Liver Extracellular Matrix in Colorectal Liver Metastasis
Simple Summary
Abstract
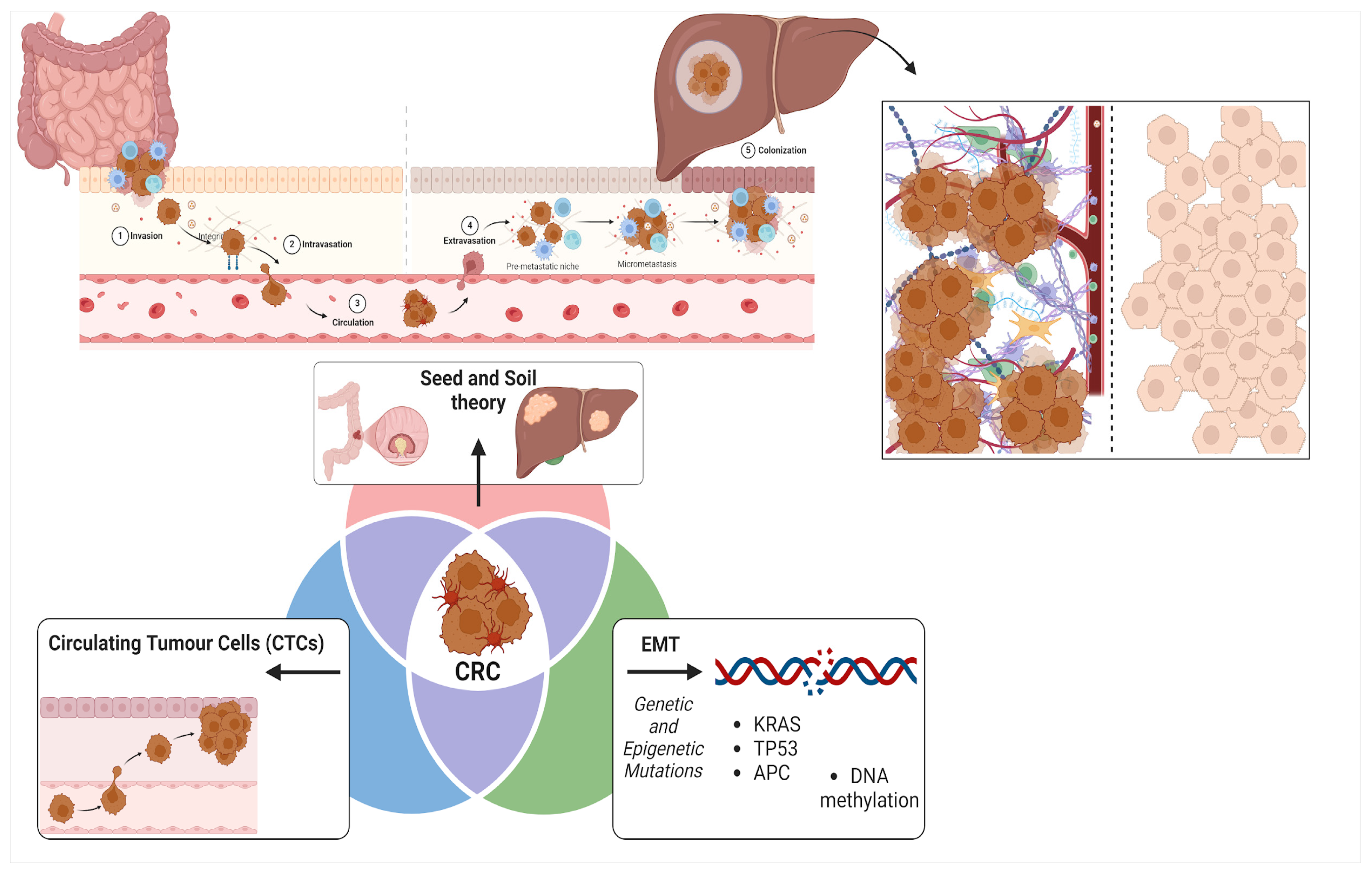
1. Introduction
- (1)
- CRC cells may undergo epithelial-mesenchymal transition (EMT), a process in which they lose their epithelial traits and acquire mesenchymal properties, thereby enhancing their ability to migrate and invade distant tissues [17]: this is principally driven by genetic mutations (such as KRAS, TP53, and APC) [18] and epigenetic alterations (like DNA methylation) [19] which influence CRC metastatic potential;
- (2)
- CRC cells can enter the bloodstream as circulating tumor cells (CTCs), where they must survive the challenges of the circulatory environment, extravasate into the liver parenchyma, and subsequently establish metastatic colonies [20];
- (3)
- CRC metastasis aligns with Paget’s ’Seed and Soil Hypothesis’ proposed in 1889 [21], suggesting that the liver provides a favorable microenvironment (“soil”) that enables CRC cells (“seeds”) to survive and proliferate, facilitated by its rich blood supply and specific extracellular matrix (ECM) components [22].
2. The Extracellular Matrix: Composition and Function in Healthy Tissue
2.1. Collagens
2.2. Glycosaminoglycans (GAGs)
2.3. Glycoproteins
2.4. Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinase (TIMPs)
3. Extracellular Matrix (ECM) in Cancer
- Pre-metastatic niche formation;
- Metastatic niche colonization;
- Movement of circulating tumoral cells (CTCs).
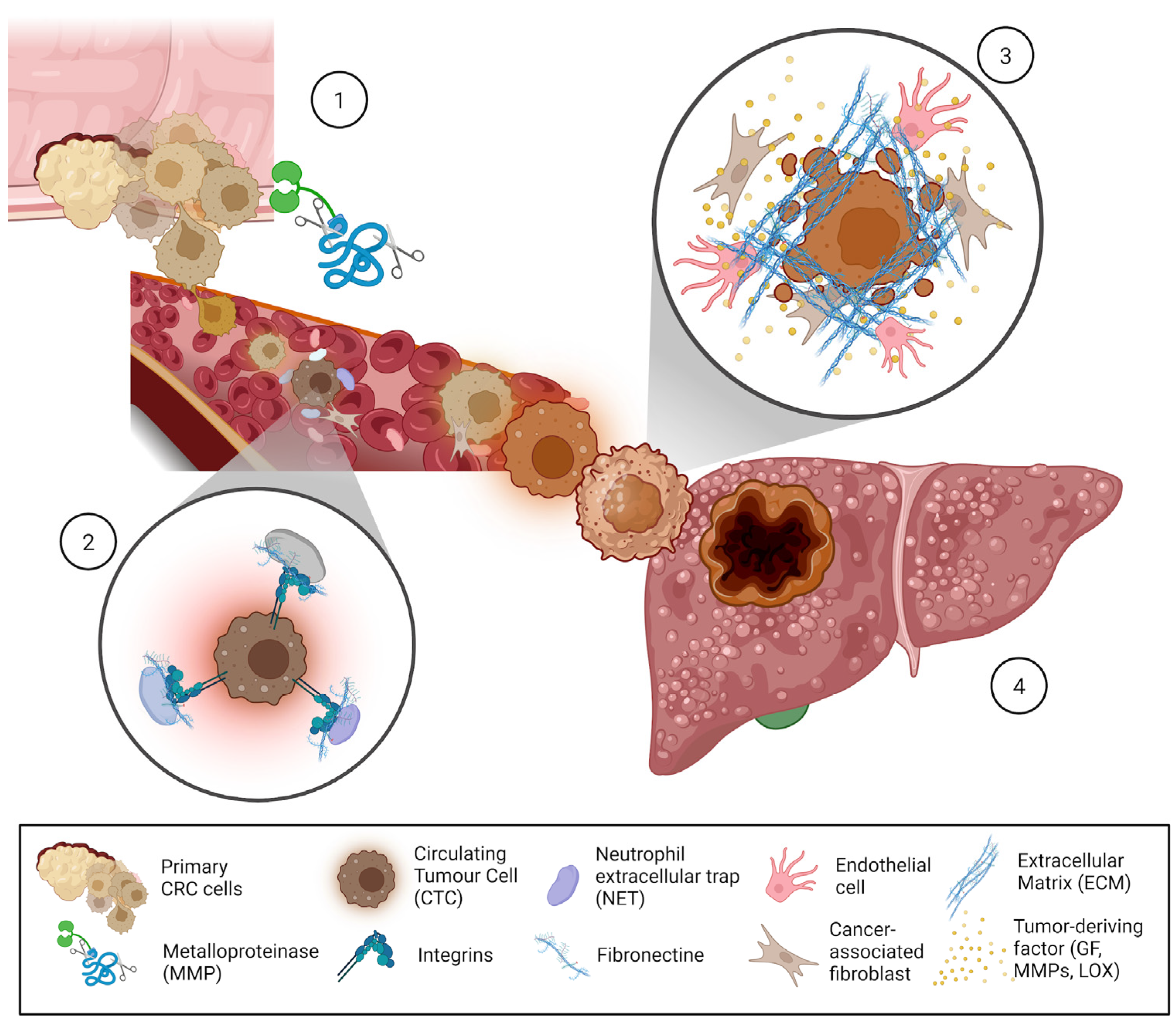
3.1. ECM Role in Pre-Metastatic Niche Formation
3.2. Cell–ECM Interactions in the Metastatic Niche
3.3. Circulating Tumoral Cells (CTCs) and ECM Interactions
4. Liver ECM in Regulating Cell Fate, and Its Role in CRC Metastasis
4.1. Unique Characteristics of the Hepatic ECM
4.2. ECM Remodeling in Normal and Pathological Liver Conditions
4.3. Influence of ECM Stiffness and Composition on Cellular Behavior
4.4. Interaction Between CRC Cells and Hepatic ECM
5. ECM Remodeling the Metastatic Niche
6. ECM and Chemoresistance
7. Methods for Studying ECM in Cancer Research
8. Therapeutic Implications and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aHSC | Activated hepatic stellate cells |
| BAPN | Beta-aminopropionitrile |
| CAF | Cancer-associated fibroblast |
| CRLM | Colorectal liver metastases |
| CRC | Colorectal cancer |
| CTCs | Circulating tumor cells |
| DLM | Decellularized liver matrix |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| EVs | Extracellular vesicles |
| FAK | Focal adhesion kinase |
| GAGs | Glycosaminoglycans |
| HA | Hyaluronic acid |
| HSCs | Hepatic stellate cells |
| KCs | Kupfferr cells |
| LOX | Lysyl oxidase |
| MMPs | Matrix metalloproteinases |
| NSCLC | Non-small cell lung cancer |
| PMN | Pre-metastatic niche |
| SHG | Second-harmonic generation imaging |
| TAFs | Tumor-associated fibroblasts |
| TGF-β | Transforming growth factor-beta |
| TIMPs | Tissue inhibitors of metalloproteinases |
| VEGF | Vascular endothelial growth factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A Review of Sex-Related Differences in Colorectal Cancer Incidence, Screening Uptake, Routes to Diagnosis, Cancer Stage and Survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef]
- Cao, W.; Qin, K.; Li, F.; Chen, W. Comparative Study of Cancer Profiles between 2020 and 2022 Using Global Cancer Statistics (GLOBOCAN). J. Natl. Cancer Cent. 2024, 4, 128–134. [Google Scholar] [CrossRef]
- Zarour, L.R.; Anand, S.; Billingsley, K.G.; Bisson, W.H.; Cercek, A.; Clarke, M.F.; Coussens, L.M.; Gast, C.E.; Geltzeiler, C.B.; Hansen, L.; et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 163–173. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal Liver Metastasis: Molecular Mechanism and Interventional Therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Strömberg, C.; Nilsson, H.; Freedman, J.; Jonas, E. Synchronous and metachronous liver metastases in patients with colorectal cancer-towards a clinically relevant definition. World J. Surg. Oncol. 2019, 17, 228. [Google Scholar] [CrossRef]
- Moris, D.; Ronnekleiv-Kelly, S.; Rahnemai-Azar, A.A.; Felekouras, E.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: A Systematic Review. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2017, 21, 1076–1085. [Google Scholar] [CrossRef]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Calon, A.; Lonardo, E.; Batlle, E. Determinants of Metastatic Competency in Colorectal Cancer. Mol. Oncol. 2017, 11, 97–119. [Google Scholar] [CrossRef] [PubMed]
- August, D.A.; Sugarbaker, P.H.; Schneider, P.D. Lymphatic Dissemination of Hepatic Metastases. Implications for the Follow-up and Treatment of Patients with Colorectal Cancer. Cancer 1985, 55, 1490–1494. [Google Scholar] [CrossRef]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xu, T.; Xue, R.; Yu, L.; Zhu, Y.; Wu, Y.; Zhang, Q.; Li, D.; Shen, S.; et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 2020, 11, 1993. [Google Scholar] [CrossRef]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef] [PubMed]
- Lenos, K.J.; Bach, S.; Ferreira Moreno, L.; Ten Hoorn, S.; Sluiter, N.R.; Bootsma, S.; Vieira Braga, F.A.; Nijman, L.E.; van den Bosch, T.; Miedema, D.M.; et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kornmann, M.; Traub, B. Role of Epithelial to Mesenchymal Transition in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 14815. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Sun, L.; Qin, H.; Fan, A.; Meng, L.; Graves-Deal, R.; Glass, S.E.; Franklin, J.L.; Liu, Q.; et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 Facilitates m6A-Dependent Stabilization of TCF7L2 mRNA and Colorectal Cancer Progression. Mol. Cancer 2022, 21, 74. [Google Scholar] [CrossRef]
- Li, W.; Guo, L.; Tang, W.; Ma, Y.; Wang, X.; Shao, Y.; Zhao, H.; Ying, J. Identification of DNA Methylation Biomarkers for Risk of Liver Metastasis in Early-Stage Colorectal Cancer. Clin. Epigenetics 2021, 13, 126. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wen, Z.; Lai, Y.; Kamila, K.; Gao, J.; Xu, W.-Y.; Gong, C.; Chen, F.; Shi, L.; et al. Genome Wide Identification of Novel DNA Methylation Driven Prognostic Markers in Colorectal Cancer. Sci. Rep. 2024, 14, 15654. [Google Scholar] [CrossRef]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, W.; Qian, H.; Sun, Y. Intracellular and Extracellular Factors of Colorectal Cancer Liver Metastasis: A Pivotal Perplex to Be Fully Elucidated. Cancer Cell Int. 2022, 22, 341. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Dhal, A.; Zambon, J.P.; Li, P.; Orlando, G.; Atala, A.; Soker, S. Current Achievements and Future Perspectives in Whole-Organ Bioengineering. Stem Cell Res. Ther. 2015, 6, 107. [Google Scholar] [CrossRef]
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.-W.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. S1), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt 24, 4195–4200. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Han, X.; Xia, K.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Compositional and Structural Analysis of Glycosaminoglycans in Cell-Derived Extracellular Matrices. Glycoconj. J. 2019, 36, 141–154. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Xia, K.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Glycosaminoglycan Disaccharide Compositional Analysis of Cell-Derived Extracellular Matrices Using Liquid Chromatography-Tandem Mass Spectrometry. Methods Cell Biol. 2020, 156, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.F.; Gilpin, C.J.; Baldock, C.; Ziese, U.; Koster, A.J.; Kadler, K.E. Corneal Collagen Fibril Structure in Three Dimensions: Structural Insights into Fibril Assembly, Mechanical Properties, and Tissue Organization. Proc. Natl. Acad. Sci. USA 2001, 98, 7307–7312. [Google Scholar] [CrossRef]
- Gillies, A.R.; Chapman, M.A.; Bushong, E.A.; Deerinck, T.J.; Ellisman, M.H.; Lieber, R.L. High Resolution Three-Dimensional Reconstruction of Fibrotic Skeletal Muscle Extracellular Matrix. J. Physiol. 2017, 595, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Rojkind, M.; Ponce-Noyola, P. The extracellular matrix of the liver. Coll. Relat. Res. 1982, 2, 151–175. [Google Scholar] [CrossRef]
- Sowbhagya, R.; Muktha, H.; Ramakrishnaiah, T.N.; Surendra, A.S.; Sushma, S.M.; Tejaswini, C.; Roopini, K.; Rajashekara, S. Collagen as the Extracellular Matrix Biomaterials in the Arena of Medical Sciences. Tissue Cell 2024, 90, 102497. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Katari, R.; Tamburrini, R.; Duisit, J.; Orlando, G. Glycosaminoglycans as a Measure of Outcome of Cell-on-Scaffold Seeding (Decellularization) Technology. Expert. Rev. Med. Devices 2016, 13, 1067–1068. [Google Scholar] [CrossRef][Green Version]
- Gray, A.L.; Pun, N.; Ridley, A.J.L.; Dyer, D.P. Role of Extracellular Matrix Proteoglycans in Immune Cell Recruitment. Int. J. Exp. Pathol. 2022, 103, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Heide, F.; Koch, M.; Stetefeld, J. Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies. Pharmaceuticals 2023, 16, 471. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin Is a Pivotal Effector in the Extracellular Matrix and Tumour Microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef]
- Brückner, G.; Morawski, M.; Arendt, T. Aggrecan-Based Extracellular Matrix Is an Integral Part of the Human Basal Ganglia Circuit. Neuroscience 2008, 151, 489–504. [Google Scholar] [CrossRef]
- Islam, S.; Watanabe, H. Versican: A Dynamic Regulator of the Extracellular Matrix. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2020, 68, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, Their Fibrillogenesis, and in Vivo Functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005041. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. Tissue Inhibitors of Metalloproteinases in Cell Signaling: Metalloproteinase-Independent Biological Activities. Sci. Signal 2008, 1, re6. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. CB 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of Exosomes on Pre-Metastatic Niche Formation in Tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef]
- Lobb, R.J.; Lima, L.G.; Möller, A. Exosomes: Key Mediators of Metastasis and Pre-Metastatic Niche Formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The Extracellular Matrix as Hallmark of Cancer and Metastasis: From Biomechanics to Therapeutic Targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef]
- Ormseth, B.; Onuma, A.; Zhang, H.; Tsung, A. The Hepatic Pre-Metastatic Niche. Cancers 2022, 14, 3731. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Cox, T.R.; Bird, D.; Baker, A.-M.; Barker, H.E.; Ho, M.W.-Y.; Lang, G.; Erler, J.T. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res. 2013, 73, 1721–1732. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Tan, X.; Du, Y.; Wei, Y.; Liu, S. Extracellular Vesicle-Mediated Pre-Metastatic Niche Formation via Altering Host Microenvironments. Front. Immunol. 2024, 15, 1367373. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, H.; Kim, J.; Choi, D.-H.; Shin, Y.; Kang, Y.G.; Kim, B.-M.; Seo, S.-U.; Chung, S.; Seok, S.H. Macrophages-Triggered Sequential Remodeling of Endothelium-Interstitial Matrix to Form Pre-Metastatic Niche in Microfluidic Tumor Microenvironment. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2019, 6, 1900195. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic Interplay between the Collagen Scaffold and Tumor Evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef]
- Kalluri, R. Basement Membranes: Structure, Assembly and Role in Tumour Angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Ting, D.T.; Wittner, B.S.; Ligorio, M.; Vincent Jordan, N.; Shah, A.M.; Miyamoto, D.T.; Aceto, N.; Bersani, F.; Brannigan, B.W.; Xega, K.; et al. Single-Cell RNA Sequencing Identifies Extracellular Matrix Gene Expression by Pancreatic Circulating Tumor Cells. Cell Rep. 2014, 8, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C.; Hikosaka, A.; Acuff, H.B.; Martin, M.D.; Kawai, N.; Singh, R.K.; Vargo-Gogola, T.C.; Begtrup, J.L.; Peterson, T.E.; Fingleton, B.; et al. MMP-7 Promotes Prostate Cancer-Induced Osteolysis via the Solubilization of RANKL. Cancer Cell 2005, 7, 485–496. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating Tumor Cells: Biology and Clinical Significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Allen-Mersh, T.G.; McCullough, T.K.; Patel, H.; Wharton, R.Q.; Glover, C.; Jonas, S.K. Role of Circulating Tumour Cells in Predicting Recurrence after Excision of Primary Colorectal Carcinoma. Br. J. Surg. 2007, 94, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-Y.; Huang, P.-S.; Chu, P.-Y.; Nguyen, T.N.A.; Hung, H.-Y.; Hsieh, C.-H.; Wu, M.-H. Current Applications and Future Directions of Circulating Tumor Cells in Colorectal Cancer Recurrence. Cancers 2024, 16, 2316. [Google Scholar] [CrossRef]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the Hallmarks of Cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Upadhyay, J.; Shekarriz, B.; Nemeth, J.A.; Dong, Z.; Cummings, G.D.; Fridman, R.; Sakr, W.; Grignon, D.J.; Cher, M.L. Membrane Type 1-Matrix Metalloproteinase (MT1-MMP) and MMP-2 Immunolocalization in Human Prostate: Change in Cellular Localization Associated with High-Grade Prostatic Intraepithelial Neoplasia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 4105–4110. [Google Scholar]
- Zhang, Y.; Luo, J.; Yang, W.; Ye, W.C. CircRNAs in colorectal cancer: Potential biomarkers and therapeutic targets. Cell Death Dis. 2023, 14, 353. [Google Scholar] [CrossRef]
- Liu, D.; Shen, M.; Liu, Z.; Chen, D.; Pan, Y.; Zhang, L.; Xu, X. SP1-induced circ_0017552 modulates colon cancer cell proliferation and apoptosis via up-regulation of NET1. Cancer Genet. 2024, 286–287, 1–10. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, X.; Liu, Y.; Cao, W.; Zhang, F.; Zhang, S.; Li, H.; Ning, L.; Fu, L.; Niu, Y.; et al. Tumor-Derived Matrix Metalloproteinase-13 (MMP-13) Correlates with Poor Prognoses of Invasive Breast Cancer. BMC Cancer 2008, 8, 83. [Google Scholar] [CrossRef]
- Bedossa, P.; Paradis, V. Liver Extracellular Matrix in Health and Disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.L.; Northall, E.; Papakyriacou, P.; Safranska, K.; Sorensen, K.K.; Lalor, P.F. Decellularization of the Human Liver to Generate Native Extracellular Matrix for Use in Automated Functional Assays with Stellate Cells. Methods Mol. Biol. Clifton NJ 2023, 2669, 233–244. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The Nature and Biology of Basement Membranes. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J. Extracellular Matrix in the Trabecular Meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef]
- Goddard, E.T.; Hill, R.C.; Barrett, A.; Betts, C.; Guo, Q.; Maller, O.; Borges, V.F.; Hansen, K.C.; Schedin, P. Quantitative Extracellular Matrix Proteomics to Study Mammary and Liver Tissue Microenvironments. Int. J. Biochem. Cell Biol. 2016, 81 Pt A, 223–232. [Google Scholar] [CrossRef]
- Krasny, L.; Paul, A.; Wai, P.; Howard, B.A.; Natrajan, R.C.; Huang, P.H. Comparative Proteomic Assessment of Matrisome Enrichment Methodologies. Biochem. J. 2016, 473, 3979–3995. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Willacy, O.; Madsen, C.D.; Rafaeva, M.; Heumüller, S.E.; Bock, F.; Sengle, G.; Koch, M.; Imhof, T.; Zaucke, F.; et al. Decellularization and Antibody Staining of Mouse Tissues to Map Native Extracellular Matrix Structures in 3D. Nat. Protoc. 2020, 15, 2140, Erratum in Nat. Protoc. 2019, 14, 3395–3425. [Google Scholar] [CrossRef]
- Payen, V.L.; Lavergne, A.; Alevra Sarika, N.; Colonval, M.; Karim, L.; Deckers, M.; Najimi, M.; Coppieters, W.; Charloteaux, B.; Sokal, E.M.; et al. Single-cell RNA sequencing of human liver reveals hepatic stellate cell heterogeneity. JHEP Rep. Innov. Hepatol. 2021, 3, 100278. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.; Machesky, L.M. The Liver Metastatic Niche: Modelling the Extracellular Matrix in Metastasis. Dis. Model. Mech. 2021, 14, dmm048801. [Google Scholar] [CrossRef]
- Wu, X.; Cai, J.; Zuo, Z.; Li, J. Collagen Facilitates the Colorectal Cancer Stemness and Metastasis through an Integrin/PI3K/AKT/Snail Signaling Pathway. Biomed. Pharmacother. 2019, 114, 108708. [Google Scholar] [CrossRef]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Burnier, J.V.; Wang, N.; Michel, R.P.; Hassanain, M.; Li, S.; Lu, Y.; Metrakos, P.; Antecka, E.; Burnier, M.N.; Ponton, A.; et al. Type IV Collagen-Initiated Signals Provide Survival and Growth Cues Required for Liver Metastasis. Oncogene 2011, 30, 3766–3783. [Google Scholar] [CrossRef]
- Gulubova, M.V. Collagen Type IV, Laminin, Alpha-Smooth Muscle Actin (alphaSMA), Alpha1 and Alpha6 Integrins Expression in the Liver with Metastases from Malignant Gastrointestinal Tumours. Clin. Exp. Metastasis 2004, 21, 485–494. [Google Scholar] [CrossRef]
- Vaniotis, G.; Rayes, R.F.; Qi, S.; Milette, S.; Wang, N.; Perrino, S.; Bourdeau, F.; Nyström, H.; He, Y.; Lamarche-Vane, N.; et al. Collagen IV-Conveyed Signals Can Regulate Chemokine Production and Promote Liver Metastasis. Oncogene 2018, 37, 3790–3805. [Google Scholar] [CrossRef]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin Is an Essential Determinant of Arterial Morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Meng, X.; Guo, Z. Elastin Structure, Synthesis, Regulatory Mechanism and Relationship with Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 596702. [Google Scholar] [CrossRef]
- Kanta, J. Elastin in the Liver. Front. Physiol. 2016, 7, 491. [Google Scholar] [CrossRef]
- Oda, H.; Nozawa, K.; Hitomi, Y.; Kakinuma, A. Laminin-Rich Extracellular Matrix Maintains High Level of Hepatocyte Nuclear Factor 4 in Rat Hepatocyte Culture. Biochem. Biophys. Res. Commun. 1995, 212, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seki, E. Hyaluronan in Liver Fibrosis: Basic Mechanisms, Clinical Implications, and Therapeutic Targets. Hepatol. Commun. 2023, 7, e0083. [Google Scholar] [CrossRef]
- Wei, B.; Zhou, X.; Liang, C.; Zheng, X.; Lei, P.; Fang, J.; Han, X.; Wang, L.; Qi, C.; Wei, H. Human Colorectal Cancer Progression Correlates with LOX-Induced ECM Stiffening. Int. J. Biol. Sci. 2017, 13, 1450–1457. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; Ferreira, B.Z.F.; Nader, H.B.; Lopes, C.C. Heparan Sulfate Proteoglycans as Targets for Cancer Therapy: A Review. Cancer Biol. Ther. 2020, 21, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the Matrisome--an Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef] [PubMed]
- McQuitty, C.E.; Williams, R.; Chokshi, S.; Urbani, L. Immunomodulatory Role of the Extracellular Matrix Within the Liver Disease Microenvironment. Front. Immunol. 2020, 11, 574276. [Google Scholar] [CrossRef]
- Mazza, G.; Telese, A.; Al-Akkad, W.; Frenguelli, L.; Levi, A.; Marrali, M.; Longato, L.; Thanapirom, K.; Vilia, M.G.; Lombardi, B.; et al. Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-Β1 Epithelial Mesenchymal Transition. Cells 2019, 9, 83. [Google Scholar] [CrossRef]
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. Matrix Metalloproteinases Induce Extracellular Matrix Degradation through Various Pathways to Alleviate Hepatic Fibrosis. Biomed. Pharmacother. 2023, 161, 114472. [Google Scholar] [CrossRef]
- Roy, A.M.; Iyer, R.; Chakraborty, S. The Extracellular Matrix in Hepatocellular Carcinoma: Mechanisms and Therapeutic Vulnerability. Cell Rep. Med. 2023, 4, 101170. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Sakai, K.; Moriya, K.; Sasaki, T.; Keene, D.R.; Akhtar, R.; Miyazono, T.; Yasumura, S.; Watanabe, M.; Morishita, S.; et al. Molecular Mechanism Responsible for Fibronectin-Controlled Alterations in Matrix Stiffness in Advanced Chronic Liver Fibrogenesis. J. Biol. Chem. 2016, 291, 72–88. [Google Scholar] [CrossRef]
- Zhong, B.; Cheng, B.; Huang, X.; Xiao, Q.; Niu, Z.; Chen, Y.-F.; Yu, Q.; Wang, W.; Wu, X.-J. Colorectal Cancer-Associated Fibroblasts Promote Metastasis by up-Regulating LRG1 through Stromal IL-6/STAT3 Signaling. Cell Death Dis. 2021, 13, 16. [Google Scholar] [CrossRef]
- Sun, H.; Santoro, S.A.; Zutter, M.M. Downstream Events in Mammary Gland Morphogenesis Mediated by Reexpression of the Alpha2beta1 Integrin: The Role of the Alpha6 and Beta4 Integrin Subunits. Cancer Res. 1998, 58, 2224–2233. [Google Scholar]
- Maltseva, D.; Raygorodskaya, M.; Knyazev, E.; Zgoda, V.; Tikhonova, O.; Zaidi, S.; Nikulin, S.; Baranova, A.; Turchinovich, A.; Rodin, S.; et al. Knockdown of the A5 Laminin Chain Affects Differentiation of Colorectal Cancer Cells and Their Sensitivity to Chemotherapy. Biochimie 2020, 174, 107–116. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.; Lim, S.Y.; Yuzhalin, A.; Lucotti, S.; Vermeer, J.A.F.; Jones, K.; Chen, J.; Muschel, R.J. Tumour-Derived Laminin A5 (LAMA5) Promotes Colorectal Liver Metastasis Growth, Branching Angiogenesis and Notch Pathway Inhibition. Cancers 2019, 11, 630. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Dzamba, B.J.; DeSimone, D.W. Extracellular Matrix (ECM) and the Sculpting of Embryonic Tissues. Curr. Top. Dev. Biol. 2018, 130, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Nguyen Ho-Bouldoires, T.H.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in Tumor Progression: The Dark Side of the Force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.-C.; Meng, Z. Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression. Front. Cell Dev. Biol. 2021, 9, 673599. [Google Scholar] [CrossRef]
- Huang, C.; Gan, D.; Luo, F.; Wan, S.; Chen, J.; Wang, A.; Li, B.; Zhu, X. Interaction Mechanisms Between the NOX4/ROS and RhoA/ROCK1 Signaling Pathways as New Anti- Fibrosis Targets of Ursolic Acid in Hepatic Stellate Cells. Front. Pharmacol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, X.; He, X.; Hu, Z.; Huang, H.; Chen, J.; Chen, K.; Zhao, S.; Wei, P.; Li, D. Liver Metastasis from Colorectal Cancer: Pathogenetic Development, Immune Landscape of the Tumour Microenvironment and Therapeutic Approaches. J. Exp. Clin. Cancer Res. 2023, 42, 177. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, X.; Lu, J.; Salfenmoser, M.; Wirsik, N.M.; Schleussner, N.; Imle, A.; Freire Valls, A.; Radhakrishnan, P.; Liang, J.; et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell 2020, 37, 800–817.e7. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Lorenzini, S.; Bird, T.G.; Boulter, L.; Bellamy, C.; Samuel, K.; Aucott, R.; Clayton, E.; Andreone, P.; Bernardi, M.; Golding, M.; et al. Characterisation of a Stereotypical Cellular and Extracellular Adult Liver Progenitor Cell Niche in Rodents and Diseased Human Liver. Gut 2010, 59, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Dituri, F.; Gigante, G.; Scialpi, R.; Mancarella, S.; Fabregat, I.; Giannelli, G. Proteoglycans in Cancer: Friends or Enemies? A Special Focus on Hepatocellular Carcinoma. Cancers 2022, 14, 1902. [Google Scholar] [CrossRef]
- Rokavec, M.; Öner, M.G.; Li, H.; Jackstadt, R.; Jiang, L.; Lodygin, D.; Kaller, M.; Horst, D.; Ziegler, P.K.; Schwitalla, S.; et al. IL-6R/STAT3/miR-34a Feedback Loop Promotes EMT-Mediated Colorectal Cancer Invasion and Metastasis. J. Clin. Investig. 2014, 124, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Vanaclocha, F. The Liver Prometastatic Reaction of Cancer Patients: Implications for Microenvironment-Dependent Colon Cancer Gene Regulation. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2011, 4, 163–180. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The Integrin-Collagen Connection—A Glue for Tissue Repair? J. Cell Sci. 2016, 129, 1284. [Google Scholar] [CrossRef]
- Arimori, T.; Miyazaki, N.; Mihara, E.; Takizawa, M.; Taniguchi, Y.; Cabañas, C.; Sekiguchi, K.; Takagi, J. Structural Mechanism of Laminin Recognition by Integrin. Nat. Commun. 2021, 12, 4012. [Google Scholar] [CrossRef]
- Takizawa, M.; Arimori, T.; Taniguchi, Y.; Kitago, Y.; Yamashita, E.; Takagi, J.; Sekiguchi, K. Mechanistic Basis for the Recognition of Laminin-511 by A6β1 Integrin. Sci. Adv. 2017, 3, e1701497. [Google Scholar] [CrossRef]
- Heslin, M.J.; Yan, J.; Johnson, M.R.; Weiss, H.; Diasio, R.B.; Urist, M.M. Role of Matrix Metalloproteinases in Colorectal Carcinogenesis. Ann. Surg. 2001, 233, 786–792. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.-A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver Metastases. Nat. Rev. Dis. Primer 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Y.; Ma, X.-M.; Luan, X.-H.; Liuyang, Z.-Y.; Hong, Y.-Y.; Dai, Y.; Dong, Q.-H.; Wang, G.-Y. BMI-1 Activates Hepatic Stellate Cells to Promote the Epithelial-Mesenchymal Transition of Colorectal Cancer Cells. World J. Gastroenterol. 2023, 29, 3606–3621. [Google Scholar] [CrossRef] [PubMed]
- Marvin, D.L.; Heijboer, R.; Ten Dijke, P.; Ritsma, L. TGF-β Signaling in Liver Metastasis. Clin. Transl. Med. 2020, 10, e160. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-X.; Gong, W.-Z.; Zhou, K.; Xiao, Z.-G.; Hou, F.-T.; Huang, T.; Zhang, L.; Dong, H.-Y.; Zhang, W.-L.; Liu, Y.; et al. CXCR4/TGF-Β1 Mediated Hepatic Stellate Cells Differentiation into Carcinoma-Associated Fibroblasts and Promoted Liver Metastasis of Colon Cancer. Cancer Biol. Ther. 2020, 21, 258–268. [Google Scholar] [CrossRef]
- Chen, C.; Yao, X.; Xu, Y.; Zhang, Q.; Wang, H.; Zhao, L.; Wen, G.; Liu, Y.; Jing, L.; Sun, X. Dahuang Zhechong Pill Suppresses Colorectal Cancer Liver Metastasis via Ameliorating Exosomal CCL2 Primed Pre-Metastatic Niche. J. Ethnopharmacol. 2019, 238, 111878. [Google Scholar] [CrossRef]
- Kitamura, T.; Fujishita, T.; Loetscher, P.; Revesz, L.; Hashida, H.; Kizaka-Kondoh, S.; Aoki, M.; Taketo, M.M. Inactivation of Chemokine (C-C Motif) Receptor 1 (CCR1) Suppresses Colon Cancer Liver Metastasis by Blocking Accumulation of Immature Myeloid Cells in a Mouse Model. Proc. Natl. Acad. Sci. USA 2010, 107, 13063–13068. [Google Scholar] [CrossRef]
- Rao, V.S.; Gu, Q.; Tzschentke, S.; Lin, K.; Ganig, N.; Thepkaysone, M.-L.; Wong, F.C.; Polster, H.; Seifert, L.; Seifert, A.M.; et al. Extravesicular TIMP-1 Is a Non-Invasive Independent Prognostic Marker and Potential Therapeutic Target in Colorectal Liver Metastases. Oncogene 2022, 41, 1809–1820. [Google Scholar] [CrossRef]
- Li, C.; Qin, F.; Wang, W.; Ni, Y.; Gao, M.; Guo, M.; Sun, G. hnRNPA2B1-Mediated Extracellular Vesicles Sorting of miR-122-5p Potentially Promotes Lung Cancer Progression. Int. J. Mol. Sci. 2021, 22, 12866. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-Delivered EGFR Regulates Liver Microenvironment to Promote Gastric Cancer Liver Metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S. Significance of the Tumor Microenvironment in Liver Cancer Progression. Crit. Rev. Oncog. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Giguelay, A.; Turtoi, E.; Khelaf, L.; Tosato, G.; Dadi, I.; Chastel, T.; Poul, M.-A.; Pratlong, M.; Nicolescu, S.; Severac, D.; et al. The Landscape of Cancer-Associated Fibroblasts in Colorectal Cancer Liver Metastases. Theranostics 2022, 12, 7624–7639. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Z.; Lai, M. Targeting the Devil: Strategies against Cancer-Associated Fibroblasts in Colorectal Cancer. Transl. Res. J. Lab. Clin. Med. 2024, 270, 81–93. [Google Scholar] [CrossRef]
- Holle, A.W.; Young, J.L.; Spatz, J.P. In Vitro Cancer Cell-ECM Interactions Inform in Vivo Cancer Treatment. Adv. Drug Deliv. Rev. 2016, 97, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-K.; Strauss, R.; Richter, M.; Yun, C.-O.; Lieber, A. Strategies to Increase Drug Penetration in Solid Tumors. Front. Oncol. 2013, 3, 193. [Google Scholar] [CrossRef]
- Jurj, A.; Ionescu, C.; Berindan-Neagoe, I.; Braicu, C. The Extracellular Matrix Alteration, Implication in Modulation of Drug Resistance Mechanism: Friends or Foes? J. Exp. Clin. Cancer Res. 2022, 41, 276. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Han, Y.; Cao, C.; Zhang, Z.; Li, L.; Xiao, C.; Guo, H.; Wang, L.; Han, L.; et al. Extracellular Matrix Physical Properties Govern the Diffusion of Nanoparticles in Tumor Microenvironment. Proc. Natl. Acad. Sci. USA 2023, 120, e2209260120. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A New Role for the PI3K/Akt Signaling Pathway in the Epithelial-Mesenchymal Transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.X.; Yuan, W.J.; Wang, H.Z.; Li, Y.X. Extracellular matrix stiffness and tumor-associated macrophage polarization: New fields affecting immune exclusion. Cancer Immunol. Immunother. CII 2024, 73, 115. [Google Scholar] [CrossRef]
- van Huizen, N.A.; Coebergh van den Braak, R.R.J.; Doukas, M.; Dekker, L.J.M.; IJzermans, J.N.M.; Luider, T.M. Up-Regulation of Collagen Proteins in Colorectal Liver Metastasis Compared with Normal Liver Tissue. J. Biol. Chem. 2019, 294, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Poskus, M.D.; Stylianopoulos, T.; Zervantonakis, I.K. Beyond matrix stiffness: Targeting force-induced cancer drug resistance. Trends Cancer 2023, 9, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Blockhuys, S.; Agarwal, N.R.; Hildesjö, C.; Jarlsfelt, I.; Wittung-Stafshede, P.; Sun, X.-F. Second Harmonic Generation for Collagen I Characterization in Rectal Cancer Patients with and without Preoperative Radiotherapy. J. Biomed. Opt. 2017, 22, 106006. [Google Scholar] [CrossRef]
- Tanaka, K.; Okigami, M.; Toiyama, Y.; Morimoto, Y.; Matsushita, K.; Kawamura, M.; Hashimoto, K.; Saigusa, S.; Okugawa, Y.; Inoue, Y.; et al. In Vivo Real-Time Imaging of Chemotherapy Response on the Liver Metastatic Tumor Microenvironment Using Multiphoton Microscopy. Oncol. Rep. 2012, 28, 1822–1830. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Lee, J.W.; Juliano, R.L. Alpha5beta1 Integrin Protects Intestinal Epithelial Cells from Apoptosis through a Phosphatidylinositol 3-Kinase and Protein Kinase B-Dependent Pathway. Mol. Biol. Cell 2000, 11, 1973–1987. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Hou, S.; Wang, J.; Li, W.; Hao, X.; Hang, Q. Roles of Integrins in Gastrointestinal Cancer Metastasis. Front. Mol. Biosci. 2021, 8, 708779. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Pirozzi, M.; Miceli, C.C.; Cocule, M.; Caraglia, M.; Boccellino, M.; Vitale, P.; De Falco, V.; Farese, S.; Zotta, A.; et al. TGF-β Modulated Pathways in Colorectal Cancer: New Potential Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 7400. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Increased Stiffness of the Tumor Microenvironment in Colon Cancer Stimulates Cancer Associated Fibroblast-Mediated Prometastatic Activin A Signaling. Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P. CRISPR screening and cell line IC50 data reveal novel key genes for trametinib resistance. Clin. Exp. Med. 2024, 25, 21. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, S.; Fan, Z.; Wang, Y.; Liu, H.; Cao, H.; Sun, G.; Huang, T.; Cai, H.; Pan, H.; et al. CHSY1 promotes CD8+ T cell exhaustion through activation of succinate metabolism pathway leading to colorectal cancer liver metastasis based on CRISPR/Cas9 screening. J. Exp. Clin. Cancer Res. 2023, 42, 248. [Google Scholar] [CrossRef]
- Takeda, H.; Kataoka, S.; Nakayama, M.; Ali, M.A.E.; Oshima, H.; Yamamoto, D.; Park, J.W.; Takegami, Y.; An, T.; Jenkins, N.A.; et al. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc. Natl. Acad. Sci. USA 2019, 116, 15635–15644. [Google Scholar] [CrossRef]
- García-Gareta, E.; Pérez, M.Á.; García-Aznar, J.M. Decellularization of Tumours: A New Frontier in Tissue Engineering. J. Tissue Eng. 2022, 13, 20417314221091682. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized Human Liver as a Natural 3D-Scaffold for Liver Bioengineering and Transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef]
- Zhou, P.; Lessa, N.; Estrada, D.C.; Severson, E.B.; Lingala, S.; Zern, M.A.; Nolta, J.A.; Wu, J. Decellularized Liver Matrix as a Carrier for the Transplantation of Human Fetal and Primary Hepatocytes in Mice. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2011, 17, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Varinelli, L.; Guaglio, M.; Brich, S.; Zanutto, S.; Belfiore, A.; Zanardi, F.; Iannelli, F.; Oldani, A.; Costa, E.; Chighizola, M.; et al. Decellularized Extracellular Matrix as Scaffold for Cancer Organoid Cultures of Colorectal Peritoneal Metastases. J. Mol. Cell Biol. 2023, 14, mjac064. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-Based 3D Cell Culture Models in Cancer Research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Gentilin, E.; D’Angelo, E.; Agostini, M.; Astolfi, L. Decellularized Normal and Cancer Tissues as Tools for Cancer Research. Cancer Gene Ther. 2022, 29, 879–888. [Google Scholar] [CrossRef]
- Atat, O.E.; Farzaneh, Z.; Pourhamzeh, M.; Taki, F.; Abi-Habib, R.; Vosough, M.; El-Sibai, M. 3D modeling in cancer studies. Hum.Cell. 2022, 35, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Jailkhani, N.; Clauser, K.R.; Mak, H.H.; Rickelt, S.; Tian, C.; Whittaker, C.A.; Tanabe, K.K.; Purdy, S.R.; Carr, S.A.; Hynes, R.O. Proteomic Profiling of Extracellular Matrix Components from Patient Metastases Identifies Consistently Elevated Proteins for Developing Nanobodies That Target Primary Tumors and Metastases. Cancer Res. 2023, 83, 2052–2065. [Google Scholar] [CrossRef]
- Karlsson, S.; Nyström, H. The extracellular matrix in colorectal cancer and its metastatic settling—Alterations and biological implications. Crit. Rev. Oncol./Hematol. 2022, 175, 103712. [Google Scholar] [CrossRef]
- Steward, W.P.; Thomas, A.L. Marimastat: The Clinical Development of a Matrix Metalloproteinase Inhibitor. Expert Opin. Investig. Drugs 2000, 9, 2913–2922. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix Metalloproteinases as Novel Biomarkers and Potential Therapeutic Targets in Human Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Huang, J.; Li, X.; You, D.; Wang, L.; Ma, X. Prognostic Value of Matrix Metalloproteinase-2 Protein and Matrix Metalloproteinase-9 Protein in Colorectal Cancer: A Meta-Analysis. BMC Cancer 2024, 24, 1065. [Google Scholar] [CrossRef]
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parry, G.C.; Parikh, A.A.; McCarty, M.F.; Bucana, C.D.; Mazar, A.P.; Ellis, L.M. Inhibition of Integrin Alpha5beta1 Function with a Small Peptide (ATN-161) plus Continuous 5-FU Infusion Reduces Colorectal Liver Metastases and Improves Survival in Mice. Int. J. Cancer 2003, 104, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhu, Y.; Meng, T.; Liu, Y.; Hong, Y.; Yuan, M.; Yuan, H.; Hu, F. Targeting High Expressed A5β1 Integrin in Liver Metastatic Lesions To Resist Metastasis of Colorectal Cancer by RPM Peptide-Modified Chitosan-Stearic Micelles. Mol. Pharm. 2018, 15, 1653–1663. [Google Scholar] [CrossRef]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin Potentiates the Effect of Oxaliplatin Preventing Outgrowth of Colon Cancer Metastasis, Binds to A5β1 Integrin and Suppresses FAK/ERK/NF-κB Signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef]
- Alghisi, G.C.; Rüegg, C. Vascular Integrins in Tumor Angiogenesis: Mediators and Therapeutic Targets. Endothel. J. Endothel. Cell Res. 2006, 13, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Ray, A.M.; Dontenwill, M. Integrin A5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers 2013, 5, 27–47. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell. Signal. 2009, 21, 665–674. [Google Scholar] [CrossRef]
- Reinmuth, N.; Liu, W.; Ahmad, S.A.; Fan, F.; Stoeltzing, O.; Parikh, A.A.; Bucana, C.D.; Gallick, G.E.; Nickols, M.A.; Westlin, W.F.; et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003, 63, 2079–2087. [Google Scholar]
- Hong, Y.; Rao, Y. Current Status of Nanoscale Drug Delivery Systems for Colorectal Cancer Liver Metastasis. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 114, 108764. [Google Scholar] [CrossRef] [PubMed]
| Molecule/Protein Name | Molecular Structure (Subtypes) | Biological Function in Healthy Liver | Tissue Distribution in Liver |
|---|---|---|---|
| Collagen Type I | Fibrillar (I, III) | Provides tensile strength, structural integrity | Perisinusoidal space |
| Collagen Type IV | Non-fibrillar | Maintains basement membrane integrity, supports cell adhesion | Sinusoidal endothelium |
| Elastin | Fibers | Provides tissue elasticity and maintains vascular integrity | Blood vessels |
| Fibronectin | Glycoprotein | Facilitates cell adhesion, migration, and tissue repair | ECM, hepatocytes |
| Laminin | Heterotrimer (α, β, γ) | Supports cell adhesion and differentiation | Basement membrane of sinusoids |
| Hyaluronic acid | Non-sulfated GAG | Regulates hydration and cellular motility | Hepatic stellate cells |
| Heparan sulfate | Sulfated GAG | Modulates growth factor signaling and maintains liver homeostasis | Basement membrane |
| Decorin | Proteoglycan | Controls collagen fibrillogenesis | ECM |
| Molecule/Protein Name | Molecular Structure (Subtypes) | Biological Function in CRLM | Tissue Distribution in Metastasis |
|---|---|---|---|
| Collagen Type I | Fibrillar (I, III) | Increases rigidity, promotes cancer invasion | Tumor stroma |
| Collagen Type IV | Non-fibrillar | Facilitates cancer cell extravasation and colonization | Tumor basement membrane |
| Elastin | Fibers | Increased vascular stiffness, promotes abnormal vasculature | Metastatic blood vessels |
| Fibronectin | Glycoprotein | Enhances tumor adhesion, migration, and angiogenesis | Tumor ECM |
| Laminin | Heterotrimer (α, β, γ) | Facilitates tumor invasion and survival in metastatic sites | Metastatic niche |
| Hyaluronic acid | Non-sulfated GAG | Promotes cell motility, immune evasion | Tumor stroma |
| Heparan sulfate | Sulfated GAG | Sequester growth factors, enhance tumor growth | Tumor-associated basement membrane |
| Decorin | Proteoglycan | Loss of decorin leads to enhanced tumor invasion | Tumor ECM |
| Laminin Type/Isoform | Chains (α, β, γ) | Role in Healthy Liver | Role in CRLM |
|---|---|---|---|
| Laminin-111 | α1, β1, γ1 | Present during early liver development; involved in hepatocyte polarization and basement membrane formation | Rare in adult liver, but cancer cells may exploit it for metastatic niche establishment |
| Laminin-211 | α2, β1, γ1 | Important for liver regeneration and muscle maintenance | Rarely found in CRLM; minimal role in metastasis |
| Laminin-332 (formerly 5) | α3, β3, γ2 | Not highly expressed in normal liver tissue | Promotes cancer cell migration, invasion, and metastasis through integrin signaling (α6β4 integrin) |
| Laminin-411 | α4, β1, γ1 | Important for liver vasculature integrity; contributes to sinusoidal endothelium and ECM maintenance | Involved in vascular remodeling and angiogenesis in CRLM, providing a supportive environment for tumor growth |
| Laminin-511 | α5, β1, γ1 | Supports hepatocyte attachment to ECM and maintains normal liver structure | Upregulated in the metastatic liver microenvironment, enhances the adhesion and migration of colorectal cancer cells |
| Laminin-521 | α5, β2, γ1 | Maintains structural integrity of the liver’s vascular basement membrane | Involved in metastatic cancer cell colonization, promoting survival of colorectal cancer cells in liver metastases |
| Laminin-332 | α3, β3, γ2 | Rare in normal adult liver, mostly in basal membranes | Facilitates cancer cell invasion and metastasis through interaction with integrins (e.g., α6β4) and promotes tumor cell survival in the metastatic niche |
| Laminin-111 (Developmental) | α1, β1, γ1 | Expressed during liver development; aids in tissue differentiation and basement membrane formation | Abnormally re-expressed in some liver metastases, contributing to the establishment of a supportive metastatic niche |
| MMP | Healthy Liver | Colorectal Liver Metastasis (CRLM) |
|---|---|---|
| MMP-2 | Present in latent form; involved in normal ECM turnover | Overexpressed and activated; facilitates tumor cell invasion leading to the breakdown of ECM barriers |
| MMP-7 | Low expression; limited role in normal liver function | Upregulated; associated with aggressive tumor behavior, progression, and poor prognosis |
| MMP-9 | Detected in latent form; participates in ECM maintenance | Elevated levels, especially in active form; promote metastasis |
| Trial ID/ Study References | Target | Drug/ Intervention | Type | N * | Key Findings | Status/ Results |
|---|---|---|---|---|---|---|
| NCT04755907 | 3D colorectal cancer models and organoids | Same chemotherapy drugs as the corresponding patients | Preclinical | 120 | Response to adjuvant chemotherapy evaluated according to DFS | unknown |
| NCT03131778 | MMP9 | Laparoscopic vs open liver resection | Clinical | 40 | Evaluate differences in inflammatory response | completed |
| NCT00835679 | EGFR FAK | Cetuximab Dasatinib | Clinical | 9 | Reduction in at least 1 biomarker of the pathway inhibited | closed prematurely (slow accrual) |
| NCT01008475 | integrin αν heterodimers | EMD 525797 (Abituzumab) with cetuximab and Irinotecan | Clinical | 232 | Assess the tolerability of different doses (phase I) and explore the efficacy and tolerability (phase II) | completed, primary PFS endpoint not met, accepted tolerability |
| NCT03170960 | tyrosin kinase (PD-1) | Cabozantinib (XL184) | Clinical | 1732 | Assess safety, tolerability, preliminary efficacy, and pharmacokinetics | active, not recruiting |
| NCT02837263 | tyrosin kinase (PD-1) | Pembrolizumab with stereotactic body radiotherapy (SBRT) | Clinical | 18 | Recurrence rate at 1 year following clearance of metastatic disease | completed |
| NCT04508140 | tyrosin kinase (PD-1) | BO-112 with Pembrolizumab | Clinical | 18 | Reverse the primary resistance that microsatellite stability presents to the PD-1 inhibitors | terminated, low recruitment rate |
| NCT04046445 | tyrosin kinase (PD-1) and IgG4 | TP128 and BI 754091 (Ezabenlimab) | Clinical | 96 | Evaluate the safety and tolerability | active, not recruiting |
| NCT02298946 | tyrosin kinase (PD-1) | AMP-224 plus SBRT | Clinical | 17 | Evaluate whether the anti-tumor immunity can be enhanced by radiation therapy | completed |
| NCT06504901 | tyrosin kinase (PD-1) | Tislelizumab Interleukin-2 Capecitabine Oxaliplatin Neupogen | Clinical | 30 | Overcoming the limitations of single-agent immunotherapy through multifaceted immune modulation | not yet recruiting |
| NCT06280495 | tyrosin kinase (PD-1) | Serpulimumab and bevacizumab | Clinical | 156 | Enhancing the immune microenvironment in the liver, increasing T lymphocyte infiltration, and consequently improving the post-op prognosis in resectable CRLM | recruiting |
| NCT06199232 | tyrosin kinase (PD-1) | HAIC + targeted therapy + Tislelizumab | Clinical | 47 | Efficacy and safety of targeted treatment based on ctDNA genotyping as salvage treatment for advanced CRCLM failed from the standard systemic treatment | recruiting |
| NCT06590259 | tyrosin kinase (PD-1) | Sintilimab + mFOLFOX6 or FOLFIRI + bevacizumab or cetuximab | Clinical | 20 | Evaluate the efficacy and safety of multi-mode ablation combined with systemic therapy | recruiting |
| NCT06794086 | tyrosin kinase (PD-1) | PD-1 Monoclonal Antibody plus SBRT | Clinical | 24 | Improve the objective response rate (ORR), achieve better long-term survival benefits, and enhance quality of life in unresectable CRLM | recruiting |
| NCT06045286 | tyrosin kinase (PD-1) | Radiation: High- and low-dose radiotherapy plus PD-1 inhibitors | Clinical | 30 | Investigate the efficacy and safety of failed second-line immunotherapy or above | recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morabito, M.; Thibodot, P.; Gigandet, A.; Compagnon, P.; Toso, C.; Berishvili, E.; Lacotte, S.; Peloso, A. Liver Extracellular Matrix in Colorectal Liver Metastasis. Cancers 2025, 17, 953. https://doi.org/10.3390/cancers17060953
Morabito M, Thibodot P, Gigandet A, Compagnon P, Toso C, Berishvili E, Lacotte S, Peloso A. Liver Extracellular Matrix in Colorectal Liver Metastasis. Cancers. 2025; 17(6):953. https://doi.org/10.3390/cancers17060953
Chicago/Turabian StyleMorabito, Marika, Pauline Thibodot, Anthony Gigandet, Philippe Compagnon, Christian Toso, Ekaterine Berishvili, Stéphanie Lacotte, and Andrea Peloso. 2025. "Liver Extracellular Matrix in Colorectal Liver Metastasis" Cancers 17, no. 6: 953. https://doi.org/10.3390/cancers17060953
APA StyleMorabito, M., Thibodot, P., Gigandet, A., Compagnon, P., Toso, C., Berishvili, E., Lacotte, S., & Peloso, A. (2025). Liver Extracellular Matrix in Colorectal Liver Metastasis. Cancers, 17(6), 953. https://doi.org/10.3390/cancers17060953







