In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy
Simple Summary
Abstract
1. Introduction
2. Methods
- Published in English with an available abstract
- Addressed at least one of the following topics: CKM, metabolic syndrome, SGLT2 inhibitors (SGLT2i), PCSK9 inhibitors (PCSK9i), soluble guanylate cyclase activators (SGCa), GLP-1 receptor agonists, diet, inflammation, diabetes, cardiotoxicity, cardioprotection, and cancer
3. CMK Syndrome: Pathogenesis and Clinical Implications
3.1. Neurohormonal Activation
3.2. Chronic Inflammation
3.3. Hemodynamic Abnormalities
3.4. Metabolic Dysregulation
3.5. Fibrosis
3.6. Gut–Kidney–Heart Axis
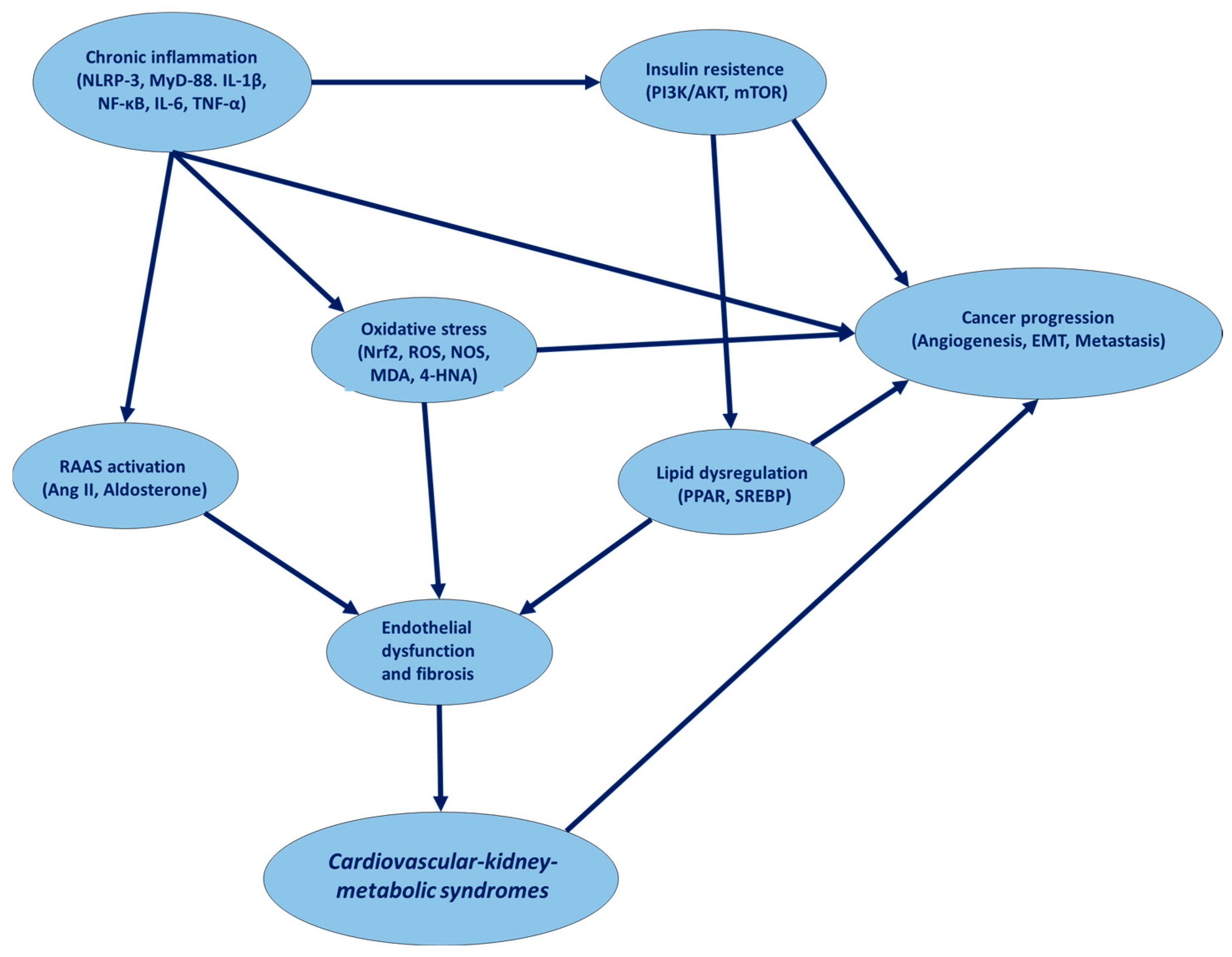
4. Common Molecular Pathways Involved in CKM Syndrome and Cancer
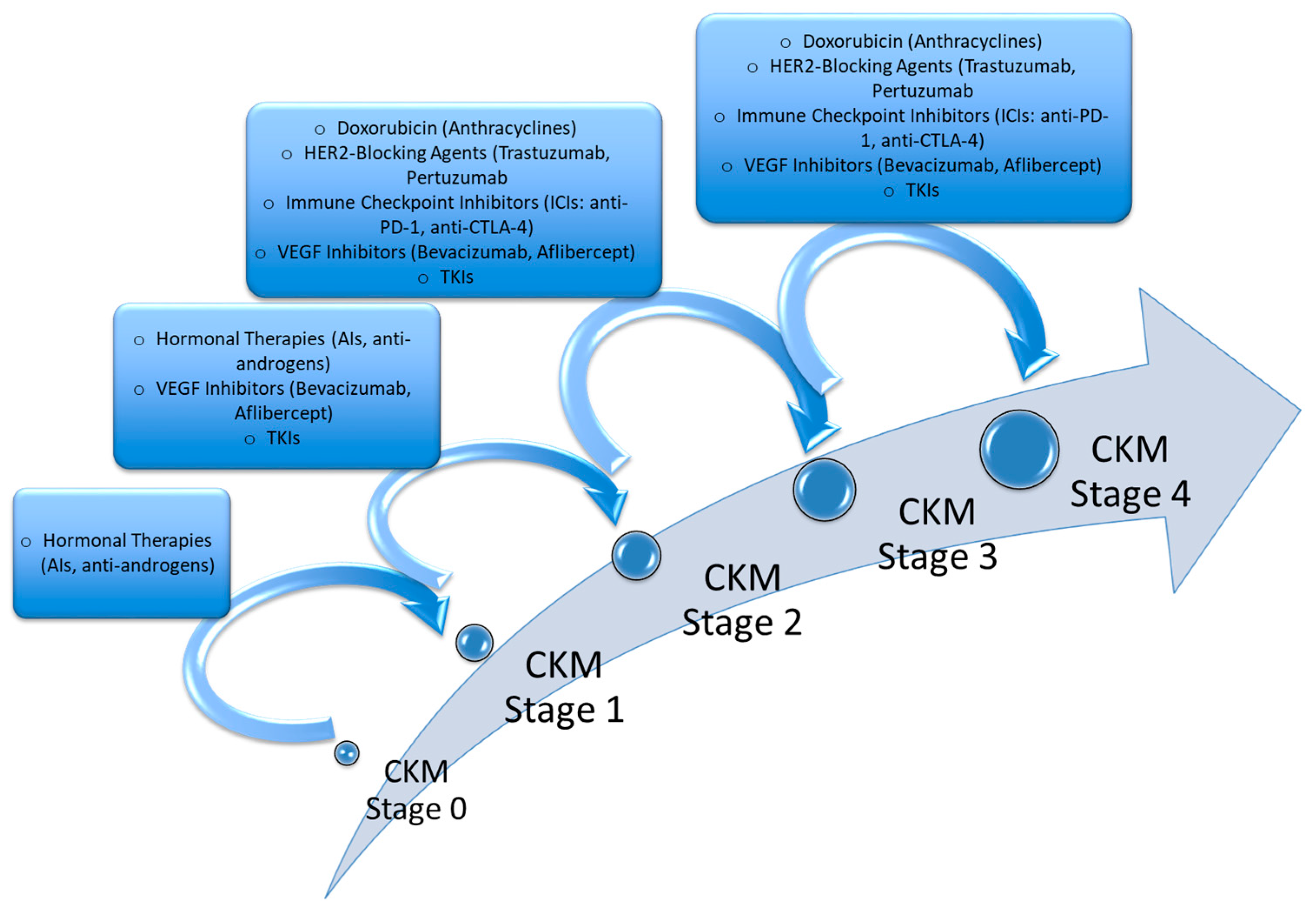
5. Anticancer Therapies and Risk of CKM Syndrome
5.1. How Do HER2-Blocking Agents (e.g., Trastuzumab, Pertuzumab) Exacerbate CKM Syndrome?
5.2. How Do Anthracyclines (e.g., Doxorubicin, Epirubicin) Exacerbate CKM Syndrome?
5.3. Do VEGF Inhibitors (e.g., Bevacizumab, Aflibercept) Increase the Risk of CKM?
5.4. Do Hormonal Therapies (e.g., Aromatase Inhibitors, Anti-Androgens) Increase the Risk of CKM Syndrome?
5.5. Does Immunotherapy (ICIs) Pose Additional Cardiovascular Risks for CKM Syndrome Patients?
6. Pharmacological and Non-Pharmacological Therapies for CKM in Cancer Patients
6.1. Sodium-Glucose Co-Transporter 2 Inhibitors (SGLT2i)
6.2. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors (PCSK9i)
6.3. Soluble Guanylate Cyclase Activators
6.4. Glucagon-like Peptide-1 Receptor Agonists
6.5. Anti-Inflammatory Diet
7. Discussion
- Baseline Risk Stratification (Pre-Therapy): Involving the assessment of traditional risk factors (hypertension, diabetes, obesity, dyslipidemia), a proper screening for subclinical cardiac dysfunction (echocardiography, GLS strain imaging), and renal function and metabolic assessment (eGFR, albuminuria, fasting glucose, lipid profile).
- During Therapy: Involving surveillance for emerging CKM components, a regular cardiac biomarker evaluation (troponins, NT-proBNP), metabolic changes (HbA1c, insulin resistance through HOMA score, visceral fat, fatty liver index), and renal function monitoring (GFR decline >10% signals early nephropathy).
- Post-Therapy Follow-up: Identifying long-term risk through annual screening for cardiovascular, renal, and metabolic complications; imaging for latent cardiomyopathy (MRI, global longitudinal strain imaging); early intervention strategies (involving SGLT2 inhibitors, GLP-1 agonists, RAAS inhibitors, and others).
- Risk-Based Stratification Models: Develop structured risk assessment algorithms integrating AI-driven predictive models to identify high-risk patients before, during, and after cancer therapy.
- CKM-Focused Therapeutic Interventions through:
- Pharmacological Strategies: SGLT2i and/or GLP-1 receptor agonists for cardio–renal–metabolic protection; statins or PCSK9i for cholesterol reduction; beta-blockers and RAAS inhibitors for cardioprotection.
- Dietary and Lifestyle Modifications: Implementing plant-based and Mediterranean diets at low glycemic and insulinemic indices (which have been shown to reduce CKM burden by ~30%) [215].
- Structured daily exercise interventions personalized to each cancer survivor.
- Cardio-Oncology Integration into Survivorship Clinics: Establish multidisciplinary CKM clinics in oncology centers; standardized follow-up plans for early detection of cardiovascular and renal complications; real-time digital monitoring (wearables, telemedicine) for high-risk patients.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moslehi, J.J. Cardio-Oncology: A New Clinical Frontier and Novel Platform for Cardiovascular Investigation. Circulation 2024, 150, 513–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kostakou, P.M.; Kouris, N.T.; Kostopoulos, V.S.; Damaskos, D.S.; Olympios, C.D. Cardio-oncology: A new and developing sector of research and therapy in the field of cardiology. Heart Fail. Rev. 2019, 24, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C. An overview of cardiovascular-kidney-metabolic syndrome. Am. J. Manag. Care 2024, 30 (Suppl. S10), S181–S188. [Google Scholar] [CrossRef] [PubMed]
- Claudel, S.E.; Verma, A. Cardiovascular-kidney-metabolic syndrome: A step toward multidisciplinary and inclusive care. Cell Metab. 2023, 35, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Guldan, M.; Ozbek, L.; Copur, S.; Covic, A.S.; Covic, A. Exploring the nexus: The place of kidney diseases within the cardiovascular-kidney-metabolic syndrome spectrum. Eur. J. Intern. Med. 2024, 127, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Ostrominski, J.W.; Vaduganathan, M. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults, 2011–2020. JAMA 2024, 331, 1858–1860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, R.; Wang, R.; He, J.; Wang, L.; Chen, H.; Niu, X.; Sun, Y.; Guan, Y.; Gong, Y.; Zhang, L.; et al. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages by Social Determinants of Health. JAMA Netw. Open 2024, 7, e2445309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, S.; M’Pembele, R.; Matute, P.; Kotfis, K.; Larmann, J.; Lurati Buse, G. Cardiovascular-Kidney-Metabolic Syndrome: Association with Adverse Events After Major Noncardiac Surgery. Anesth. Analg. 2024, 139, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Zarifa, A.; Lopez-Mattei, J.; Palaskas, N.; Iliescu, C.; Durand, J.B.; Kim, P.Y. Immune Checkpoint Inhibitors (ICIs)-Related Cardiotoxicity. Adv. Exp. Med. Biol. 2020, 1244, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Maurea, N.; Coppola, C.; Piscopo, G.; Galletta, F.; Riccio, G.; Esposito, E.; De Lorenzo, C.; De Laurentiis, M.; Spallarossa, P.; Mercuro, G. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J. Cardiovasc. Med. 2016, 17 (Suppl. S1), e19–e26. [Google Scholar] [CrossRef] [PubMed]
- Siaravas, K.C.; Katsouras, C.S.; Sioka, C. Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forte, V.; Pandey, A.; Abdelmessih, R.; Forte, G.; Whaley-Connell, A.; Sowers, J.R.; McFarlane, S.I. Obesity, Diabetes, the Cardiorenal Syndrome, and Risk for Cancer. Cardiorenal. Med. 2012, 2, 143–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, R.; Wang, R.; He, J.; Wang, L.; Chen, H.; Wang, Y.; An, P.; Li, K.; Ren, F.; Xu, W.; et al. Associations of cardiovascular-kidney-metabolic syndrome stages with premature mortality and the role of social determinants of health. J. Nutr. Health Aging 2025, 29, 100504. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Cui, L.; Shu, R.; Song, H.; Wang, J.; Chen, S.; Liu, B.; Shi, H.; Gao, H.; et al. Association between different stages of cardiovascular-kidney-metabolic syndrome and the risk of all-cause mortality. Atherosclerosis 2024, 397, 118585. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. The Renin-Angiotensin System and Cardiovascular-Kidney-Metabolic Syndrome: Focus on Early-Life Programming. Int. J. Mol. Sci. 2024, 25, 3298. [Google Scholar] [CrossRef]
- Claudel, S.E.; Schmidt, I.M.; Waikar, S.S.; Verma, A. Cumulative Incidence of Mortality Associated with Cardiovascular-Kidney-Metabolic (CKM) Syndrome. J. Am. Soc. Nephrol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Laghlam, D.; Jozwiak, M.; Nguyen, L.S. Renin-Angiotensin-Aldosterone System and Immunomodulation: A State-of-the-Art Review. Cells 2021, 10, 1767. [Google Scholar] [CrossRef]
- Petramala, L.; Gigante, A.; Sarlo, F.; Servello, A.; Circosta, F.; Marino, L.; Ciccarelli, A.; Cavallaro, G.; Letizia, C. Relevance of obesity-related organ damage and metabolic syndrome classification in cardiovascular and renal risk stratification in patients with essential hypertension. Front. Cardiovasc. Med. 2024, 11, 1369090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ewen, S.; Ukena, C.; Linz, D.; Schmieder, R.E.; Böhm, M.; Mahfoud, F. The sympathetic nervous system in chronic kidney disease. Curr. Hypertens. Rep. 2013, 15, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Mancia, G. Sympathetic activation in cardiovascular disease: Evidence, clinical impact and therapeutic implications. Eur. J. Clin. Investig. 2015, 45, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Drager, L.F. Sympathetic overactivity, hypertension and cardiovascular disease: State of the art. Curr. Med. Res. Opin. 2024, 40 (Suppl. S1), 5–13. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Sympathetic nervous system and hypertension: New evidences. Auton. Neurosci. 2022, 238, 102954. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. RAAS inhibition and mortality in hypertension. Glob. Cardiol. Sci. Pract. 2013, 2013, 269–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bavishi, C.; Bangalore, S.; Messerli, F.H. Renin Angiotensin Aldosterone System Inhibitors in Hypertension: Is There Evidence for Benefit Independent of Blood Pressure Reduction? Prog. Cardiovasc. Dis. 2016, 59, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension-Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Masi, S.; Taddei, S. The renin-angiotensin-aldosterone system: A crossroad from arterial hypertension to heart failure. Heart Fail. Rev. 2020, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, S.; Zhao, R.; Shen, P.; Zhu, X.; Yang, Y.; Duan, C.; Wang, Y.; Ni, H.; Zhou, L.; et al. Association between systemic immune-inflammation index and cardiovascular-kidney-metabolic syndrome. Sci. Rep. 2024, 14, 19151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, Y.; Wang, W.; Xie, S.; Xu, Y.; Lin, Z. Joint association of the inflammatory marker and cardiovascular-kidney-metabolic syndrome stages with all-cause and cardiovascular disease mortality: A national prospective study. BMC Public Health 2025, 25, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, S.C.; Yang, W.V. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit. Rev. Oncol. Hematol. 2016, 108, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Park, S.; Lakatta, E.G. RAGE signaling in inflammation and arterial aging. Front. Biosci. 2009, 14, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miletić, T.; Kovacević-Jovanović, V.; Vujić, V.; Stanojević, S.; Mitić, K.; Lazarević-Macanović, M.; Dimitrijević, M. Reactive oxygen species (ROS), but not nitric oxide (NO), contribute to strain differences in the susceptibility to experimental arthritis in rats. Immunobiology 2007, 212, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.J.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients with Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Shen, C.; Kong, W.; Zhou, X.; Fan, H.; Zhang, Y.; Liu, Z.; Zheng, L. Association between the triglyceride glucose-body mass index and future cardiovascular disease risk in a population with Cardiovascular-Kidney-Metabolic syndrome stage 0–3: A nationwide prospective cohort study. Cardiovasc. Diabetol. 2024, 23, 292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bobbert, P.; Jenke, A.; Bobbert, T.; Kühl, U.; Rauch, U.; Lassner, D.; Scheibenbogen, C.; Poller, W.; Schultheiss, H.P.; Skurk, C. High leptin and resistin expression in chronic heart failure: Adverse outcome in patients with dilated and inflammatory cardiomyopathy. Eur. J. Heart Fail. 2012, 14, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef] [PubMed]
- Guldan, M.; Unlu, S.; Abdel-Rahman, S.M.; Ozbek, L.; Gaipov, A.; Covic, A.; Soler, M.J.; Covic, A.; Kanbay, M. Understanding the Role of Sex Hormones in Cardiovascular Kidney Metabolic Syndrome: Toward Personalized Therapeutic Approaches. J. Clin. Med. 2024, 13, 4354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massy, Z.A.; Drueke, T.B. Combination of Cardiovascular, Kidney, and Metabolic Diseases in a Syndrome Named Cardiovascular-Kidney-Metabolic, with New Risk Prediction Equations. Kidney Int. Rep. 2024, 9, 2608–2618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014, 2014, 689360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382, Erratum in J. Vet. Intern. Med. 2019, 33, 2551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vendeville, N.; Lepage, M.A.; Festa, M.C.; Mavrakanas, T.A. Clinical Outcomes of Renin-Angiotensin-Aldosterone Blockade in Patients with Advanced Chronic Kidney Disease: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2024, 40, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Ishibashi, K.; Morishita, Y. Systematic Review and Meta-Analysis of Renin-Angiotensin-Aldosterone System Blocker Effects on the Development of Cardiovascular Disease in Patients with Chronic Kidney Disease. Front. Pharmacol. 2021, 12, 662544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pecoits-Filho, R.; Fliser, D.; Tu, C.; Zee, J.; Bieber, B.; Wong, M.M.Y.; Port, F.; Combe, C.; Lopes, A.A.; Reichel, H.; et al. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J. Clin. Hypertens. 2019, 21, 991–1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P.; STOP ACEi Trial Investigators. Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Siragy, H.M.; Carey, R.M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 2010, 31, 541–550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kittelson, K.S.; Junior, A.G.; Fillmore, N.; da Silva Gomes, R. Cardiovascular-kidney-metabolic syndrome—An integrative review. Prog. Cardiovasc. Dis. 2024, 87, 26–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batko, K.; Sączek, A.; Banaszkiewicz, M.; Małyszko, J.; Koc-Żórawska, E.; Żórawski, M.; Niezabitowska, K.; Siek, K.; Bętkowska-Prokop, A.; Małyszko, J.A.; et al. Comprehensive assessment of cardiovascular-kidney-metabolic (CKM) syndrome: Novel tools for assessment of cardiovascular risk and kidney outcomes in long-term kidney transplant patients. Kardiol. Pol. 2024, 82, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [PubMed] [PubMed Central]
- Xiao, S.; Wagner, L.; Schmidt, R.J.; Baylis, C. Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001, 59, 1466–1472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Lei, L.; Wang, W.; Ding, W.; Yu, Y.; Pu, B.; Peng, Y.; Li, Y.; Zhang, L.; Guo, Y. Social Risk Profile and Cardiovascular-Kidney-Metabolic Syndrome in US Adults. J. Am. Heart Assoc. 2024, 13, e034996. [Google Scholar] [CrossRef] [PubMed]
- de Haas, E.C.; Oosting, S.F.; Lefrandt, J.D.; Wolffenbuttel, B.H.; Sleijfer, D.T.; Gietema, J.A. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010, 11, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Kaneko, H.; Suzuki, Y.; Okada, A.; Matsuoka, S.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Metabolic syndrome and cardiovascular disease in cancer survivors. J Cachexia Sarcopenia Muscle 2024, 15, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Huang, X.; Liang, J.; Zhang, J.; Fu, J.; Xie, W.; Zheng, F. Association of cardiovascular-kidney-metabolic health and social connection with the risk of depression and anxiety. Psychol. Med. 2024, 54, 4203–4211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Wu, W.; Li, D.; Guo, Y.; Ding, H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine 2010, 37, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Delarue, J.; Magnan, C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.D.; Sparks, C.E.; Adeli, K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, S.; Takahashi, S.; Masamura, K.; Kanehara, H.; Sakai, J.; Tohda, G.; Okada, E.; Oida, K.; Iwasaki, T.; Hattori, H.; et al. Evidence of macrophage foam cell formation by very low-density lipoprotein receptor: Interferon-gamma inhibition of very low-density lipoprotein receptor expression and foam cell formation in macrophages. Circulation 2001, 103, 1142–1147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gajewska, A.; Wasiak, J.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. SGLT2 Inhibitors in Kidney Diseases-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4959. [Google Scholar] [CrossRef]
- Mende, C.W. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv. Ther. 2022, 39, 148–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, L.; Huang, Z. Elevated triglyceride glucose index is associated with advanced cardiovascular kidney metabolic syndrome. Sci. Rep. 2024, 14, 31352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, A.R.; Salama, A.H.; Aleem, Z.; Alfakeer, H.; Alnemr, L.; Shareef, A.M.M. The Promising Frontier of Cardiometabolic Syndrome: A New Paradigm in Cardiology. Cureus 2023, 15, e45542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schreibing, F.; Anslinger, T.M.; Kramann, R. Fibrosis in Pathology of Heart and Kidney: From Deep RNA-Sequencing to Novel Molecular Targets. Circ. Res. 2023, 132, 1013–1033. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.H.; Tham, C.L.; Harith, H.H.; Firdaus, N.; Israf, D.A. TGF-β-induced fibrosis: A review on the underlying mechanism and potential therapeutic strategies. Eur. J. Pharmacol. 2021, 911, 174510. [Google Scholar] [CrossRef] [PubMed]
- Mulsow, J.J.; Watson, R.W.; Fitzpatrick, J.M.; O’Connell, P.R. Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Ann. Surg. 2005, 242, 880–887, discussion 887–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzarino, M.; Cetin, E.; Bartosova, M.; Marinovic, I.; Ipseiz, N.; Hughes, T.R.; Schmitt, C.P.; Ramji, D.P.; Labéta, M.O.; Raby, A.C. Therapeutic targeting of chronic kidney disease-associated DAMPs differentially contributing to vascular pathology. Front. Immunol. 2023, 14, 1240679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yoo, T.H. TGF-β Inhibitors for Therapeutic Management of Kidney Fibrosis. Pharmaceuticals 2022, 15, 1485. [Google Scholar] [CrossRef]
- Chiuariu, T.; Șalaru, D.; Ureche, C.; Vasiliu, L.; Lupu, A.; Lupu, V.V.; Șerban, A.M.; Zăvoi, A.; Benchea, L.C.; Clement, A.; et al. Cardiac and Renal Fibrosis, the Silent Killer in the Cardiovascular Continuum: An Up-to-Date. J. Cardiovasc. Dev. Dis. 2024, 11, 62. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.B. Reactive oxygen species and matrix remodeling in diabetic kidney. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S246–S249. [Google Scholar] [CrossRef] [PubMed]
- La Russa, A.; Serra, R.; Faga, T.; Crugliano, G.; Bonelli, A.; Coppolino, G.; Bolignano, D.; Battaglia, Y.; Ielapi, N.; Costa, D.; et al. Kidney Fibrosis and Matrix Metalloproteinases (MMPs). Front. Biosci. 2024, 29, 192. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cui, H.; Wang, Y.; Ju, F.; Cai, Y.; Gang, X.; Wang, G. The role of lipotoxicity in kidney disease: From molecular mechanisms to therapeutic prospects. Biomed. Pharmacother. 2023, 161, 114465. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Fontanesi, F.; Merscher, S.; Fornoni, A. The Vicious Cycle of Renal Lipotoxicity and Mitochondrial Dysfunction. Front. Physiol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wende, A.R.; Abel, E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta. 2010, 1801, 311–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumura, I.; Ishikawa, J.; Nakajima, K.; Oritani, K.; Tomiyama, Y.; Miyagawa, J.; Kato, T.; Miyazaki, H.; Matsuzawa, Y.; Kanakura, Y. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol. Cell Biol. 1997, 17, 2933–2943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumida, K.; Kovesdy, C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol. Int. 2019, 106, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stockler-Pinto, M.B.; Fouque, D.; Soulage, C.O.; Croze, M.; Mafra, D. Indoxyl sulfate and p-cresyl sulfate in chronic kidney disease. Could these toxins modulate the antioxidant Nrf2-Keap1 pathway? J. Ren. Nutr. 2014, 24, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, T.; Macrina, L.; Premaschi, S.; Bologna, A.; Magni, G.; Foligno, N.; Avino, M.; Belloni, C.; Palmieri, N.; Conte, F.; et al. Serum concentrations of free indoxyl and p-cresyl sulfate are associated with mineral metabolism variables and cardiovascular risk in hemodialysis patients. J. Nephrol. 2022, 35, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, T.; Fujieda, A.; Itoh, Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE 2018, 13, e0193342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmar, J.; de la Puente-Secades, S.; Floege, J.; Noels, H.; Jankowski, J.; Orth-Alampour, S. Uremic Toxins Affecting Cardiovascular Calcification: A Systematic Review. Cells 2020, 9, 2428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Chamieh, C.; Liabeuf, S.; Massy, Z. Uremic Toxins and Cardiovascular Risk in Chronic Kidney Disease: What Have We Learned Recently beyond the Past Findings? Toxins 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, M.M.; Islam, F.; -Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez Báez, A.; Ayala, G.; Pedroza-Saavedra, A.; González-Sánchez, H.M.; Chihu Amparan, L. Phosphorylation Codes in IRS-1 and IRS-2 Are Associated with the Activation/Inhibition of Insulin Canonical Signaling Pathways. Curr. Issues Mol. Biol. 2024, 46, 634–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, C.; Timóteo-Ferreira, F.; Minchiotti, G.; Brunelli, S.; Guardiola, O. Cellular interactions and microenvironment dynamics in skeletal muscle regeneration and disease. Front. Cell Dev. Biol. 2024, 12, 1385399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, X.; Zeng, Y.; Huang, J.; Chen, X.; Kang, H.; Huang, S. Tumor-Derived Sarcopenia Factors Are Diverse in Different Tumor Types: A Pan-Cancer Analysis. Biomedicines 2024, 12, 329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; Passariello, M.; Coppola, C.; Rea, D.; Barbieri, A.; Scherillo, M.; Monti, M.G.; Iaffaioli, R.V.; De Laurentiis, M.; Ascierto, P.A.; et al. Cardiotoxicity and pro-inflammatory effects of the immune checkpoint inhibitor Pembrolizumab associated to Trastuzumab. Int. J. Cardiol. 2019, 292, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ago, T.; Kuroda, J.; Kamouchi, M.; Sadoshima, J.; Kitazono, T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system-Review and perspective-. Circ. J. 2011, 75, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Nostrand, J.L.; Hellberg, K.; Luo, E.C.; Van Nostrand, E.L.; Dayn, A.; Yu, J.; Shokhirev, M.N.; Dayn, Y.; Yeo, G.W.; Shaw, R.J. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes. Dev. 2020, 34, 1330–1344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishop, T.; Ratcliffe, P.J. HIF hydroxylase pathways in cardiovascular physiology and medicine. Circ. Res. 2015, 117, 65–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut-Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef]
- Osei Baah, F.; Sharda, S.; Davidow, K.; Jackson, S.; Kernizan, D.; Jacobs, J.A.; Baumer, Y.; Schultz, C.L.; Baker-Smith, C.M.; Powell-Wiley, T.M. Social Determinants of Health in Cardio-Oncology: Multi-Level Strategies to Overcome Disparities in Care: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 331–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; De Laurentiis, M.; Cocco, S.; Rea, G.; Bonelli, A.; Caronna, A.; Lombari, M.C.; Conforti, G.; Berretta, M.; Botti, G.; et al. NLRP3 as Putative Marker of Ipilimumab-Induced Cardiotoxicity in the Presence of Hyperglycemia in Estrogen-Responsive and Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7802. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Di Mauro, A.; Cipullo, C.; Paccone, A.; Barbieri, A.; Palma, G.; Luciano, A.; Buccolo, S.; Bisceglia, I.; et al. Immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. Front. Cardiovasc. Med. 2022, 9, 930797, Erratum in Front. Cardiovasc. Med. 2023, 10, 1129873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caldiroli, L.; Armelloni, S.; Eskander, A.; Messa, P.; Rizzo, V.; Margiotta, E.; Cesari, M.; Vettoretti, S. Association between the uremic toxins indoxyl-sulfate and p-cresyl-sulfate with sarcopenia and malnutrition in elderly patients with advanced chronic kidney disease. Exp. Gerontol. 2021, 147, 111266. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.R.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.W.; Song, S.H. Sarcopenia in chronic kidney disease: From bench to bedside. Korean J. Intern. Med. 2023, 38, 303–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fradley, M.G.; Nguyen, N.H.K.; Madnick, D.; Chen, Y.; DeMichele, A.; Makhlin, I.; Dent, S.; Lefebvre, B.; Carver, J.; Upshaw, J.N.; et al. Adverse Cardiovascular Events Associated with Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Metastatic Breast Cancer. J. Am. Heart Assoc. 2023, 12, e029361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, G.; Cioffi, G.; Di Lenarda, A.; Tuccia, F.; Bovelli, D.; Di Tano, G.; Alunni, G.; Gori, S.; Faggiano, P.; Tarantini, L. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern. Emerg. Med. 2012, 7, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Cesana, E.; Donatelli, F.; Cammarota, R.; Bucci, E.O.; Baravelli, M.; Anzà, C.; Noonan, D.M. Cardio-oncology in targeting the HER receptor family: The puzzle of different cardiotoxicities of HER2 inhibitors. Future Cardiol. 2011, 7, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Dent, S.F.; Morse, A.; Burnette, S.; Guha, A.; Moore, H. Cardiovascular Toxicity of Novel HER2-Targeted Therapies in the Treatment of Breast Cancer. Curr. Oncol. Rep. 2021, 23, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, A.B.; Zhang, Y.; Tian, P.; Meng, T.T.; Chen, J.L.; Zhang, D.; Zheng, Y.; Su, G.H. Metabolic syndrome and cardiovascular disease among adult cancer patients: Results from NHANES 2007–2018. BMC Public Health 2024, 24, 2259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mauro, C.; Capone, V.; Cocchia, R.; Cademartiri, F.; Riccardi, F.; Arcopinto, M.; Alshahid, M.; Anwar, K.; Carafa, M.; Carbone, A.; et al. Cardiovascular Side Effects of Anthracyclines and HER2 Inhibitors among Patients with Breast Cancer: A Multidisciplinary Stepwise Approach for Prevention, Early Detection, and Treatment. J. Clin. Med. 2023, 12, 2121. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, M.; Azizi, Y.; Shayan, M.; Nezamoleslami, S.; Eslami, F.; Farjoo, M.H.; Dehpour, A.R. Doxorubicin-Induced Cardiotoxicity: An Overview on Pre-clinical Therapeutic Approaches. Cardiovasc. Toxicol. 2022, 22, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Vaitiekus, D.; Muckiene, G.; Vaitiekiene, A.; Maciuliene, D.; Vaiciuliene, D.; Ambrazeviciute, G.; Sereikaite, L.; Verikas, D.; Jurkevicius, R.; Juozaityte, E. Impact of Arterial Hypertension on Doxorubicin-Based Chemotherapy-Induced Subclinical Cardiac Damage in Breast Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.C.; Radin, M.J.; Heller, L.; Rogers, L.K.; Tobias, A.; Matise, I.; Wang, Q.; Apple, F.S.; McCune, S.A. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol. Appl. Pharmacol. 2013, 273, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Bressler, N.M.; Avery, R.L.; Bakri, S.J.; Boyer, D.S.; Brown, D.M.; Dugel, P.U.; Freund, K.B.; Glassman, A.R.; Kim, J.E.; et al. Comparison of Aflibercept, Bevacizumab, and Ranibizumab for Treatment of Diabetic Macular Edema: Extrapolation of Data to Clinical Practice. JAMA Ophthalmol. 2016, 134, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, S.J.H.; Petrie, M.C.; Myles, R.C.; Touyz, R.M.; Lang, N.N. Cardiotoxic effects of angiogenesis inhibitors. Clin. Sci. 2021, 135, 71–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mihalcea, D.; Memis, H.; Mihaila, S.; Vinereanu, D. Cardiovascular Toxicity Induced by Vascular Endothelial Growth Factor Inhibitors. Life 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallerio, P.; Orenti, A.; Tosi, F.; Maistrello, M.; Palazzini, M.; Cingarlini, S.; Colombo, P.; Bertuzzi, M.; Spina, F.; Amatu, A.; et al. Major adverse cardiovascular events associated with VEGF-targeted anticancer tyrosine kinase inhibitors: A real-life study and proposed algorithm for proactive management. ESMO Open 2022, 7, 100338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.I.; Yu, J.H.; Anh, S.G.; Lee, H.W.; Jeong, J.; Lee, K.S. Aromatase Inhibitors and Newly Developed Nonalcoholic Fatty Liver Disease in Postmenopausal Patients with Early Breast Cancer: A Propensity Score-Matched Cohort Study. Oncologist 2019, 24, e653–e661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Redig, A.J.; Munshi, H.G. Care of the cancer survivor: Metabolic syndrome after hormone-modifying therapy. Am. J. Med. 2010, 123, 87.e1–87.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, E.S.; Sood, S.; Kiss, N.; Daly, R.M.; Nicoll, A.J.; Roberts, S.K.; Baguley, B.J. The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review. Curr. Oncol. 2022, 30, 48–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Peng, C.C.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Review of Shared Cardiometabolic Risk Factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Arcaro, G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007, 191, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Mann, M.C.; Holroyd-Leduc, J.M.; Wilton, S.B.; James, M.T.; Seely, E.W.; Ahmed, S.B. The effect of hormone therapy on all-cause and cardiovascular mortality in women with chronic kidney disease: Protocol for a systematic review and meta-analysis. Syst. Rev. 2015, 4, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minegishi, S.; Horita, N.; Ishigami, T.; Hibi, K. Cardiotoxicity Associated with Immune Checkpoint Inhibitors. Cancers 2023, 15, 5487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Lombari, M.C.; Conforti, G.; Libutti, M.; Iaffaioli, R.V.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9169–9171. [Google Scholar] [CrossRef] [PubMed]
- Sury, K.; Perazella, M.A.; Shirali, A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol. 2018, 14, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Cozma, A.; Sporis, N.D.; Lazar, A.L.; Buruiana, A.; Ganea, A.M.; Malinescu, T.V.; Berechet, B.M.; Fodor, A.; Sitar-Taut, A.V.; Vlad, V.C.; et al. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10948. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Y.; Zhang, Y.; Wang, W.; Wang, Y.; Lu, Z.; Zhang, Y.; Lei, H.; Li, D.; Long, B.; et al. Association of immune checkpoint inhibitors therapy with arterial thromboembolic events in cancer patients: A retrospective cohort study. Cancer Med. 2023, 12, 18531–18541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inno, A.; Chiampan, A.; Lanzoni, L.; Verzè, M.; Molon, G.; Gori, S. Immune Checkpoint Inhibitors and Atherosclerotic Vascular Events in Cancer Patients. Front. Cardiovasc. Med. 2021, 8, 652186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, M.; Yu, T.; Zhang, Z.; Zhang, J.; Wang, R.; Tse, G.; Liu, T.; Zhong, L. ICIs-Related Cardiotoxicity in Different Types of Cancer. J. Cardiovasc. Dev. Dis. 2022, 9, 203. [Google Scholar] [CrossRef]
- Zarifa, A.; Lopez-Mattei, J.; Palaskas, N.L.; Iliescu, C.; Durand, J.B.; Kim, P.Y. Immune Checkpoint Inhibitor (ICI)-Related Cardiotoxicity. Adv. Exp. Med. Biol. 2021, 1342, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Avula, V.; Sharma, G.; Kosiborod, M.N.; Vaduganathan, M.; Neilan, T.G.; Lopez, T.; Dent, S.; Baldassarre, L.; Scherrer-Crosbie, M.; Barac, A.; et al. SGLT2 Inhibitor Use and Risk of Clinical Events in Patients with Cancer Therapy-Related Cardiac Dysfunction. JACC Heart Fail. 2024, 12, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 159–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gongora, C.A.; Drobni, Z.D.; Quinaglia Araujo Costa Silva, T.; Zafar, A.; Gong, J.; Zlotoff, D.A.; Gilman, H.K.; Hartmann, S.E.; Sama, S.; Nikolaidou, S.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Cardiac Outcomes Among Patients Treated with Anthracyclines. JACC Heart Fail. 2022, 10, 559–567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.; Ota, T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte 2018, 7, 121–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosokawa, Y.; Ogawa, W. SGLT2 inhibitors for genetic and acquired insulin resistance: Considerations for clinical use. J. Diabetes Investig. 2020, 11, 1431–1433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delanaye, P.; Scheen, A.J. The diuretic effects of SGLT2 inhibitors: A comprehensive review of their specificities and their role in renal protection. Diabetes Metab. 2021, 47, 101285. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef]
- Kristensen, D.K.; Mose, F.H.; Buus, N.H.; Duus, C.L.; Mårup, F.H.; Bech, J.N.; Nielsen, S.F. SGLT2 inhibition improves endothelium-independent vasodilatory function in type 2 diabetes: A double-blind, randomized, placebo-controlled crossover trial. Diabetes Obes. Metab. 2025, 27, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Piperidou, A.; Sarafidis, P.; Boutou, A.; Thomopoulos, C.; Loutradis, C.; Alexandrou, M.E.; Tsapas, A.; Karagiannis, A. The effect of SGLT-2 inhibitors on albuminuria and proteinuria in diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2019, 37, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: A systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, J.B.; McCullough, P.A. Roles for SGLT2 Inhibitors in Cardiorenal Disease. Cardiorenal Med. 2022, 12, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Lamina, C.; Kollerits, B.; Schachtl-Riess, J.F.; Schultheiss, U.T.; Forer, L.; Sekula, P.; Kotsis, F.; Eckardt, K.U.; Kronenberg, F.; et al. PCSK9 and Cardiovascular Disease in Individuals with Moderately Decreased Kidney Function. Clin. J. Am. Soc. Nephrol. 2022, 17, 809–818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaro, J.M.; Villanego, F.; Naranjo, J.; Orellana, C.; Vigara, L.A.; Narváez, C.E.; Torrado, J.; Cazorla, J.M.; Rodríguez, C.; Mazuecos, A. Treatment with PCSK9 inhibitors in patients with chronic kidney disease at very high cardiovascular risk. Nefrologia 2023, 43 (Suppl. S2), 133–135. [Google Scholar] [CrossRef] [PubMed]
- Dutka, M.; Zimmer, K.; Ćwiertnia, M.; Ilczak, T.; Bobiński, R. The role of PCSK9 in heart failure and other cardiovascular diseases-mechanisms of action beyond its effect on LDL cholesterol. Heart Fail. Rev. 2024, 29, 917–937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hummelgaard, S.; Vilstrup, J.P.; Gustafsen, C.; Glerup, S.; Weyer, K. Targeting PCSK9 to tackle cardiovascular disease. Pharmacol. Ther. 2023, 249, 108480. [Google Scholar] [CrossRef] [PubMed]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 Promotes Cardiovascular Diseases: Recent Evidence about Its Association with Platelet Activation-Induced Myocardial Infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Igweonu-Nwakile, E.O.; Ali, S.; Paul, S.; Yakkali, S.; Teresa Selvin, S.; Thomas, S.; Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; Abu Jad, A.A.; et al. A Systematic Review on the Safety and Efficacy of PCSK9 Inhibitors in Lowering Cardiovascular Risks in Patients with Chronic Kidney Disease. Cureus 2022, 14, e29140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Kang, Y.; Chen, L. Activation mechanism of human soluble guanylate cyclase by stimulators and activators. Nat. Commun. 2021, 12, 5492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Giordano, V.; Arianna, R.; Barbato, M.; Izzo, F.; Maurea, C.; et al. The sGCa Vericiguat Exhibit Cardioprotective and Anti-Sarcopenic Effects through NLRP-3 Pathways: Potential Benefits for Anthracycline-Treated Cancer Patients. Cancers 2024, 16, 1487. [Google Scholar] [CrossRef]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394, Erratum in Handb. Exp. Pharmacol. 2021, 264, 425. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Wheeler, D.C.; Debén, F.M.; Speeckaert, M.; Thomas, D.; Berger, M.; Klein, S.; Friedrichs, F.; Paraschin, K.; Schmieder, R.E. The soluble guanylate cyclase activator runcaciguat significantly improves albuminuria in patients with chronic kidney disease: A randomized placebo-controlled clinical trial. Nephrol. Dial. Transplant. 2024, gfae261. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Liang, B. Soluble Guanylate Cyclase Activators and Stimulators in Patients with Heart Failure. Curr. Cardiol. Rep. 2023, 25, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, T.; Lang, C.C.; Petty, R.D.; Baxter, M.A. Cardiotoxicity and Chemotherapy-The Role of Precision Medicine. Diseases. 2021, 9, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinhart, G.A.; Harrison, P.C.; Lincoln, K.; Chen, H.; Sun, P.; Hill, J.; Qian, H.S.; McHugh, M.C.; Clifford, H.; Ng, K.J.; et al. The novel, clinical-stage soluble guanylate cyclase activator BI 685509 protects from disease progression in models of renal injury and disease. J. Pharmacol. Exp. Ther. 2023, 384, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, F.B.; Xu, M.; Erdoğan, C.; Fei, L.; Mathar, I.; Dietz, L.; Sandner, P.; Seeliger, E.; Boral, S.; Bonk, J.S.; et al. Activating soluble guanylyl cyclase attenuates ischemic kidney damage. Kidney Int. 2025, 107, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Giordano, V.; Giacobbe, I.; Scherillo, M.; Gabrielli, D.; Maurea, C.; Barbato, M.; et al. Glucagon-like Peptide 1 Receptor Agonists in Cardio-Oncology: Pathophysiology of Cardiometabolic Outcomes in Cancer Patients. Int. J. Mol. Sci. 2024, 25, 11299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacIsaac, R.J.; Trevella, P.; Ekinci, E.I. Glucagon-like peptide-1 receptor agonists and kidney outcomes. J. Diabetes. 2024, 16, e13609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natale, P.; Green, S.C.; Tunnicliffe, D.J.; Pellegrino, G.; Toyama, T.; Strippoli, G.F. Glucagon-like peptide 1 (GLP-1) receptor agonists for people with chronic kidney disease and diabetes. Cochrane Database Syst. Rev. 2025, 2, CD015849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michos, E.D.; Bakris, G.L.; Rodbard, H.W.; Tuttle, K.R. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: A review of their kidney and heart protection. Am. J. Prev. Cardiol. 2023, 14, 100502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Marsico, F.; Paolillo, S.; Gargiulo, P.; Bruzzese, D.; Dell’Aversana, S.; Esposito, I.; Renga, F.; Esposito, L.; Marciano, C.; Dellegrottaglie, S.; et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: A meta-analysis of randomized controlled trials. Eur. Heart J. 2020, 41, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, C.J.; Sakach, J.; Maples, J.M.; Fulton, J.; Chippior, J.; O’Donnell, B.; O’Malley, D.M.; Chambers, L.M. Glucagon-like peptide-1 (GLP-1) receptor agonists for weight management: A review for the gynecologic oncologist. Gynecol. Oncol. 2024, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Song, J.; Chi, K.Y.; Chang, Y.C.; Xanthavanij, N.; Chang, Y.; Hsia, Y.P.; Chiang, C.H.; Ghamari, A.; Reynolds, K.L.; et al. Glucagon-like Peptide-1 Agonists Reduce Cardiovascular Events in Cancer Patients on Immune Checkpoint Inhibitors. Eur. J. Cancer 2025, 216, 115170. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.C.; Chang, L.S.; McGuire, D.K.; Plutzky, J.; Aroda, V.R.; Vaduganathan, M. Use of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes and Cardiovascular Disease: A Review. JAMA Cardiol. 2020, 5, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellissimo, M.P.; Carbone, S.; He, J.; Jordan, J.H.; Ambale-Venkatesh, B.; Lima, J.A.; LaRose, J.G.; Salloum, F.N.; Bandyopadhyay, D.; Hundley, W.G. Higher diet quality relates to better cardiac function in cancer survivors: The multi-ethnic study of atherosclerosis. Prog. Cardiovasc. Dis. 2023, 81, 10–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alizadehasl, A.; Malekzadeh Moghani, M.; Mirzaei, H.; Keshvari, M.; Fadaei, F.; Cramer, H.; Pasalar, M.; Heydarirad, G. Cardioprotective Diet to Prevent Anthracycline-Induced Cardiotoxicity in Patients with Breast Cancer: A Randomized Open-Label Controlled Trial. J. Integr. Complement. Med. 2024, 30, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Venturini, E.; Gilchrist, S.; Corsi, E.; DI Lorenzo, A.; Cuomo, G.; D’Ambrosio, G.; Pacileo, M.; D’Andrea, A.; Canale, M.L.; Iannuzzo, G.; et al. The core components of cardio-oncology rehabilitation. Panminerva Med. 2021, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Clar, C.; Al-Khudairy, L.; Loveman, E.; Kelly, S.A.; Hartley, L.; Flowers, N.; Germanò, R.; Frost, G.; Rees, K. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 7, CD004467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, S.; Frost, G.; Whittaker, V.; Summerbell, C. Low glycaemic index diets for coronary heart disease. Cochrane Database Syst. Rev. 2004, CD004467, Update in Cochrane Database Syst. Rev. 2017, 7, CD004467. [Google Scholar] [CrossRef] [PubMed]
- de Lima, K.; Mazur, C.E.; Vicente Cavagnari, M.A.; Castilho, A.J.; Schiessel, D.L. Omega-3 supplementation effects on cardiovascular risk and inflammatory profile in chronic kidney disease patients in hemodialysis treatment: An intervention study. Clin. Nutr. ESPEN 2023, 58, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wang, C.L.; Liu, K.L.; Yeh, C.N.; Chiang, T.I. Omega-3 Fatty Acids Improve Chronic Kidney Disease-Associated Pruritus and Inflammation. Medicina 2022, 58, 796. [Google Scholar] [CrossRef]
- Burris, J.; Shikany, J.M.; Rietkerk, W.; Woolf, K. A Low Glycemic Index and Glycemic Load Diet Decreases Insulin-like Growth Factor-1 among Adults with Moderate and Severe Acne: A Short-Duration, 2-Week Randomized Controlled Trial. J. Acad. Nutr. Diet. 2018, 118, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lanza, E.; Ross, A.C.; Albert, P.S.; Colburn, N.H.; Rovine, M.J.; Bagshaw, D.; Ulbrecht, J.S.; Hartman, T.J. A high-legume low-glycemic index diet reduces fasting plasma leptin in middle-aged insulin-resistant and -sensitive men. Eur. J. Clin. Nutr. 2011, 65, 415–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goff, L.M.; Cowland, D.E.; Hooper, L.; Frost, G.S. Low glycaemic index diets and blood lipids: A systematic review and meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Sciarretta, F.; Turchi, R.; Li, B.H.; Rosina, M.; Ceci, V.; Guidobaldi, G.; Arena, S.; D’Ambrosio, C.; Audano, M.; et al. Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue. Redox Biol. 2020, 36, 101633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łagowska, K.; Drzymała-Czyż, S. A low glycemic index, energy-restricted diet but not Lactobacillus rhamnosus supplementation changes fecal short-chain fatty acid and serum lipid concentrations in women with overweight or obesity and polycystic ovary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Chang, A.R.; Appel, L.J.; Anderson, C.A.; Crews, D.C.; Thomas, L.; Charleston, J.; Miller, E.R., 3rd. Effect of glycemic index and carbohydrate intake on kidney function in healthy adults. BMC Nephrol. 2016, 17, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picard, K.; Senior, P.A.; Adame Perez, S.; Jindal, K.; Richard, C.; Mager, D.R. Low Mediterranean Diet scores are associated with reduced kidney function and health related quality of life but not other markers of cardiovascular risk in adults with diabetes and chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Halimi, J.M.; Rossignol, P.; Sarafidis, P.; De Caterina, R.; Giugliano, R.; Zannad, F. From Cardiorenal Syndrome to Chronic Cardiovascular and Kidney Disorder: A Conceptual Transition. Clin. J. Am. Soc. Nephrol. 2024, 19, 813–820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonsu, J.M.; Guha, A.; Charles, L.; Yildiz, V.O.; Wei, L.; Baker, B.; Brammer, J.E.; Awan, F.; Lustberg, M.; Reinbolt, R.; et al. Reporting of Cardiovascular Events in Clinical Trials Supporting FDA Approval of Contemporary Cancer Therapies. J. Am. Coll. Cardiol. 2020, 75, 620–628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batra, G.; Ghukasyan Lakic, T.; Lindbäck, J.; Held, C.; White, H.D.; Stewart, R.A.H.; Koenig, W.; Cannon, C.P.; Budaj, A.; Hagström, E.; et al. Interleukin 6 and Cardiovascular Outcomes in Patients with Chronic Kidney Disease and Chronic Coronary Syndrome. JAMA Cardiol. 2021, 6, 1440–1445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.A.; Liu, Y. Activation of the NF-κB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Oxid. Med. Cell Longev. 2020, 2020, 5803639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ki, Y.W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, L.; Dong, N.; Li, F. NLRP3 inflammasome: The rising star in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 927061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vlad, C.E.; Foia, L.; Popescu, R.; Ivanov, I.; Luca, M.C.; Delianu, C.; Toma, V.; Statescu, C.; Rezus, C.; Florea, L. Apolipoproteins A and B and PCSK9: Nontraditional Cardiovascular Risk Factors in Chronic Kidney Disease and in End-Stage Renal Disease. J. Diabetes Res. 2019, 2019, 6906278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubin, R.F.; Shah, S.J. Soluble Guanylate Cyclase Stimulators: A Novel Treatment Option for Heart Failure Associated with Cardiorenal Syndromes? Curr. Heart Fail. Rep. 2016, 13, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Bakis, H.; Chauveau, P.; Combe, C.; Pfirmann, P. Mediterranean Diet for Cardiovascular Risk Reduction in Chronic Kidney Disease. Adv. Kidney Dis. Health 2023, 30, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology—Strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Shubrook, J.H.; Neumiller, J.J. Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D. J. Fam. Pract. 2023, 72 (Suppl. S6), S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R. Correction to: Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2024, 149, e1023, Erratum for Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Stage | Clinical Characteristics | Pathophysiological Features | CVD Risk Level |
|---|---|---|---|
| Stage 0 | No evidence of metabolic, renal, or cardiovascular dysfunction | Normal metabolic and hemodynamic function | Low |
| Stage 1 | Central obesity with early metabolic alterations | Adipose tissue dysfunction, mild insulin resistance, low-grade inflammation | Borderline |
| Stage 2 | Established metabolic syndrome and/or early kidney dysfunction | Hypertension, dyslipidemia, hyperglycemia, increased renal stress | Intermediate |
| Stage 3 | Subclinical cardiovascular disease with metabolic and/or renal impairment | Endothelial dysfunction, left ventricular remodeling, increased arterial stiffness | High |
| Stage 4 | Overt cardiovascular disease with advanced metabolic and renal dysfunction | Atherosclerosis, heart failure, chronic kidney disease progression | Very High |
| Database | Search String |
|---|---|
| Medline | “CKM AND cardiovascular diseases” OR “CKM AND cancer” OR “(CKM OR Metabolic Syndrome OR obesity) AND cardiology” OR “(CKM OR Metabolic Syndrome OR obesity) AND cardio-oncology” OR “(SGLT2i OR PCSK9i OR SGCa OR GLP-1 receptor agonist OR diet) AND CKM” |
| EMBASE | “CKM AND cardiovascular diseases” OR “CKM AND cancer” OR “(CKM OR Metabolic Syndrome OR obesity) AND cardiology” OR “(CKM OR Metabolic Syndrome OR obesity) AND cardio-oncology” OR “(SGLT2i OR PCSK9i OR SGCa OR GLP-1 receptor agonist OR diet) AND CKM” |
| Clincal Aspect | Clinical Impact in Cancer Patients |
|---|---|
| Cardiovascular (CV) Risk | Increased risk of heart failure, arrhythmias, and atherosclerosis due to shared risk factors (hypertension, diabetes, obesity). |
| Kidney Dysfunction | Cancer therapies (e.g., chemotherapy, immunotherapy) may worsen CKD, leading to nephrotoxicity and increased mortality. |
| Metabolic Dysregulation | Insulin resistance, dyslipidemia, and obesity may accelerate cancer progression and worsen treatment response. |
| Cancer Therapy Toxicity | CKM syndrome exacerbates toxic effects of chemotherapy, targeted therapies, and immunotherapies, increasing MACE events. |
| Survival and Outcomes | Higher risk of treatment interruptions, complications, and reduced overall survival. |
| Inflammation and Immunity | Chronic inflammation (via metabolic and renal dysfunction) may promote cancer growth and reduce efficacy of immunotherapy. |
| Therapeutic Challenges | Need for multidisciplinary care; balancing cancer treatment with CV, renal, and metabolic management. |
| Biochemical Pathway | Cardiovascular Disease | Cancer | Relevance to CKM Syndromes | Key Signaling Molecules |
|---|---|---|---|---|
| Renin–Angiotensin–Aldosterone System (RAAS) | Hypertension, heart failure, atherosclerosis | Tumor angiogenesis, cancer cell proliferation | Dysregulated RAAS promotes endothelial dysfunction, fibrosis, and metabolic disturbances | Angiotensin II, AT1R, Aldosterone, ACE, Renin |
| Insulin Signaling Pathway | Insulin resistance, diabetic cardiomyopathy | Hyperinsulinemia-driven tumorigenesis (e.g., colorectal, breast cancer) | CKM syndrome involves metabolic syndrome, leading to hyperinsulinemia and inflammation | IRS-1/2, PI3K, AKT, mTOR, GLUT4 |
| Inflammatory Pathways (NF-κB, IL-6, TNF-α) | Chronic inflammation, atherosclerosis, myocardial fibrosis | Tumor-associated inflammation, immune evasion | Systemic inflammation links CKM syndrome with endothelial dysfunction and fibrosis | NF-κB, IL-6, TNF-α, IL-1β, COX-2 |
| Oxidative Stress Pathway (Nrf2, ROS, NOX) | Endothelial dysfunction, atherosclerosis | DNA damage, cancer progression | Oxidative stress accelerates CKM pathology via mitochondrial dysfunction and apoptosis | Nrf2, Keap1, ROS, NOX, SOD, GSH |
| Lipid Metabolism Pathway (PPAR, SREBP, LXR) | Dyslipidemia, atherosclerosis | Lipid-driven cancer proliferation (e.g., prostate, breast cancer) | CKM syndromes involve altered lipid metabolism, driving cardiovascular and oncogenic risks | PPAR-γ, SREBP-1, LXR, LDLR, HMGCR |
| AMPK/mTOR Pathway | Metabolic stress, heart failure, atherosclerosis | Cancer cell survival, metabolic adaptation | AMPK dysfunction in CKM syndromes leads to metabolic inflexibility and cardiovascular damage | AMPK, mTOR, ULK1, TSC2, Raptor |
| TGF-β/SMAD Pathway | Cardiac fibrosis, hypertrophy, kidney fibrosis | Epithelial–mesenchymal transition (EMT), metastasis | CKM-related organ fibrosis and metabolic dysfunction | TGF-β, SMAD2/3, SMAD7, α-SMA |
| HIF-1α Pathway | Hypoxia-induced vascular dysfunction | Hypoxia-driven tumorigenesis, angiogenesis | Hypoxia exacerbates CKM-related ischemic injury and metabolic imbalances | HIF-1α, VEGF, PHD2, VHL |
| Gut Microbiota and TMAO Pathway | Atherosclerosis, hypertension | Inflammation-driven carcinogenesis | Dysbiosis in CKM syndromes promotes metabolic endotoxemia and inflammation | TMAO, FMO3, LPS, SCFA |
| Uremic Toxins and Endothelial Dysfunction (Indoxyl Sulfate, p-Cresol) | Kidney injury, vascular calcification | Chronic inflammation, cancer promotion | CKM syndrome includes chronic kidney disease, exacerbating cardiovascular and oncogenic risks | Indoxyl sulfate, p-cresol, Klotho, eNOS |
| Therapy | Influence on CKM Progression | Stage Transition |
|---|---|---|
| Hormonal Therapies (AIs, anti-androgens) | Increase central obesity, insulin resistance, and dyslipidemia, promoting metabolic syndrome and early kidney dysfunction. | Stage 0 → Stage 1 → Stage 2 |
| Doxorubicin (Anthracyclines) | Induces cardiotoxicity via oxidative stress, mitochondrial damage, and endothelial dysfunction, contributing to subclinical and overt cardiovascular disease. | Stage 2 → Stage 3 → Stage 4 |
| HER2-Blocking Agents (Trastuzumab, Pertuzumab) | Dysregulate cardioprotective signaling, increasing the risk of heart failure, microvascular ischemia, and left ventricular dysfunction, particularly in patients with pre-existing CKM risk factors. | Stage 2 → Stage 3 → Stage 4 |
| VEGF Inhibitors (Bevacizumab, Aflibercept) TKIs | Promote hypertension, endothelial dysfunction, arterial stiffness, and renal dysfunction, worsening metabolic and cardiovascular disease. | Stage 1 → Stage 2 → Stage 3 → Stage 4 |
| Immune Checkpoint Inhibitors (anti-PD-1, anti-CTLA-4) | Exacerbate systemic inflammation, leading to myocarditis, endothelial dysfunction, and renal immune-mediated damage, accelerating CKM progression. | Stage 2 → Stage 3 → Stage 4 |
| Therapeutic Approach | Clinical Outcomes | Key Signaling Pathways Involved | Mechanisms of Action |
|---|---|---|---|
| SGLT2 Inhibitors (SGLT2i) (e.g., empagliflozin, dapagliflozin) | ↓ Heart failure hospitalization, ↓ CKD progression, ↓ MACE (major adverse cardiovascular events), ↓ BP, ↓ Body weight | AMPK/mTOR, NHE3 inhibition, RAAS inhibition, oxidative stress reduction | Increases glucose and sodium excretion, reduces cardiac and renal stress, improves mitochondrial efficiency, reduces fibrosis and inflammation |
| PCSK9 Inhibitors (PCSK9i) (e.g., evolocumab, alirocumab) | ↓ LDL cholesterol, ↓ Atherosclerosis progression, ↓ MACE, ↓ Inflammatory markers | LDL receptor recycling, anti-inflammatory pathways (NF-κB, IL-6) | Blocks PCSK9-mediated LDL receptor degradation, enhances LDL clearance, reduces vascular inflammation |
| GLP-1 Receptor Agonists (GLP1-RA) (e.g., semaglutide, liraglutide) | ↓ Body weight, ↓ Atherosclerosis, ↓ MACE, ↓ BP, ↓ CKD progression | cAMP/PKA, PI3K/AKT, AMPK activation, anti-inflammatory (NF-κB inhibition), endothelial NO production | Increases insulin secretion, reduces appetite, enhances endothelial function, reduces inflammation, improves lipid metabolism |
| Soluble Guanylate Cyclase (sGC) Activators (e.g., vericiguat, riociguat) | ↓ Heart failure exacerbations, ↑ Endothelial function, ↓ Fibrosis | NO- cGMP/PKG pathway, anti-fibrotic, anti-inflammatory | Enhances vasodilation, reduces vascular stiffness, improves cardiac and renal function |
| Diet (Mediterranean, Low-Carb, Plant-Based) | ↓ Obesity, ↓ BP, ↓ Atherosclerosis, ↓ Insulin resistance, ↓ CKD risk | PPAR activation, AMPK activation, gut microbiota–TMAO modulation, anti-inflammatory (NF-κB, IL-6 suppression) | Modifies lipid metabolism, reduces oxidative stress, improves endothelial function, modulates gut microbiota |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Barbato, M.; Maurea, C.; Canale, M.L.; Paccone, A.; Inno, A.; et al. In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy. Cancers 2025, 17, 1169. https://doi.org/10.3390/cancers17071169
Quagliariello V, Berretta M, Bisceglia I, Giacobbe I, Iovine M, Barbato M, Maurea C, Canale ML, Paccone A, Inno A, et al. In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy. Cancers. 2025; 17(7):1169. https://doi.org/10.3390/cancers17071169
Chicago/Turabian StyleQuagliariello, Vincenzo, Massimiliano Berretta, Irma Bisceglia, Ilaria Giacobbe, Martina Iovine, Matteo Barbato, Carlo Maurea, Maria Laura Canale, Andrea Paccone, Alessandro Inno, and et al. 2025. "In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy" Cancers 17, no. 7: 1169. https://doi.org/10.3390/cancers17071169
APA StyleQuagliariello, V., Berretta, M., Bisceglia, I., Giacobbe, I., Iovine, M., Barbato, M., Maurea, C., Canale, M. L., Paccone, A., Inno, A., Scherillo, M., Oliva, S., Cadeddu Dessalvi, C., Mauriello, A., Fonderico, C., Maratea, A. C., Gabrielli, D., & Maurea, N. (2025). In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy. Cancers, 17(7), 1169. https://doi.org/10.3390/cancers17071169











