The Impact of Homelessness on Lung Cancer Survival and Healthcare Utilization in the Hungarian Universal Healthcare System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Sample
2.3. Statistical Analyses
3. Results
3.1. Sample Descriptives
3.2. Healthcare Costs
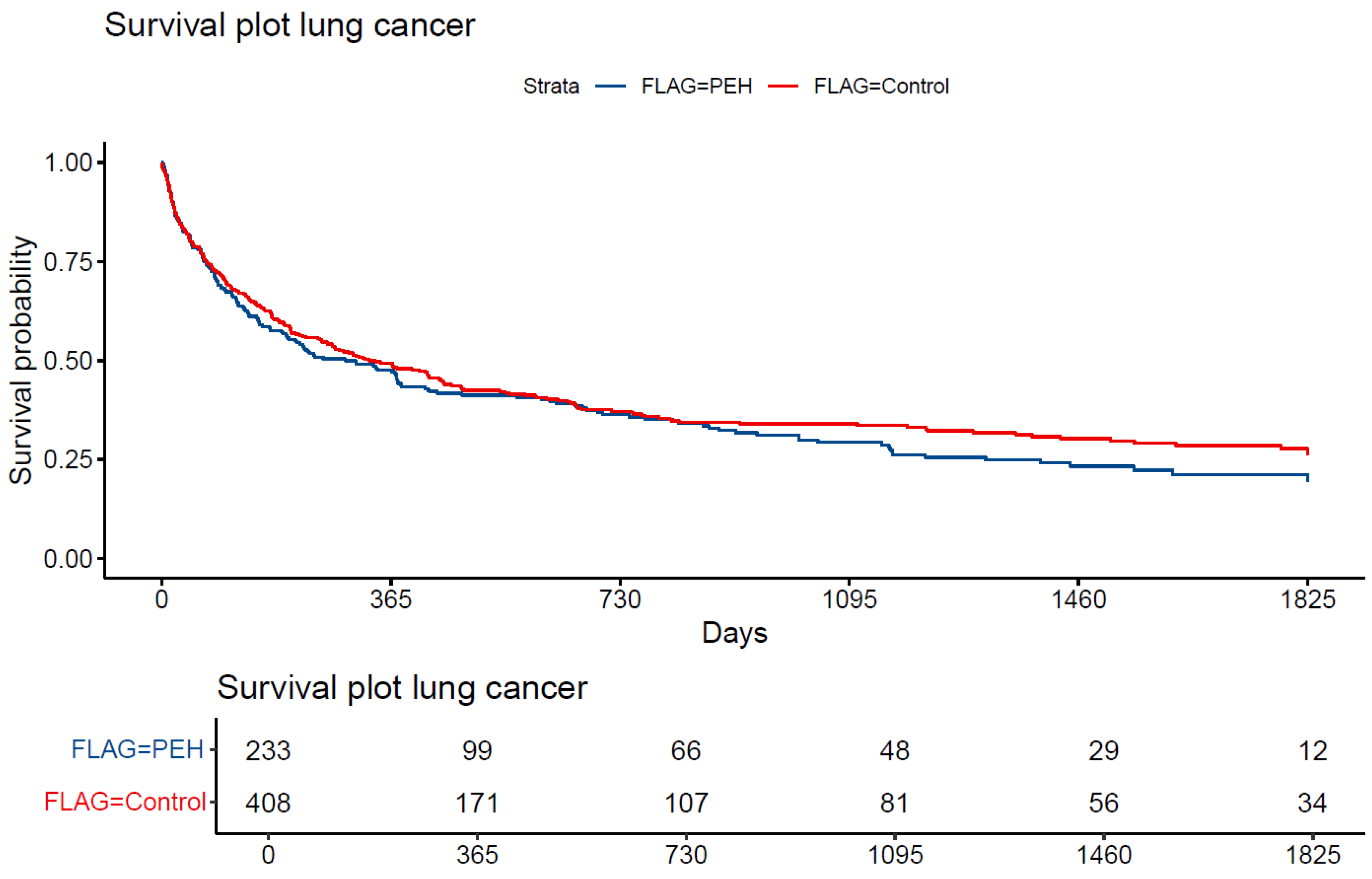
3.3. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Cancer ICD 10 Code | Percentage of All Cancer Cases |
|---|---|
| C34 | 19.8% |
| C79 | 6.4% |
| C32 | 6.0% |
| C78 | 5.9% |
| C77 | 4.6% |
| C10 | 3.4% |
| C53 | 3.1% |
| C13 | 2.9% |
| C18 | 2.5% |
| C67 | 2.5% |

References
- Fornaro, M.; Dragioti, E.; De Prisco, M.; Billeci, M.; Mondin, A.M.; Calati, R.; Smith, L.; Hatcher, S.; Kaluzienski, M.; Fiedorowicz, J.G.; et al. Homelessness and health-related outcomes: An umbrella review of observational studies and randomized controlled trials. BMC Med. 2021, 20, 224. [Google Scholar] [CrossRef]
- Tipple, G.; Speak, S. Definitions of homelessness in developing countries. Habitat Int. 2005, 29, 337–352. [Google Scholar] [CrossRef]
- Lung cancer n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 29 August 2024).
- Redondo-Sánchez, D.; Petrova, D.; Rodríguez-Barranco, M.; Fernández-Navarro, P.; Jiménez-Moleón, J.J.; Sánchez, M.-J. Socio-Economic Inequalities in Lung Cancer Outcomes: An Overview of Systematic Reviews. Cancers 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; I Heath, E.; Pappas, L.M.; Ruterbusch, J.J.; Gorski, D.H.; A Triest, J.; Park, H.K.; Beebe-Dimmer, J.L.; Schwartz, A.G.; Cote, M.L.; et al. The Epidemiology of Cancer Among Homeless Adults in Metropolitan Detroit. JNCI Cancer Spectr. 2019, 3, pkz006. [Google Scholar] [CrossRef]
- OECD Data Explorer Healthcare coverage n.d. Available online: https://data-explorer.oecd.org/vis?fs[0]=Topic%2C1%7CHealth%23HEA%23%7CHealthcare%20coverage%23HEA_COV%23&pg=0&fc=Topic&bp=true&snb=1&vw=tl&df[ds]=dsDisseminateFinalDMZ&df[id]=DSD_HEALTH_PROT%40DF_HEALTH_PROT&df[ag]=OECD.ELS.HD&df[vs]=1.0&pd=2010%2C&dq=HUN.A..PT_POP.&ly[cl]=TIME_PERIOD&to[TIME_PERIOD]=false (accessed on 12 June 2024).
- Tasks of the National Health Insurance Fund of Hungary (Hungarian acronym: NEAK)—NEAK n.d. Available online: https://www.neak.gov.hu/oldalak/nyelvi-oldalak/english (accessed on 7 March 2025).
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (10th ed.) 2019. Available online: https://icd.who.int/browse10/2019/en (accessed on 24 March 2025).
- Menyhárt, O.; Fekete, J.T.; Győrffy, B. Demographic shift disproportionately increases cancer burden in an aging nation: Current and expected incidence and mortality in Hungary up to 2030. Clin. Epidemiol. 2018, 10, 1093–1108. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Prognostic/Clinical Prediction Models: Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Tutorials in Biostatistics. Stat. Methods Clin. Stud. 2005, 1, 223–249. [Google Scholar] [CrossRef]
- Hartman, N.; Kim, S.; He, K.; Kalbfleisch, J.D. Pitfalls of the concordance index for survival outcomes. Stat. Med. 2023, 42, 2179–2190. [Google Scholar] [CrossRef]
- Kuhn, R.; Culhane, D.P. Applying Cluster Analysis to Test a Typology of Homelessness by Pattern of Shelter Utilization: Results from the Analysis of Administrative Data. Am. J. Community Psychol. 1998, 26, 207–232. [Google Scholar] [CrossRef]
- Exchange Rates and PPPs by Indicator, Country and Year. UNECE Statistical Database n.d. Available online: https://w3.unece.org/PXWeb2015/pxweb/en/STAT/STAT__20-ME__6-MEER/30_en_MECCExchPPPsNEWY_r.px/ (accessed on 1 September 2024).
- Statistics—Eurostat n.d. Available online: https://ec.europa.eu/eurostat/databrowser/view/ERT_BIL_EUR_A/default/table?lang=en (accessed on 24 February 2025).
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Kumar, M.; Sonker, P.K.; Saroj, A.; Jain, A.; Bhattacharjee, A.; Saroj, R.K. Parametric survival analysis using R: Illustration with lung cancer data. Cancer Rep. 2020, 3, e1210. [Google Scholar] [CrossRef]
- Tájékoztató a Közadatok Újrahasznosításáról—NEAK n.d. Available online: http://www.neak.gov.hu/felso_menu/rolunk/kozerdeku_adatok/kozadatok_igenylese/kozadat_ujrahasznositas (accessed on 20 February 2025).
- OECD Data Explorer—Annual Purchasing Power Parities and exchange rates n.d. Available online: https://data-explorer.oecd.org/vis?tm=purchasing%20power&pg=0&snb=44&vw=tb&df[ds]=dsDisseminateFinalDMZ&df[id]=DSD_NAMAIN10%40DF_TABLE4&df[ag]=OECD.SDD.NAD&df[vs]=2.0&dq=A.EU27_2020%2BEA20%2BHUN...PPP_B1GQ.......&lom=LASTNPERIODS&lo=10&to[TIME_PERIOD]=false (accessed on 24 February 2025).
- May, L.; Shows, K.; Nana-Sinkam, P.; Li, H.; Landry, J.W. Sex Differences in Lung Cancer. Cancers 2023, 15, 3111. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Yap, M.L.; Cheng, E.S.; Ngo, P.J.; Vaneckova, P.; Karikios, D.; Canfell, K.; Weber, M.F. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings from a Large Australian Cohort. J. Thorac. Oncol. 2022, 17, 688–699. [Google Scholar] [CrossRef]
- Barry, R.; Anderson, J.; Tran, L.; Bahji, A.; Dimitropoulos, G.; Ghosh, S.M.; Kirkham, J.; Messier, G.; Patten, S.B.; Rittenbach, K.; et al. Prevalence of Mental Health Disorders Among Individuals Experiencing Homelessness: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2024, 81, 691–699. [Google Scholar] [CrossRef]
- Soar, K.; Dawkins, L.; Robson, D.; Cox, S. Smoking amongst adults experiencing homelessness: A systematic review of prevalence rates, interventions and the barriers and facilitators to quitting and staying quit. J. Smok. Cessat. 2020, 15, 94–108. [Google Scholar] [CrossRef]
- Baggett, T.P.; Chang, Y.; Porneala, B.C.; Bharel, M.; Singer, D.E.; Rigotti, N.A. Disparities in Cancer Incidence, Stage, and Mortality at Boston Health Care for the Homeless Program. Am. J. Prev. Med. 2015, 49, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Tănase, B.C.; Zob, D.L.; Gheorghe, A.S.; Lungulescu, C.V.; Dumitrescu, E.A.; Stănculeanu, D.L.; Manolescu, L.S.C.; Popescu, O.; Ibraim, E.; et al. The Bidirectional Relationship between Pulmonary Tuberculosis and Lung Cancer. Int. J. Environ. Res. Public Health 2023, 20, 1282. [Google Scholar] [CrossRef]
- Dias, M.; Gaio, R.; Sousa, P.; Abranches, M.; Gomes, M.; Oliveira, O.; Correia-Neves, M.; Ferreira, E.; Duarte, R. Tuberculosis among the homeless: Should we change the strategy? Int. J. Tuberc. Lung Dis. 2017, 21, 327–332. [Google Scholar] [CrossRef]
- Inotai, A.; Abonyi-Tóth, Z.; Rokszin, G.; Vokó, Z. Prognosis, Cost, and Occurrence of Colorectal, Lung, Breast, and Prostate Cancer in Hungary. Value Heal. Reg. Issues 2015, 7, 1–8. [Google Scholar] [CrossRef]
- McGuire, A.; Martin, M.; Lenz, C.; Sollano, J.A. Treatment cost of non-small cell lung cancer in three European countries: Comparisons across France, Germany, and England using administrative databases. J. Med. Econ. 2015, 18, 525–532. [Google Scholar] [CrossRef]
- Sloan, J.A.; Zhao, X.; Novotny, P.J.; Wampfler, J.; Garces, Y.; Clark, M.M.; Yang, P. Relationship Between Deficits in Overall Quality of Life and Non–Small-Cell Lung Cancer Survival. J. Clin. Oncol. 2012, 30, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Flike, K.; Aronowitz, T. Factors That Influence Quality of Life in People Experiencing Homelessness: A Systematic Mixed Studies Review. J. Am. Psychiatr. Nurses Assoc. 2022, 28, 128–153. [Google Scholar] [CrossRef] [PubMed]
- Concannon, K.F.; Thayer, J.H.; Wu, Q.V.; Jenkins, I.C.; Baik, C.S.; Linden, H.M. Outcomes Among Homeless Patients with Non–Small-Cell Lung Cancer: A County Hospital Experience. JCO Oncol. Pr. 2020, 16, e1004–e1014. [Google Scholar] [CrossRef]
- Joiner, K.A.; Lin, J.; Pantano, J. Upcoding in medicare: Where does it matter most? Health Econ. Rev. 2024, 14, 1. [Google Scholar] [CrossRef]
- Bowblis, J.R.; Brunt, C.S. Medicare Skilled Nursing Facility Reimbursement and Upcoding. Health Econ. 2014, 23, 821–840. [Google Scholar] [CrossRef]
- Travis, W.D.; Rush, W.; Flieder, D.B.; Falk, R.; Fleming, M.V.; Gal, A.A.; Koss, M.N. Survival Analysis of 200 Pulmonary Neuroendocrine Tumors with Clarification of Criteria for Atypical Carcinoid and Its Separation from Typical Carcinoid. Am. J. Surg. Pathol. 1998, 22, 934–944. [Google Scholar] [CrossRef]
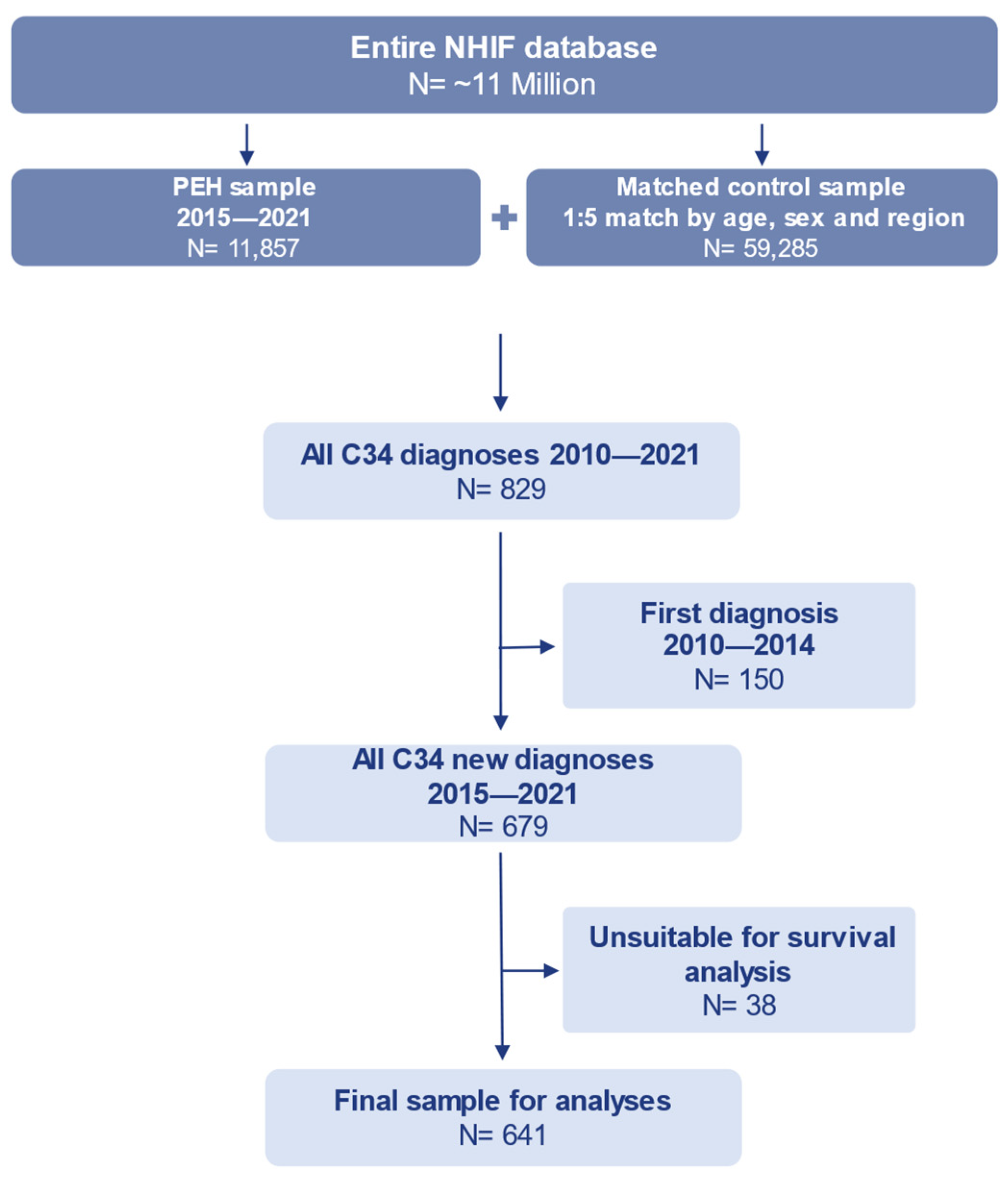
| All | New Lung Cancer Cases | |||
|---|---|---|---|---|
| PEH Sample | Control Sample | PEH Group | Control Group | |
| Total (N/%) | 11,857 (17%) | 59,285 (83%) | 233 (36%) | 408 (64%) |
| Males (N/%) | 9095 (77%) | 45,475 (77%) | 196 (82%) | 372 (85%) |
| Females (N/%) | 2762 (23%) | 13,810 (23%) | 43 (18%) | 68 (15%) |
| Age in 2015 (mean ± SD) | 43 (±12) | 43 (±12) | 53 (±7) | 55 (±7) |
| Males | 44 (±12) | 44 (±12) | 53 (±7) | 56 (±7) |
| Females | 41 (±12) | 41 (±12) | 53 (±6) | 53 (±6) |
| Age at C34 diagnosis (mean ± SD) | - | - | 56 (±7) | 58 (±7) |
| Males | - | - | 56 (±7) | 58 (±7) |
| Females | - | - | 56 (±6) | 56 (±6) |
| Number of years registered as homeless (mean ± SD) | 2.1 (±1.5) | - | 1.9 (±1.3) | - |
| Percentage of time registered as homeless (mean ± SD) | 33% (±25) | - | 40% (±29) | - |
| Homelessness Length Index (HLI, N/%): | ||||
| HLI 1 | 8095 (68% †) | - | 138 (59% ‡) | - |
| HLI 2 | 2292 (19% †) | - | 52 (22% ‡) | - |
| HLI 3 | 1470 (12% †) | - | 43 (19% ‡) | - |
| HUF | EUR | PPP EUR | Difference PEH vs. Control | Percentage of Total Costs | |||||
|---|---|---|---|---|---|---|---|---|---|
| PEH | Control | PEH | Control | PEH | Control | PEH | Control | ||
| Average C34 associated total healthcare costs per patient | 1,136,568 | 2,115,777 | 3564 | 6635 | 12,052 | 22,436 | −46% | 100% | 100% |
| Average C34 associated total healthcare costs per patient year | 675,562 | 1,276,978 | 2119 | 4004 | 7163 | 13,541 | −47% | 100% | 100% |
| Average C34 associated inpatient costs per patient year | 295,223 | 366,925 | 926 | 1151 | 3131 | 3890 | −20% | 44% | 29% |
| Average C34 associated pharmaceuticals costs per patient year | 19,119 | 100,084 | 60 | 314 | 203 | 1,061 | −81% | 3% | 8% |
| Average C34 associated outpatient costs per patient year | 15,008 | 26,245 | 47 | 82 | 159 | 278 | −43% | 2% | 2% |
| Average C34 associated radio-diagnostics costs per patient year | 15,410 | 29,328 | 48 | 92 | 163 | 311 | −47% | 2% | 2% |
| Average C34 associated other costs per patient year | 330,802 | 754,397 | 1037 | 2366 | 3508 | 8000 | −56% | 49% | 59% |
| CPH | HR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Age at diagnosis | 1.03 (1.01–1.05) * | 1.03 (1.01–1.05) * | 1.03 (1.01–1.05) * | 1.03 (1.02–1.05) * | 1.04 (1.02–1.05) * | 1.03 (1.02–1.05) * |
| Female sex | 0.83 (0.63–1.02) | 0.83 (0.63–1.02) | 0.83 (0.63–1.01) | 0.88 (0.67–1.16) | 0.89 (0.67–1.18) | 0.92 (0.69–1.21) |
| Homelessness yes/no | - | 1.02 (0.99–1.47) | - | - | - | - |
| Homelessness in years | - | - | 0.99 (0.91–1.07) | - | - | - |
| HLI | ||||||
| 1 | - | - | - | 0.96 (0.75–1.24) | 0.98 (0.76–1.26) | 1.03 (0.80–1.32) |
| 2 | - | - | - | 1.59 (1.18–2.16) * | 1.51 (1.11–2.05) * | 1.47 (1.08–2.00) * |
| 3 | - | - | - | 1.66 (1.15–2.41) * | 1.50 (1.03–2.18) * | 1.47 (1.01–2.14) * |
| Total annualized lung cancer associated healthcare costs (per HUF 100,000) | - | - | - | - | 0.97 (0.96–0.98) * | 0.97 (0.96–0.98) * |
| Metastatic cancer | - | - | - | - | - | 2.37 (1.92–2.93) * |
| Concordance index | 0.563 | 0.565 | 0.563 | 0.584 | 0.675 | 0.690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heilig, D.; Szabó, Á.; Fadgyas-Freyler, P.; Simon, J. The Impact of Homelessness on Lung Cancer Survival and Healthcare Utilization in the Hungarian Universal Healthcare System. Cancers 2025, 17, 1158. https://doi.org/10.3390/cancers17071158
Heilig D, Szabó Á, Fadgyas-Freyler P, Simon J. The Impact of Homelessness on Lung Cancer Survival and Healthcare Utilization in the Hungarian Universal Healthcare System. Cancers. 2025; 17(7):1158. https://doi.org/10.3390/cancers17071158
Chicago/Turabian StyleHeilig, Daniel, Ákos Szabó, Petra Fadgyas-Freyler, and Judit Simon. 2025. "The Impact of Homelessness on Lung Cancer Survival and Healthcare Utilization in the Hungarian Universal Healthcare System" Cancers 17, no. 7: 1158. https://doi.org/10.3390/cancers17071158
APA StyleHeilig, D., Szabó, Á., Fadgyas-Freyler, P., & Simon, J. (2025). The Impact of Homelessness on Lung Cancer Survival and Healthcare Utilization in the Hungarian Universal Healthcare System. Cancers, 17(7), 1158. https://doi.org/10.3390/cancers17071158






