromosomes 1q,3p, 6p, 8q, 10p,12q, 18p and18q were associated with poor lipiodol deposition. TFx outperformed AFP levels in predicting tumor burden and therapeutic outcomes (Sensitivity: 85.3%, Specificity: 94.4%). |
| Muraoka, 2021 [15] | 67 HCC patients: 32 TACE, 35 TKIs | cfDNA hTERT promoter mutations for predicting responses | cfDNA (hTERT promoter mutation) | cfDNA by dPCR; analysis of mutant vs. wild-type cfDNA changes | TACE—Mutant cfDNA rate increased post-TACE (33% to 73%, (p = 0.001). Post-TACE correlations: mutant cfDNA changes with tumor necrosis (p < 0.001) and wild-type cfDNA changes with AST changes (p < 0.001). TKIs—Mutant cfDNA levels peaked within 1 week only in responders, who had longer PFS (10 vs. 3.4 months, p = 0.004). |
| Nakatsuka, 2021 [62] | 100 HCC patients: TACE: 32, MTAs (lenvatinib, sorafenib, regorafenib): 35, RFA: 33 | cfDNA levels and mutation profiles for tumor response and treatment outcomes | cfDNA, ctDNA, TERT promoter mutations | cfDNA levels measured pre- and post-treatment; TERT mutations detected using ddPCR; ultra-deep sequencing (22,000× coverage) | Baseline cfDNA—High (>70.7 ng/mL) vs. low baseline cfDNA: shorter OS (5.5 vs. 14 mos, p < 0.001). Post-treatment—cfDNA levels increased post-TACE (49 to 249 ng/mL, p < 0.001) and post-RFA (39 to 96 ng/mL, p < 0.001); rate of TERT mutations increased post-TACE (45% to 57%) and post-RFA (42% to 55%). Post-MTA—cfDNA levels increased after initiation of MTA; >1.5-fold cfDNA increase within 1 week: longer PFS (10 vs. 3.4 mos, p = 0.004). Lenvatinib response: Associated with mutations in genes like AMER1, MLL3 and NOTCH2 identified by ultra-deep sequencing. |
| Li, 2022 [63] | 60 HCC patients treated with primary liver resection | TP53 mutations in exosomal cfDNA and HCC prognosis and treatment response | TP53 mutationstatus | Exosome extraction; TP53 mutations detected using ddPCR | High- vs. low-frequency mutations (MD/TD ≥67% vs. <67%): shorter RFS (68 vs. 368 days, p < 0.01). High-frequency mutation: poor prognosis, though patients with better pathological characteristics (HR: 4.61; p = 0.003). |
| Kim, 2023 [64] | 37 patients with advanced HCC under-going RT | cfDNA for prediction of treatment response in advanced HCC treated with RT | cfDNA genomic instability score (I-score) | cfDNA analysis at pre-RT and 1 week post-RT, whole-genome sequencing, genomic instability scoring | Genomic instability—I-score: predictive of PFS (AUC = 0.71; sensitivity = 50%, specificity = 91%). Pre-RT I-score—Pre-treatment I-score (≥6.3) was associated with worse PFS (HR = 2.69, p = 0.017) and correlated with tumor burden. Post-RT I-score—High I-score (≥6.2): poor responses (non-complete response, p = 0.034). Dynamic changes in I-score—Delta I-score ratio reflected treatment effects, with negative/positive ratios in responders/non-responders. |
| Campani, 2024 [56] | 173 HCC patients and 56 controls (including cirrhotic patients) | ctDNA as biomarker for tumor biology and treatment monitoring | ctDNA | NGS on MiSeq and droplet based digital PCR for TERT, TP53, CTNNB1, PIK3CA NFE2L2 mutations | ctDNA mutations—40% of active HCC, 14.6% of inactive HCC and 1.8% of controls.
ctDNA mutations post-treatment—Detection prior to and 24 h after percutaneous ablation and persistence throughout the initial four cycles of atezolizumab + bevacizumab: lower OS and RFS. |
6.2. Systemic Therapies (Table 7)
| First Author, Year [Reference] | Study Population | Main Objective | Marker Type | Methodology | Key Findings |
|---|---|---|---|---|---|
| Oh, 2019 [67] | 151 HCC patients receiving sorafenib | cfDNA levels, genome-wide CNAs, VEGFA amplification for prognosis post sorafenib | cfDNA levels, genome-wide CNAs (I-score) and VEGFA amplification | WGS of cfDNA; VEGFA analysis via ddPCR | cfDNA—Higher cfDNA linked to worse TTP (2.2 vs. 4.1 mos, HR: 1.71) and OS (4.1 vs. 14.8 mos, HR: 3.50). Genome-Wide CNAs (I-score)—Higher I-score: worse TTP (2.2 vs. 4.1 mos, HR: 2.09, p < 0.0001) and OS (4.6 vs. 14.8 mos, HR: 3.35). VEGFA amplification—VEGFA amplification levels: higher in HCC, but no significant correlation with treatment outcomes (DCR, TTP, or OS). |
| Mohamed, 2024 [68] | 44 HCC patients receiving nivolumab | ctDNA as a biomarker for predicting OS and PFS | ctDNA alte-rations in TP53, PIK3CA, BRCA1, CCND1 and CTNNB1 genes | CLIA-certified Guardant360 platform targeting 74 cancer-related using NGS | Mutation profiles—PIK3CA and KIT mutations: associated with shorter PFS (p < 0.0004). CTNNB1 mutation: associated with longer PFS (p = 0.04). Mutations in PIK3CA, BRCA1 and CCND1 amplification: correlated with shorter OS (p < 0.0001, p < 0.0001 and p = 0.01, respectively). |
| Felden, 2020 [69] | 51 HCC patients undergoing systemic therapy | ctDNA mutations as predictors of systemic therapy response | ctDNA alterations in TERT, TP53, CTNNB1, PI3K/MTOR pathway genes | Ultra-deep sequencing, digital droplet PCR | Mutation profiles—PI3K/MTOR mutations linked to TKI resistance (PFS: 2.1 vs. 3.7 mos, p < 0.001); WNT mutations not predictive of CPI response. Serial ctDNA profiling enabled treatment monitoring. |
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- EASL. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.; Villanueva, A.; Singal, A.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Zhang, J.; Monga, S.P.; Dhanasekaran, R. Battle of the biopsies: Role of tissue and liquid biopsy in hepatocellular carcinoma. J. Hepatol. 2024, 80, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef]
- Chan, Y.T.; Zhang, C.; Wu, J.; Lu, P.; Xu, L.; Yuan, H.; Feng, Y.; Chen, Z.S.; Wang, N. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol. Cancer 2024, 23, 189. [Google Scholar] [CrossRef]
- Papatheodoridi, A.; Karakousis, N.; Lembessis, P.; Chatzigeorgiou, A.; Papatheodoridis, G.V. The Significance of Circulating Cell-Free DNA Markers in Chronic Hepatitis B Patients with Hepatocellular Carcinoma. Pathogens 2021, 10, 1524. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Zhou, J.; Zhao, H.; Zhang, H.; Wang, G. Diagnostic value of circulating cell-free DNA levels for hepatocellular carcinoma. Int. J. Infect. Dis. 2018, 67, 92–97. [Google Scholar] [PubMed]
- Tran, N.H.; Kisiel, J.; Roberts, L.R. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep. 2021, 3, 100304. [Google Scholar] [PubMed]
- Sun, X.; Feng, W.; Cui, P.; Ruan, R.; Ma, W.; Han, Z.; Sun, J.; Pan, Y.; Zhu, J.; Zhong, X.; et al. Detection and monitoring of HBV-related hepatocellular carcinoma from plasma cfDNA fragmentation profiles. Genomics 2022, 114, 110502. [Google Scholar] [PubMed]
- Kaseb, A.O.; Sanchez, N.S.; Sen, S.; Kelley, R.K.; Tan, B.; Bocobo, A.G.; Lim, K.H.; Abdel-Wahab, R.; Uemura, M.; Pestana, R.C.; et al. Molecular Profiling of Hepatocellular Carcinoma Using Circulating Cell-Free DNA. Clin. Cancer Res. 2019, 25, 6107–6118. [Google Scholar] [CrossRef]
- Muraoka, M.; Maekawa, S.; Katoh, R.; Komiyama, Y.; Nakakuki, N.; Takada, H.; Matsuda, S.; Suzuki, Y.; Sato, M.; Tatsumi, A.; et al. Usefulness of Cell-Free Human Telomerase Reverse Transcriptase Mutant DNA Quantification in Blood for Predicting Hepatocellular Carcinoma Treatment Efficacy. Hepatol. Commun. 2021, 5, 1927–1938. [Google Scholar]
- Yang, J.; Lin, N.; Niu, M.; Yin, B. Circulating tumor DNA mutation analysis: Advances in its application for early diagnosis of hepatocellular carcinoma and therapeutic efficacy monitoring. Aging 2024, 16, 11460–11474. [Google Scholar]
- Banini, B.A.; Sanyal, A.J. The use of cell free DNA in the diagnosis of HCC. Hepatoma Res. 2019, 5, 34. [Google Scholar]
- Iizuka, N.; Sakaida, I.; Moribe, T.; Fujita, N.; Miura, T.; Stark, M.; Tamatsukuri, S.; Ishitsuka, H.; Uchida, K.; Terai, S.; et al. Elevated levels of circulating cell-free DNA in the blood of patients with hepatitis C virus-associated hepatocellular carcinoma. Anticancer. Res. 2006, 26, 4713–4719. [Google Scholar]
- Iida, M.; Iizuka, N.; Sakaida, I.; Moribe, T.; Fujita, N.; Miura, T.; Tamatsukuri, S.; Ishitsuka, H.; Uchida, K.; Terai, S.; et al. Relation between serum levels of cell-free DNA and inflammation status in hepatitis C virus-related hepatocellular carcinoma. Oncol. Rep. 2008, 20, 761–765. [Google Scholar]
- Tokuhisa, Y.; Iizuka, N.; Sakaida, I.; Moribe, T.; Fujita, N.; Miura, T.; Tamatsukuri, S.; Ishitsuka, H.; Uchida, K.; Terai, S.; et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Br. J. Cancer 2007, 97, 1399–1403. [Google Scholar] [CrossRef]
- Elzehery, R.; Effat, N.; El Farahaty, R.; Elsayed Farag, R.; Abo-Hashem, E.M.; Elhelaly, R. Circulating Cell-Free DNA and DNA Integrity as Molecular Diagnostic Tools in Hepatocellular Carcinoma. Am. J. Clin. Pathol. 2022, 158, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Lu, C.; Li, F.; Yu, X.; Ai, L.; Wu, B.; Gong, X.; Zhou, W.; Liang, X.; Zhan, J.; et al. Monitoring Hepatocellular Carcinoma Using Tumor Content in Circulating Cell-Free DNA. Clin. Cancer Res. 2024, 30, 2772–2779. [Google Scholar]
- Jiang, P.; Chan, C.W.; Chan, K.C.; Cheng, S.H.; Wong, J.; Wong, V.W.; Wong, G.L.; Chan, S.L.; Mok, T.S.; Chan, H.L.; et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, E1317–E1325. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Zhang, X.; Zhou, S.L.; Cao, Y.; Huang, X.W.; Fan, J.; Yang, X.R.; Zhou, J. Plasma Circulating Cell-free DNA Integrity as a Promising Biomarker for Diagnosis and Surveillance in Patients with Hepatocellular Carcinoma. J. Cancer 2016, 7, 1798–1803. [Google Scholar] [PubMed]
- Papatheodoridi, A.; Chatzigeorgiou, A.; Chrysavgis, L.; Lembessis, P.; Loglio, A.; Facchetti, F.; Cholongitas, E.; Koutsilieris, M.; Lampertico, P.; Papatheodoridis, G. Circulating cell-free DNA species affect the risk of hepatocellular carcinoma in treated chronic hepatitis B patients. J. Viral Hepat. 2021, 28, 464–474. [Google Scholar]
- Kamal, M.M.; Abdelaziz, A.O.; El-Baz, H.N.; Mohamed, G.M.; Saleh, S.S.; Nabeel, M.M.; Elbaz, T.M.; Lithy, R.; Shousha, H.I. Plasma cell-free DNA integrity index and hepatocellular carcinoma treated or not with direct-acting antivirals: A case-control study. Arab. J. Gastroenterol. 2022, 23, 39–44. [Google Scholar] [CrossRef]
- Xu, R.-h.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161. [Google Scholar] [CrossRef]
- Luo, B.; Ma, F.; Liu, H.; Hu, J.; Rao, L.; Liu, C.; Jiang, Y.; Kuangzeng, S.; Lin, X.; Wang, C.; et al. Cell-free DNA methylation markers for differential diagnosis of hepatocellular carcinoma. BMC Med. 2022, 20, 8. [Google Scholar]
- Wang, P.; Song, Q.; Ren, J.; Zhang, W.; Wang, Y.; Zhou, L.; Wang, D.; Chen, K.; Jiang, L.; Zhang, B.; et al. Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection. Sci. Transl. Med. 2022, 14, eabp8704. [Google Scholar]
- Phan, T.H.; Chi Nguyen, V.T.; Thi Pham, T.T.; Nguyen, V.C.; Ho, T.D.; Quynh Pham, T.M.; Tran, T.H.; Nguyen, T.D.; Khang Le, N.D.; Nguyen, T.H.; et al. Circulating DNA methylation profile improves the accuracy of serum biomarkers for the detection of nonmetastatic hepatocellular carcinoma. Future Oncol. 2022, 18, 4399–4413. [Google Scholar]
- Deng, Z.; Ji, Y.; Han, B.; Tan, Z.; Ren, Y.; Gao, J.; Chen, N.; Ma, C.; Zhang, Y.; Yao, Y.; et al. Early detection of hepatocellular carcinoma via no end-repair enzymatic methylation sequencing of cell-free DNA and pre-trained neural network. Genome Med. 2023, 15, 93. [Google Scholar]
- Guo, P.; Zheng, H.; Li, Y.; Li, Y.; Xiao, Y.; Zheng, J.; Zhu, X.; Xu, H.; He, Z.; Zhang, Q.; et al. Hepatocellular carcinoma detection via targeted enzymatic methyl sequencing of plasma cell-free DNA. Clin. Epigenetics 2023, 15, 2. [Google Scholar]
- Wang, J.; Yang, L.; Diao, Y.; Liu, J.; Li, J.; Li, R.; Zheng, L.; Zhang, K.; Ma, Y.; Hao, X. Circulating tumour DNA methylation in hepatocellular carcinoma diagnosis using digital droplet PCR. J. Int. Med. Res. 2021, 49, 300060521992962. [Google Scholar] [PubMed]
- Lewin, J.; Kottwitz, D.; Aoyama, J.; deVos, T.; Garces, J.; Hasinger, O.; Kasielke, S.; Knaust, F.; Rathi, P.; Rausch, S.; et al. Plasma cell free DNA methylation markers for hepatocellular carcinoma surveillance in patients with cirrhosis: A case control study. BMC Gastroenterol. 2021, 21, 136. [Google Scholar]
- Lin, N.; Lin, Y.; Xu, J.; Liu, D.; Li, D.; Meng, H.; Gallant, M.A.; Kubota, N.; Roy, D.; Li, J.S.; et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 1753–1763. [Google Scholar] [PubMed]
- Han, L.Y.; Fan, Y.C.; Mu, N.N.; Gao, S.; Li, F.; Ji, X.F.; Dou, C.Y.; Wang, K. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) is a potential biomarker for hepatitis B Virus associated hepatocellular carcinoma. Int. J. Med. Sci. 2014, 11, 164–171. [Google Scholar]
- Li, F.; Fan, Y.C.; Gao, S.; Sun, F.K.; Yang, Y.; Wang, K. Methylation of serum insulin-like growth factor-binding protein 7 promoter in hepatitis B virus-associated hepatocellular carcinoma. Genes Chromosom. Cancer 2014, 53, 90–97. [Google Scholar]
- Huang, G.; Krocker, J.D.; Kirk, J.L.; Merwat, S.N.; Ju, H.; Soloway, R.D.; Wieck, L.R.; Li, A.; Okorodudu, A.O.; Petersen, J.R.; et al. Evaluation of INK4A promoter methylation using pyrosequencing and circulating cell-free DNA from patients with hepatocellular carcinoma. Clin. Chem. Lab. Med. 2014, 52, 899–909. [Google Scholar]
- Oussalah, A.; Rischer, S.; Bensenane, M.; Conroy, G.; Filhine-Tresarrieu, P.; Debard, R.; Forest-Tramoy, D.; Josse, T.; Reinicke, D.; Garcia, M.; et al. Plasma mSEPT9: A Novel Circulating Cell-free DNA-Based Epigenetic Biomarker to Diagnose Hepatocellular Carcinoma. EBioMedicine 2018, 30, 138–147. [Google Scholar]
- Kim, S.C.; Kim, D.W.; Cho, E.J.; Lee, J.Y.; Kim, J.; Kwon, C.; Kim-Ha, J.; Hong, S.K.; Choi, Y.; Yi, N.J.; et al. A circulating cell-free DNA methylation signature for the detection of hepatocellular carcinoma. Mol. Cancer 2023, 22, 164. [Google Scholar]
- Cai, J.; Chen, L.; Zhang, Z.; Zhang, X.; Lu, X.; Liu, W.; Shi, G.; Ge, Y.; Gao, P.; Yang, Y.; et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut 2019, 68, 2195–2205. [Google Scholar]
- Cai, Z.; Zhang, J.; He, Y.; Xia, L.; Dong, X.; Chen, G.; Zhou, Y.; Hu, X.; Zhong, S.; Wang, Y.; et al. Liquid biopsy by combining 5-hydroxymethylcytosine signatures of plasma cell-free DNA and protein biomarkers for diagnosis and prognosis of hepatocellular carcinoma. ESMO Open 2021, 6, 100021. [Google Scholar]
- Guo, D.Z.; Huang, A.; Wang, Y.C.; Zhou, S.; Wang, H.; Xing, X.L.; Zhang, S.Y.; Cheng, J.W.; Xie, K.H.; Yang, Q.C.; et al. Early detection and prognosis evaluation for hepatocellular carcinoma by circulating tumour DNA methylation: A multicentre cohort study. Clin. Transl. Med. 2024, 14, e1652. [Google Scholar]
- Kim, K.; Zheng, Y.; Joyce, B.T.; Nannini, D.R.; Wang, J.; Qu, Y.; Hawkins, C.A.; Okeke, E.; Lesi, O.A.; Roberts, L.R.; et al. Cell-free DNA methylation-based inflammation score as a marker for hepatocellular carcinoma among people living with HIV. Hepatol. Int. 2024. [Google Scholar] [CrossRef]
- Manea, I.; Iacob, R.; Iacob, S.; Cerban, R.; Dima, S.; Oniscu, G.; Popescu, I.; Gheorghe, L. Liquid biopsy for early detection of hepatocellular carcinoma. Front. Med. 2023, 10, 1218705. [Google Scholar]
- Jin, C.; Liu, X.; Zheng, W.; Su, L.; Liu, Y.; Guo, X.; Gu, X.; Li, H.; Xu, B.; Wang, G.; et al. Characterization of fragment sizes, copy number aberrations and 4-mer end motifs in cell-free DNA of hepatocellular carcinoma for enhanced liquid biopsy-based cancer detection. Mol. Oncol. 2021, 15, 2377–2389. [Google Scholar] [PubMed]
- Zhang, X.; Wang, Z.; Tang, W.; Wang, X.; Liu, R.; Bao, H.; Chen, X.; Wei, Y.; Wu, S.; Bao, H.; et al. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology 2022, 76, 317–329. [Google Scholar]
- Meng, Z.; Ren, Q.; Zhong, G.; Li, S.; Chen, Y.; Wu, W.; Feng, Y.; Mao, M.; Zhang, F.; Long, G. Noninvasive Detection of Hepatocellular Carcinoma with Circulating Tumor DNA Features and α-Fetoprotein. J. Mol. Diagn. 2021, 23, 1174–1184. [Google Scholar]
- Foda, Z.H.; Annapragada, A.V.; Boyapati, K.; Bruhm, D.C.; Vulpescu, N.A.; Medina, J.E.; Mathios, D.; Cristiano, S.; Niknafs, N.; Luu, H.T.; et al. Detecting Liver Cancer Using Cell-Free DNA Fragmentomes. Cancer Discov. 2023, 13, 616–631. [Google Scholar]
- Nguyen, V.C.; Nguyen, T.H.; Phan, T.H.; Tran, T.T.; Pham, T.T.T.; Ho, T.D.; Nguyen, H.H.T.; Duong, M.L.; Nguyen, C.M.; Nguyen, Q.B.; et al. Fragment length profiles of cancer mutations enhance detection of circulating tumor DNA in patients with early-stage hepatocellular carcinoma. BMC Cancer 2023, 23, 233. [Google Scholar]
- Chen, L.; Abou-Alfa, G.K.; Zheng, B.; Liu, J.F.; Bai, J.; Du, L.T.; Qian, Y.S.; Fan, R.; Liu, X.L.; Wu, L.; et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res. 2021, 31, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Chen, L.; Zhao, S.; Yang, H.; Li, Z.; Qian, Y.; Ma, H.; Liu, X.; Wang, C.; Liang, X.; et al. Novel, high accuracy models for hepatocellular carcinoma prediction based on longitudinal data and cell-free DNA signatures. J. Hepatol. 2023, 79, 933–944. [Google Scholar] [CrossRef]
- Chen, L.; Wu, T.; Fan, R.; Qian, Y.S.; Liu, J.F.; Bai, J.; Zheng, B.; Liu, X.L.; Zheng, D.; Du, L.T.; et al. Cell-free DNA testing for early hepatocellular carcinoma surveillance. EBioMedicine 2024, 100, 104962. [Google Scholar] [CrossRef]
- Wu, T.; Fan, R.; Bai, J.; Yang, Z.; Qian, Y.-S.; Du, L.-T.; Wang, C.-Y.; Wang, Y.-C.; Jiang, G.-Q.; Zheng, D.; et al. The development of a cSMART-based integrated model for hepatocellular carcinoma diagnosis. J. Hematol. Oncol. 2023, 16, 1. [Google Scholar] [CrossRef]
- Li, C.L.; Ho, M.C.; Lin, Y.Y.; Tzeng, S.T.; Chen, Y.J.; Pai, H.Y.; Wang, Y.C.; Chen, C.L.; Lee, Y.H.; Chen, D.S.; et al. Cell-Free Virus-Host Chimera DNA from Hepatitis B Virus Integration Sites as a Circulating Biomarker of Hepatocellular Cancer. Hepatology 2020, 72, 2063–2076. [Google Scholar] [CrossRef]
- Campani, C.; Imbeaud, S.; Couchy, G.; Ziol, M.; Hirsch, T.Z.; Rebouissou, S.; Noblet, B.; Nahon, P.; Hormigos, K.; Sidali, S.; et al. Circulating tumour DNA in patients with hepatocellular carcinoma across tumour stages and treatments. Gut 2024, 73, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, Y.J. Liquid biopsy for early detection and therapeutic monitoring of hepatocellular carcinoma. J. Liver Cancer 2022, 22, 103–114. [Google Scholar] [CrossRef]
- Long, G.; Fang, T.; Su, W.; Mi, X.; Zhou, L. The prognostic value of postoperative circulating cell-free DNA in operable hepatocellular carcinoma. Scand. J. Gastroenterol. 2020, 55, 1441–1446. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, K.; Wang, X.; Liu, Y.; Guo, D.; Bian, Z.; Su, L.; Liu, K.; Gu, X.; Guo, X.; et al. Multiple-level copy number variations in cell-free DNA for prognostic prediction of HCC with radical treatments. Cancer Sci. 2021, 112, 4772–4784. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Hu, Z.; Yang, Z.; Pan, Y.; Chen, J.; Wang, J.; Hu, D.; Zhou, Z.; Xu, L.; et al. Preoperative serum ctDNA predicts early hepatocellular carcinoma recurrence and response to systemic therapies. Hepatol. Int. 2022, 16, 868–878. [Google Scholar] [CrossRef]
- Dong, X.; Chen, G.; Huang, X.; Li, Z.; Peng, F.; Chen, H.; Zhou, Y.; He, L.; Qiu, L.; Cai, Z.; et al. Copy number profiling of circulating free DNA predicts transarterial chemoembolization response in advanced hepatocellular carcinoma. Mol. Oncol. 2022, 16, 1986–1999. [Google Scholar]
- Nakatsuka, T.; Nakagawa, H.; Hayata, Y.; Wake, T.; Yamada, T.; Nishibatake Kinoshita, M.; Nakagomi, R.; Sato, M.; Minami, T.; Uchino, K.; et al. Post-treatment cell-free DNA as a predictive biomarker in molecular-targeted therapy of hepatocellular carcinoma. J. Gastroenterol. 2021, 56, 456–469. [Google Scholar] [PubMed]
- Li, Y.; Wu, J.; Li, E.; Xiao, Z.; Lei, J.; Zhou, F.; Yin, X.; Hu, D.; Mao, Y.; Wu, L.; et al. TP53 mutation detected in circulating exosomal DNA is associated with prognosis of patients with hepatocellular carcinoma. Cancer Biol. Ther. 2022, 23, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Cho, E.H.; Kim, J.S.; Chie, E.K.; Kang, H.C. Plasma Circulating Cell-free DNA in Advanced Hepatocellular Carcinoma Patients Treated with Radiation Therapy. Vivo 2023, 37, 2306–2313. [Google Scholar]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar]
- Franses, J.W.; Lim, M.; Burgoyne, A.M.; Mody, K.; Lennerz, J.; Chang, J.; Imperial, R.; Dybel, S.N.; Dinh, T.M.; Masannat, J.; et al. Profile and Predictors of Blood Tumor Mutational Burden in Advanced Hepatocellular Carcinoma. Oncologist 2022, 27, e908–e911. [Google Scholar]
- Oh, C.R.; Kong, S.Y.; Im, H.S.; Kim, H.J.; Kim, M.K.; Yoon, K.A.; Cho, E.H.; Jang, J.H.; Lee, J.; Kang, J.; et al. Genome-wide copy number alteration and VEGFA amplification of circulating cell-free DNA as a biomarker in advanced hepatocellular carcinoma patients treated with Sorafenib. BMC Cancer 2019, 19, 292. [Google Scholar] [CrossRef]
- Mohamed, Y.I.; Lee, S.S.; Demir, T.; Chamseddine, S.; Hu, Z.I.; Xiao, L.; Elsayes, K.; Morris, J.S.; Wolff, R.A.; Hiatia, R.; et al. Circulating tumor DNA (ctDNA) as a biomarker of response to therapy in advanced Hepatocellular carcinoma treated with Nivolumab. Cancer Biomark. 2024, 41, 83–91. [Google Scholar] [CrossRef]
- von Felden, J.; Craig, A.J.; Garcia-Lezana, T.; Labgaa, I.; Haber, P.K.; D’Avola, D.; Asgharpour, A.; Dieterich, D.; Bonaccorso, A.; Torres-Martin, M.; et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene 2021, 40, 140–151. [Google Scholar]
- Cisneros-Villanueva, M.; Hidalgo-Pérez, L.; Rios-Romero, M.; Cedro-Tanda, A.; Ruiz-Villavicencio, C.A.; Page, K.; Hastings, R.; Fernandez-Garcia, D.; Allsopp, R.; Fonseca-Montaño, M.A.; et al. Cell-free DNA analysis in current cancer clinical trials: A review. Br. J. Cancer 2022, 126, 391–400. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [PubMed]
- Lyu, X.; Tsui, Y.M.; Ho, D.W.; Ng, I.O. Liquid Biopsy Using Cell-Free or Circulating Tumor DNA in the Management of Hepatocellular Carcinoma. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Warton, K.; Mahon, K.L.; Samimi, G. Methylated circulating tumor DNA in blood: Power in cancer prognosis and response. Endocr. Relat. Cancer 2016, 23, R157–R171. [Google Scholar]
- Li, J.; Yuan, Y.; Fu, Q.; Chen, M.; Liang, H.; Chen, X.; Long, X.; Zhang, B.; Zhao, J.; Chen, Q. Novel insights into the role of immunomodulatory extracellular vesicles in the pathogenesis of liver fibrosis. Biomark. Res. 2024, 12, 119. [Google Scholar]
| First Author, Year [Reference] | HCC Patients | Controls | Species of cfDNA | Sensitivity | Specificity | AUROC (95% CI) | Other Key Findings |
|---|---|---|---|---|---|---|---|
| Izuka, 2006 [18] | 52 HCV | 30 HCV and 18 controls | cfDNA levels measured by rtPCR (GSTP1 gene) | 69% | 93% | 0.90 (0.83–0.96) | cfDNA superior than AFP or PIVKA-II |
| Iida, 2008 [19] | 96 HCV | 100 HCV | Serum cfDNA levels | NA | NA | NA | cfDNA levels: higher in HCC than non-HCC cases, p < 0.001; High cfDNA level: association with HCC inflammatory status |
| Tokuhisa, 2007 [20] | 96 HCV | 100 HCV | cfDNA levels | NA | NA | NA | cfDNA higher in HCC vs. non-HCC pts (116 vs. 34 ng/mL, p < 0.0001) |
| Elzehery, 2022 [21] | 50 HCV | 50 HCV LC and 50 controls | cfDNA levels and cfDNA integrity (Alu247/115) | cfDNA: 82% integrity: 70% | cfDNA: 76% integrity: 88% | cfDNA: 0.83 (0.75–0.91); integrity: 0.86 (0.78–0.93) | |
| Lian, 2024 [22] | 63 HBV | 90 CHB | Genome-wide copy number and tumor content in cfDNA | 1 year pre-diagnosis 23% BCLC A: 30% | 98% | NA | High tumor content associated with tumor stage and poor survival |
| Jiang, 2015 [23] | 90 | 135 controls (103 CLD) | ctDNA size and mitochondrial DNA | Mitochondrial DNA: 80% | Mitochondrial DNA: 94% | Mitochondrial DNA: 0.93 | |
| Huang, 2016 [24] | 53 (and 19 non-HCC cancers) | 37 controls | cfDNA integrity: Alu247/Alu115 | 43% | 100% | 0.705 | cfDNA integrity: may be useful for HCC treatment surveillance |
| Papatheodoridi, 2021 [10] | 19 CHB | 38 CHB | cfDNA, Alu115, Alu247, nucleosomes and cfDNA integrity (Alu247/115) | NA | NA | NA | HCC-CHB vs. CHB—median Alu 247: 64 vs. 23, p = 0.010; Alu247/115: 1 vs. 0.7, p < 0.001 |
| Papatheodoridi, 2021 [25] | 37 CHB | 74 CHB | cfDNA levels, Alu 247 and 115, RNase P coding DNA, mitochondrial DNA, DNA methylation | NA | NA | 0.80 (0.71–0.89) for RNase P levels | Median Alu247: 123 vs. 69, p = 0.042; median RNase P: GE 68 vs. 15, p < 0.001 |
| Kamal, 2022 [26] | 80 HCV | 80 HCV LC | cfDNA integrity (Alu115/247) by rtPCR | 85% | 97.5% | NA |
| First Author, Year [Reference] | Study Population | Main Objective | Marker Type | Methodology | Key Findings |
|---|---|---|---|---|---|
| Xu, 2017 [27] | 1098 HCC patients and 835 healthy controls | ctDNA methylation markers for HCC diagnosis, treatment response and prognosis | ctDNA methyla-tion markers | Bisulfite sequencing, padlock probe capture, LASSO and random-forest feature selection | Prognostic model—eight-marker panel correlated with survival outcomes; high-risk (cp-score > −0.24): worse survival than low-risk patients Treatment response monitoring—decreased cp-scores post-treatment: better outcomes than rising scores, which correlated with tumor burden and progression. |
| Lian, 2024 [22] | 67 patients with HBV-related HCC and 90 controls | Tumor-derived cfDNA (tumor content) as biomarker for monitoring, HCC progression and prognosis | cfDNA tumor content | Shallow WGS and ichorCNA method to assess genome-wide copy number variations and tumor content in cfDNA | Tumor content and stage/survival—high tumor content in cfDNA correlated with advanced tumor stage (p < 0.001) and poorer survival (HR: 12.3, 95% CI: 1.4–107.9; p = 0.023) Post-treatment monitoring: tumor content turned negative post-surgery p = 0.027) but remained positive after TACE (p = 0.578). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatheodoridi, A.; Lekakis, V.; Chatzigeorgiou, A.; Papatheodoridis, G. The Current Role of Circulating Cell-Free DNA in the Management of Hepatocellular Carcinoma. Cancers 2025, 17, 1042. https://doi.org/10.3390/cancers17061042
Papatheodoridi A, Lekakis V, Chatzigeorgiou A, Papatheodoridis G. The Current Role of Circulating Cell-Free DNA in the Management of Hepatocellular Carcinoma. Cancers. 2025; 17(6):1042. https://doi.org/10.3390/cancers17061042
Chicago/Turabian StylePapatheodoridi, Alkistis, Vasileios Lekakis, Antonios Chatzigeorgiou, and George Papatheodoridis. 2025. "The Current Role of Circulating Cell-Free DNA in the Management of Hepatocellular Carcinoma" Cancers 17, no. 6: 1042. https://doi.org/10.3390/cancers17061042
APA StylePapatheodoridi, A., Lekakis, V., Chatzigeorgiou, A., & Papatheodoridis, G. (2025). The Current Role of Circulating Cell-Free DNA in the Management of Hepatocellular Carcinoma. Cancers, 17(6), 1042. https://doi.org/10.3390/cancers17061042







