Tumor Treating Fields and Combination Therapy in Management of Brain Oncology
Simple Summary
Abstract
1. Introduction
2. Inhibition of Cell Mitosis
3. Anti-Tumor Immune Response and Increasing Cell Membrane Permeability
4. Pre-Clinical Experiments with TTFields
5. Clinical Experiments
6. TTFields Dosage and Array Layout
7. Combination Therapy
7.1. TTFields and Chemotherapy
7.2. TTFields and Radiation Therapy
7.3. TTFields and Targeted Drug Therapy, Immunotherapy, and Others
7.4. TTField Effects on Different Cancer Cell Lines
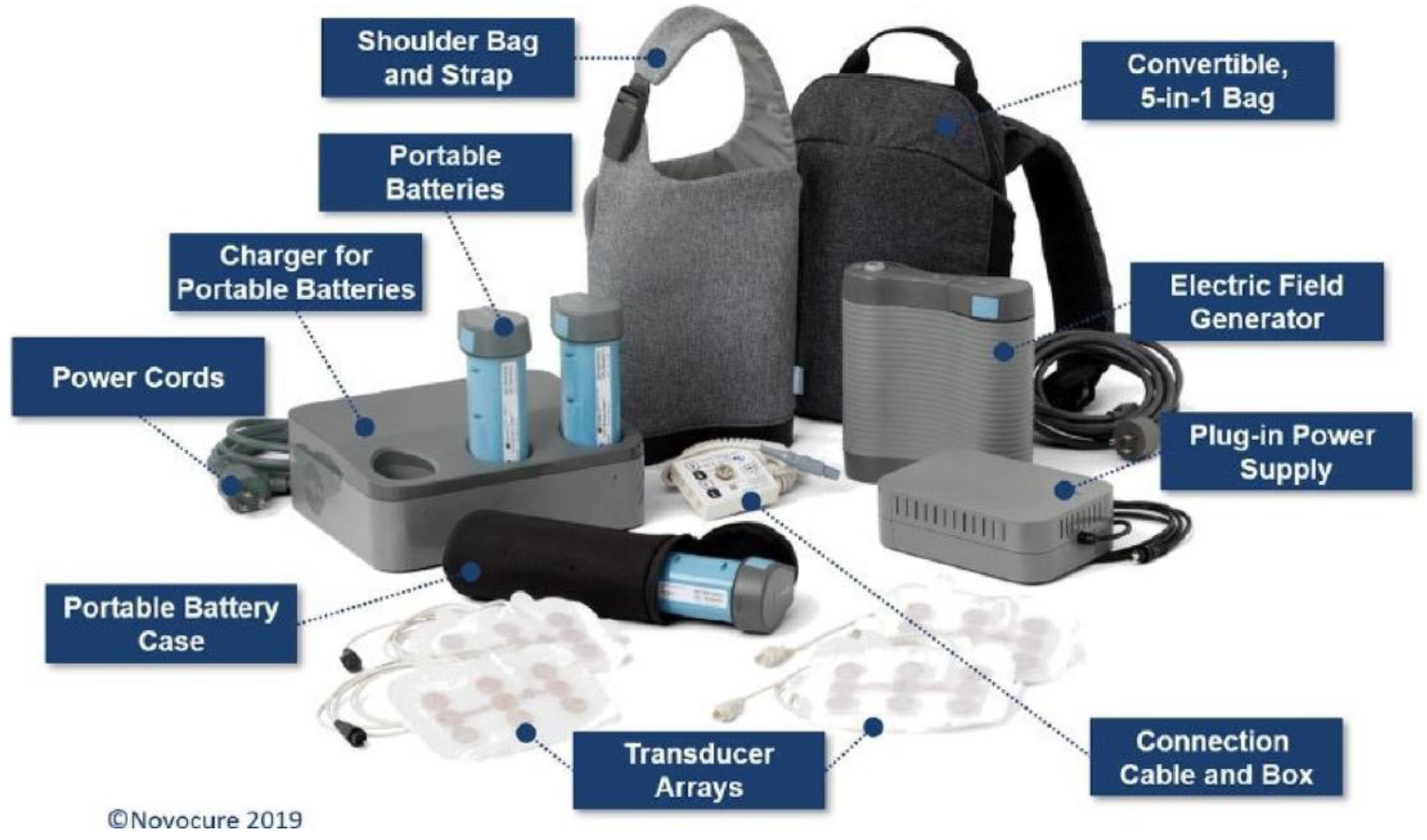
7.5. TTField Device
7.6. Adverse Effects and Contraindications in TTFields or Combination Therapy
8. Limitations and Challenges of TTFields Modalities
9. Discussion
9.1. TTFields and Combination Therapy Comparisons
9.2. TTField Monotherapy
9.3. TTFields in Combination with Chemotherapy
9.4. TTFields in Combination with Radiation Therapy
9.5. TTFields in Combination with Immunotherapy
9.6. Other Non-Invasive Therapies and TTFields
9.7. TTFields’ Cost-Effectiveness and Accessibility in Clinical Applications
9.8. Future Directions of TTField-Related Clinical Applications and Research
- (a)
- Optimization of Treatment Regimens
- (b)
- Expansion to Other Cancer Types
- (c)
- Combination with Targeted Therapies
- (d)
- Personalized Medicine
- (e)
- Long-Term Clinical Studies
- (f)
- Development of Biomarkers
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghiaseddin, A.P.; Shin, D.; Melnick, K.; Tran, D.D. Tumor treating fields in the management of patients with malignant gliomas. Curr. Treat. Options Oncol. 2020, 21, 76. [Google Scholar]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar]
- Anthony, P.; McArdle, S.; McHugh, M. Tumor treating fields: Adjuvant treatment for high-grade gliomas. Semin. Oncol. Nurs. 2018, 34, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Oborski, M.J.; Hwang, M.; Lieberman, F.S.; Mountz, J.M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [PubMed]
- Velasquez, C.; Mansouri, S.; Mora, C.; Nassiri, F.; Suppiah, S.; Martino, J.; Zadeh, G.; Fernandez-Luna, J.L. Molecular and clinical insights into the invasive capacity of glioblastoma cells. J. Oncol. 2019, 2019, 1740763. [Google Scholar]
- Rick, J.; Chandra, A.; Aghi, M.K. Tumor treating fields: A new approach to glioblastoma therapy. J. Neurooncol. 2018, 137, 447–453. [Google Scholar]
- Zhou, Y.; Xing, X.; Zhou, J.; Jiang, H.; Cen, P.; Jin, C.; Zhong, Y.; Zhou, R.; Wang, J.; Tian, M.; et al. Therapeutic potential of tumor treating fields for malignant brain tumors. Cancer Rep. 2023, 6, e1813. [Google Scholar]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.E. Cusp9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget 2014, 5, 8052–8082. [Google Scholar]
- Skaga, E.; Skaga, I.O.; Grieg, Z.; Sandberg, C.J.; Langmoen, I.A.; Vik-Mo, E.O. The efficacy of a coordinated pharmacological blockade in glioblastoma stem cells with nine repurposed drugs using the cusp9 strategy. J. Cancer Res. Clin. Oncol. 2019, 145, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Huang, J.; Gui, B.; Chen, Y.; Guo, Y.; Lian, Y.; Pan, J.; Hu, Y.; Jiang, N.; Deng, Q.; et al. Ultrasound-responsive nanobubbles for breast cancer: Synergistic sonodynamic, chemotherapy, and immune activation through the cgas-sting pathway. ACS Appl. Mater. Interfaces 2025, 17, 19317–19334. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Guo, J.; Yuan, S.J.; Li, K.; Zhang, Q.; Lei, H.M.; Wu, J.L.; Zhao, L.; Xu, Y.H.; Chen, X. Targeted sonodynamic therapy induces tumor cell quasi-immunogenic ferroptosis and macrophage immunostimulatory autophagy in glioblastoma. Biomaterials 2025, 315, 122913. [Google Scholar] [CrossRef]
- Han, N.; Hou, C.; Duan, Z.; Gu, J.; Zhang, Y.; Luo, J. 2,9-diaryl-6,13-bis(triisopropylsilylethynyl)pentacene derivatives: Synthesis and application in cancer sonodynamic therapy. Chem. Commun. 2025, 61, 5174–5177. [Google Scholar]
- Tanzhu, G.; Chen, L.; Xiao, G.; Shi, W.; Peng, H.; Chen, D.; Zhou, R. The schemes, mechanisms and molecular pathway changes of tumor treating fields (ttfields) alone or in combination with radiotherapy and chemotherapy. Cell Death Discov. 2022, 8, 416. [Google Scholar]
- Makimoto, A.; Nishikawa, R.; Terashima, K.; Kurihara, J.; Fujisaki, H.; Ihara, S.; Morikawa, Y.; Yuza, Y. Tumor-treating fields therapy for pediatric brain tumors. Neurol. Int. 2021, 13, 151–165. [Google Scholar] [CrossRef]
- Tsuboi, N.; Rivera-Caraballo, K.A.; Sahu, U.; Pacholczyk, R.; Douglass, E.; Johnson, T.S.; Wang, Q.; Kolhe, R.; Hedrick, C.C.; Munn, D.H.; et al. Blocking feedback immunosuppression of antigen presentation in brain tumor during oncolytic virotherapy with ohsv-mshpkr. Mol. Cancer Ther. 2025, 24, 444–452. [Google Scholar]
- Melendez-Vazquez, N.M.; Gomez-Manzano, C.; Godoy-Vitorino, F. Oncolytic virotherapies and adjuvant gut microbiome therapeutics to enhance efficacy against malignant gliomas. Viruses 2024, 16, 1775. [Google Scholar] [CrossRef]
- Sabahi, M.; Fathi Jouzdani, A.; Sadeghian, Z.; Dabbagh Ohadi, M.A.; Sultan, H.; Salehipour, A.; Maniakhina, L.; Rezaei, N.; Adada, B.; Mansouri, A.; et al. Car-engineered nk cells versus car t cells in treatment of glioblastoma; strength and flaws. J. Neurooncol. 2025, 171, 495–530. [Google Scholar]
- Tachi, T.; Kijima, N.; Kuroda, H.; Ikeda, S.; Murakami, K.; Nakagawa, T.; Yaga, M.; Nakagawa, K.; Utsugi, R.; Hirayama, R.; et al. Antitumor effects of intracranial injection of b7-h3-targeted car-t and car-nk cells in a patient-derived glioblastoma xenograft model. Cancer Immunol. Immunother. 2024, 73, 256. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [PubMed]
- Arvind, R.; Chandana, S.R.; Borad, M.J.; Pennington, D.; Mody, K.; Babiker, H. Tumor-treating fields: A fourth modality in cancer treatment, new practice updates. Crit. Rev. Oncol. Hematol. 2021, 168, 103535. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Robinson, A.J.J.A.; Sherman, H.G.; Hague, R.J.M.; Rahman, R.; Sanjuan-Alberte, P.; Rawson, F.J. Toward hijacking bioelectricity in cancer to develop new bioelectronic medicine. Adv. Ther. 2021, 4, 2000248. [Google Scholar] [CrossRef]
- Payne, S.L.; Ram, P.; Srinivasan, D.H.; Le, T.T.; Levin, M.; Oudin, M.J. Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer. EBioMedicine 2022, 75, 103767. [Google Scholar] [CrossRef] [PubMed]
- Karanam, N.K.; Ding, L.; Aroumougame, A.; Story, M.D. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: Implications for cancer therapy. Transl. Res. 2020, 217, 33–46. [Google Scholar]
- Moser, J.C.; Salvador, E.; Deniz, K.; Swanson, K.; Tuszynski, J.; Carlson, K.W.; Karanam, N.K.; Patel, C.B.; Story, M.; Lou, E.; et al. The mechanisms of action of tumor treating fields. Cancer Res. 2022, 82, 3650–3658. [Google Scholar] [CrossRef]
- Giladi, M.; Munster, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Blat, R.; Zielinska-Chomej, K.; Haag, P.; Bomzon, Z.; Kirson, E.D.; et al. Tumor treating fields (ttfields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 2017, 12, 206. [Google Scholar] [CrossRef]
- Wong, E.T.T.J.; Swanson, K.D. Tumor treating fields exert cellular and immunologic effects. Cancer Res. 2018, 78, 1707. [Google Scholar] [CrossRef]
- Lacroix, B.; Dumont, J. Spatial and temporal scaling of microtubules and mitotic spindles. Cells 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Schneiderman, R.S.; Volodin, A.; Shamir, R.R.; Kaynan, N.; Zeevi, E.; Koren, L.; Klein-Goldberg, A.; Paz, R.; Giladi, M.; et al. Tumor treating fields (ttfields) hinder cancer cell motility through regulation of microtubule and acting dynamics. Cancers 2020, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, R.S.G.M.; Zeevi, E.; Voloshin, T.; Shteigauz, A.; Porat, Y.; Munster, M.; Weinberg, U.; Kirson, E.D.; Palti, Y. Tumor treating fields (ttfields) inhibit cancer cell migration and invasion by inducing reorganization of the actin cytoskeleton and formation of cell adhesions. Neuro Oncol. 2018, 20, vi30. [Google Scholar]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Kaynan, N.; Davidi, S.; Porat, Y.; Shteingauz, A.; Schneiderman, R.S.; Zeevi, E.; Munster, M.; Blat, R.; Tempel Brami, C.; et al. Tumor-treating fields (ttfields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-pd-1 therapy. Cancer Immunol. Immunother. 2020, 69, 1191–1204. [Google Scholar] [CrossRef]
- Chen, D.; Le, S.B.; Hutchinson, T.E.; Calinescu, A.A.; Sebastian, M.; Jin, D.; Liu, T.; Ghiaseddin, A.; Rahman, M.; Tran, D.D. Tumor treating fields dually activate sting and aim2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Investig. 2022, 132, e149258. [Google Scholar]
- Salvador, E.; Koppl, T.; Hormann, J.; Schonharl, S.; Bugaeva, P.; Kessler, A.F.; Burek, M.; Ernestus, R.I.; Lohr, M.; Hagemann, C. Tumor treating fields (ttfields) induce cell junction alterations in a human 3d in vitro model of the blood-brain barrier. Pharmaceutics 2023, 15, 185. [Google Scholar] [CrossRef]
- Kessler, A.F.S.E.; Domröse, D.; Burek, M.; Schaeffer, C.; Tempel Brami, C.; Voloshin, T.; Giladi, M.; Ernestus, R.I.; Löhr, M.; Förster, C.; et al. Blood brain barrier (bbb) integrity is affected by tumor treating fields (ttfields) in vitro and in vivo. Int. J. Radiat. Oncol. 2019, 105, S162–S163. [Google Scholar] [CrossRef]
- Aguilar, A.A.; Ho, M.C.; Chang, E.; Carlson, K.W.; Natarajan, A.; Marciano, T.; Bomzon, Z.; Patel, C.B. Permeabilizing cell membranes with electric fields. Cancers 2021, 13, 2283. [Google Scholar] [CrossRef]
- Kim, E.H.; Song, H.S.; Yoo, S.H.; Yoon, M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 2016, 7, 65125–65136. [Google Scholar]
- McKinnon, C.A.A.; Wahedi, A.; Cook, J.A.; Plaha, P. Tumour-treating fields for high-grade glioma. Cochrane Datababse Syst. Rev. 2023, 2023, CD014979. [Google Scholar]
- Kirson, E.D.; Giladi, M.; Gurvich, Z.; Itzhaki, A.; Mordechovich, D.; Schneiderman, R.S.; Wasserman, Y.; Ryffel, B.; Goldsher, D.; Palti, Y. Alternating electric fields (ttfields) inhibit metastatic spread of solid tumors to the lungs. Clin. Exp. Metastasis 2009, 26, 633–640. [Google Scholar]
- Giladi, M.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Itzhaki, A.; Mordechovich, D.; Cahal, S.; Kirson, E.D.; Weinberg, U.; Palti, Y. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014, 14, 54–63. [Google Scholar] [PubMed]
- Gentilal, N.; Salvador, R.; Miranda, P.C. Temperature control in ttfields therapy of gbm: Impact on the duty cycle and tissue temperature. Phys. Med. Biol. 2019, 64, 225008. [Google Scholar]
- Mrugala, M.M.; Ruzevick, J.; Zlomanczuk, P.; Lukas, R.V. Tumor treating fields in neuro-oncological practice. Curr. Oncol. Rep. 2017, 19, 53. [Google Scholar] [PubMed]
- Korshoej, A.R.; Hansen, F.L.; Thielscher, A.; von Oettingen, G.B.; Sorensen, J.C.H. Impact of tumor position, conductivity distribution and tissue homogeneity on the distribution of tumor treating fields in a human brain: A computer modeling study. PLoS ONE 2017, 12, e0179214. [Google Scholar]
- Porat, Y.; Giladi, M.; Schneiderman, R.S.; Blat, R.; Shteingauz, A.; Zeevi, E.; Munster, M.; Voloshin, T.; Kaynan, N.; Tal, O.; et al. Determining the optimal inhibitory frequency for cancerous cells using tumor treating fields (ttfields). J. Vis. Exp. 2017, 123, 55820. [Google Scholar]
- Schneiderman, R.S.G.M.; Porat, Y.; Munster, M.; Weinberg, U.; Kirson, E.D.; Palti, Y. Overcoming cell size escape from tumor treating fields using a varying frequency treatment paradigm in vitro. J. Clin. Oncol. 2013, 31, e22134. [Google Scholar]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. Novottf-100a versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase iii trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar]
- Kanner, A.A.; Wong, E.T.; Villano, J.L.; Ram, Z.; Investigators, E.F. Post hoc analyses of intention-to-treat population in phase iii comparison of novottf-100a system versus best physician’s choice chemotherapy. Semin. Oncol. 2014, 41 (Suppl. S6), S25–S34. [Google Scholar]
- Mrugala, M.M.; Engelhard, H.H.; Dinh Tran, D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Annenelie Bota, D.; Rudnick, J.; Love Sumrall, A.; Zhu, J.J.; et al. Clinical practice experience with novottf-100a system for glioblastoma: The patient registry dataset (pride). Semin. Oncol. 2014, 41 (Suppl. S6), S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Schneiderman, R.S.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (ttfields). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [PubMed]
- Taphoorn, M.J.B.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar]
- Toms, S.A.; Kim, C.Y.; Nicholas, G.; Ram, Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the ef-14 phase iii trial. J. Neurooncol. 2019, 141, 467–473. [Google Scholar] [PubMed]
- Wong, E.T.P.M.; Barron, L.; Lok, E. Updated safety analysis of bevacizumab plus alternating electric fields therapy in patients with recurrent malignant gliomas. Neuro Oncol. 2015, 17, v174. [Google Scholar]
- Ballo, M.T.; Urman, N.; Lavy-Shahaf, G.; Grewal, J.; Bomzon, Z.; Toms, S. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: A large-scale numerical simulation-based analysis of data from the phase 3 ef-14 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1106–1113. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Hansen, F.L.; Mikic, N.; von Oettingen, G.; Sorensen, J.C.H.; Thielscher, A. Importance of electrode position for the distribution of tumor treating fields (ttfields) in a human brain. Identification of effective layouts through systematic analysis of array positions for multiple tumor locations. PLoS ONE 2018, 13, e0201957. [Google Scholar] [CrossRef]
- Mohan, S.; Chawla, S.; Wang, S.; Verma, G.; Skolnik, A.; Brem, S.; Peters, K.B.; Poptani, H. Assessment of early response to tumor-treating fields in newly diagnosed glioblastoma using physiologic and metabolic mri: Initial experience. CNS Oncol. 2016, 5, 137–144. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Tran, D.W.S.; Allen, A.; Sampson, D.; Chen, D.; Thomas, N.; Greene, V.; Rahman, M.; Ghiaseddin, A. Phase 2 open-labeled study of adjuvant temozolomide plus tumor treating fields plus pembrolizumab in patients with newly diagnosed glioblastoma (2-the-top). Neuro Oncol. 2019, 21, vi10. [Google Scholar]
- Goldman, S.H.E.; Lai, J.-S.; Kocak, M.; Lulla, R.; Dhall, G.; Robison, N.; Onar-Thomas, A.; Dunkel, I.J. Feasibility trial of ttfields (tumor treating fields) for children with recurrent or progressive supratentorial high-grade glioma (hgg) and ependymoma: A pediatric brain tumor consortium study: Pbtc-048. Neuro Oncol. 2018, 20, vi201–vi202. [Google Scholar]
- Lin, Y.; Chen, B. Case report: Tumor-treating fields prolongs idh-mutant anaplastic astrocytoma progression-free survival and pathological evolution to glioblastoma. Ann. Transl. Med. 2021, 9, 1804. [Google Scholar] [PubMed]
- Meletath, S.K.; Pavlick, D.; Brennan, T.; Hamilton, R.; Chmielecki, J.; Elvin, J.A.; Palma, N.; Ross, J.S.; Miller, V.A.; Stephens, P.J.; et al. Personalized treatment for a patient with a braf v600e mutation using dabrafenib and a tumor treatment fields device in a high-grade glioma arising from ganglioglioma. J. Natl. Compr. Canc. Netw. 2016, 14, 1345–1350. [Google Scholar]
- Stachelek, G.C.; Grimm, J.; Moore, J.; Huang, E.; Spoleti, N.; Redmond, K.J.; Lim, M.; Bettegowda, C.; Kleinberg, L. Tumor-treating field arrays do not reduce target volume coverage for glioblastoma radiation therapy. Adv. Radiat. Oncol. 2020, 5, 62–69. [Google Scholar]
- Karanam, N.K.; Srinivasan, K.; Ding, L.; Sishc, B.; Saha, D.; Story, M.D. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of brca1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017, 8, e2711. [Google Scholar]
- Miller, R.; Niazi, M.; Russial, O.; Poiset, S.; Shi, W. Tumor treating fields with radiation for glioblastoma: A narrative review. Chin. Clin. Oncol. 2022, 11, 40. [Google Scholar]
- Kim, E.H.; Kim, Y.H.; Song, H.S.; Jeong, Y.K.; Lee, J.Y.; Sung, J.; Yoo, S.H.; Yoon, M. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget 2016, 7, 62267–62279. [Google Scholar] [CrossRef]
- Bokstein, F.; Blumenthal, D.; Limon, D.; Harosh, C.B.; Ram, Z.; Grossman, R. Concurrent tumor treating fields (ttfields) and radiation therapy for newly diagnosed glioblastoma: A prospective safety and feasibility study. Front. Oncol. 2020, 10, 411. [Google Scholar] [CrossRef]
- Den, R.B.; Kamrava, M.; Sheng, Z.; Werner-Wasik, M.; Dougherty, E.; Marinucchi, M.; Lawrence, Y.R.; Hegarty, S.; Hyslop, T.; Andrews, D.W.; et al. A phase i study of the combination of sorafenib with temozolomide and radiation therapy for the treatment of primary and recurrent high-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 321–328. [Google Scholar]
- Jo, Y.; Kim, E.H.; Sai, S.; Kim, J.S.; Cho, J.M.; Kim, H.; Baek, J.H.; Kim, J.Y.; Hwang, S.G.; Yoon, M. Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int. J. Mol. Sci. 2018, 19, 3684. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.Y.O.T.; Kaynan, N.; Giladi, M.; Weinberg, U.; Palti, Y. Alternating electric fields (ttfields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-pd-1 therapy. Neuro Oncol. 2017, 19, vi126. [Google Scholar]
- Jo, Y.; Han, Y.I.; Lee, E.; Seo, J.; Oh, G.; Sung, H.; Gi, Y.; Kim, H.; Park, S.; Yoon, M. The combination of tumor treating fields and hyperthermia has synergistic therapeutic effects in glioblastoma cells by downregulating stat3. Am. J. Cancer Res. 2022, 12, 1423–1432. [Google Scholar]
- Neuhaus, E.; Zirjacks, L.; Ganser, K.; Klumpp, L.; Schuler, U.; Zips, D.; Eckert, F.; Huber, S.M. Alternating electric fields (ttfields) activate ca(v)1.2 channels in human glioblastoma cells. Cancers 2019, 11, 110. [Google Scholar] [CrossRef]
- Vergote, I.B.M.; Pless, M.; Ceresoli, G. Safety of ttfields applied to the torso: Meta-analysis of 176 patients from four phase i-ii trials. Int. J. Radiat. Oncol. 2018, 102, E370. [Google Scholar]
- Giladi, M.; Weinberg, U.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Voloshin, T.; Blatt, R.; Cahal, S.; Itzhaki, A.; Onn, A.; et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 2014, 41 (Suppl. S6), S35–S41. [Google Scholar]
- Pless, M.; Droege, C.; von Moos, R.; Salzberg, M.; Betticher, D. A phase i/ii trial of tumor treating fields (ttfields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 2013, 81, 445–450. [Google Scholar] [PubMed]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crino, L.; et al. Tumour treating fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (stellar): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar]
- Wang, Y.; Pandey, M.; Ballo, M.T. Integration of tumor-treating fields into the multidisciplinary management of patients with solid malignancies. Oncologist 2019, 24, e1426–e1436. [Google Scholar]
- Kinzel, A.; Ambrogi, M.; Varshaver, M.; Kirson, E.D. Tumor treating fields for glioblastoma treatment: Patient satisfaction and compliance with the second-generation optune((r)) system. Clin. Med. Insights Oncol. 2019, 13, 1179554918825449. [Google Scholar]
- Gatson, N.T.N.; Barnholtz-Sloan, J.; Drappatz, J.; Henriksson, R.; Hottinger, A.F.; Hinoul, P.; Kruchko, C.; Puduvalli, V.K.; Tran, D.D.; Wong, E.T.; et al. Tumor treating fields for glioblastoma therapy during the COVID-19 pandemic. Front. Oncol. 2021, 11, 679702. [Google Scholar]
- Trusheim, J.; Dunbar, E.; Battiste, J.; Iwamoto, F.; Mohile, N.; Damek, D.; Bota, D.A.; Connelly, J. A state-of-the-art review and guidelines for tumor treating fields treatment planning and patient follow-up in glioblastoma. CNS Oncol. 2017, 6, 29–43. [Google Scholar] [PubMed]
- Lacouture, M.E.; Anadkat, M.J.; Ballo, M.T.; Iwamoto, F.; Jeyapalan, S.A.; La Rocca, R.V.; Schwartz, M.; Serventi, J.N.; Glas, M. Prevention and management of dermatologic adverse events associated with tumor treating fields in patients with glioblastoma. Front. Oncol. 2020, 10, 1045. [Google Scholar]
- Tran, D. Real-world surveillance data for tumor treating fields affirm the tolerability of tumor treating fields for the treatment of glioblastoma in the united states. Neuro Oncol. 2018, 20, vi 198. [Google Scholar]
- Lacouture, M.E.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and management of dermatologic adverse events with the novottf-100a system, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin. Oncol. 2014, 41 (Suppl. S4), S1–S14. [Google Scholar] [PubMed]
- Onken, J.; Staub-Bartelt, F.; Vajkoczy, P.; Misch, M. Acceptance and compliance of ttfields treatment among high grade glioma patients. J. Neurooncol. 2018, 139, 177–184. [Google Scholar]
- Haj, A.D.C.; Hohenberger, C.; Brawanski, A.; Proescholdt, M.A. Ttfields for newly diagnosed glioblastoma: Impact of consultation strategy. Neuro Oncol. 2018, 20, iii265. [Google Scholar]
- Guzauskas, G.F.; Pollom, E.L.; Stieber, V.W.; Wang, B.C.M.; Garrison, L.P., Jr. Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: A cost-effectiveness study. J. Med. Econ. 2019, 22, 1006–1013. [Google Scholar]
- Bahr, O.T.G.; Fietkau, R.; Goldbrunner, R.; Glas, M.G. Tumor treating fields (ttfields) therapy in patients with glioblastoma: Long-term survival results from ttfields in germany in routine clinical care (tiger) study. J. Clin. Oncol. 2024, 42, 2036. [Google Scholar]
- Bahr, O.T.G.; Fietkau, R.; Goldbrunner, R.; Glas, M.G. P14.63 the use of ttfields for newly diagnosed gbm patients in germany in routine clinical care (tiger: Ttfields in germany in routine clinical care). Neuro Oncol. 2019, 21, iii82. [Google Scholar]
- Khagi, S.; Kotecha, R.; Gatson, N.T.N.; Jeyapalan, S.; Abdullah, H.I.; Avgeropoulos, N.G.; Batzianouli, E.T.; Giladi, M.; Lustgarten, L.; Goldlust, S.A. Recent advances in tumor treating fields (ttfields) therapy for glioblastoma. Oncologist 2025, 30, oyae227. [Google Scholar] [CrossRef] [PubMed]
- Memari, E.; Khan, D.; Alkins, R.; Helfield, B. Focused ultrasound-assisted delivery of immunomodulating agents in brain cancer. J. Control Release 2024, 367, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Patel, A.; Hanchate, K.; Robinson, E.; Edwards, A.; Shah, S.; Higgins, D.; Haworth, K.J.; Lucke-Wold, B.; Pomeranz Krummel, D.; et al. Advances in focused ultrasound for the treatment of brain tumors. Tomography 2023, 9, 1094–1109. [Google Scholar] [CrossRef]
- Noel, G.; Gondi, V. Proton therapy for tumors of the base of the skull. Chin. Clin. Oncol. 2016, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Jin, H.; Ahmad, S. Comparison of intensity-modulated radiation therapy (imrt), 3d conformal proton therapy and intensity-modulated proton therapy (impt) for the treatment of metastatic brain cancer. Med. Dosim. 2023, 48, 73–76. [Google Scholar] [CrossRef]
- Merchant, T.E.; Hoehn, M.E.; Khan, R.B.; Sabin, N.D.; Klimo, P.; Boop, F.A.; Wu, S.; Li, Y.; Burghen, E.A.; Jurbergs, N.; et al. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (rt2cr): A single-arm, phase 2 study. Lancet Oncol. 2023, 24, 523–534. [Google Scholar] [CrossRef]

| Adverse Effects | Potential Cause(s) | Treatment Recommendation(s) |
|---|---|---|
| Skin irritations/abrasions | Sensitivity to array pads | High-potency topical steroids Gauze to protect areas of irritation Cold, moist compress applications |
| Skin infection Pustules | Bacterial infection | Change cranial arrays every 3–4 days Wash scalp and shift array positions Topical antibiotics Treatment interruptions for 2–7 days |
| Hyperhidrosis | Excessive sweating High ambient temperatures Intense activity | Avoid using ointments that cause sweating High-potency topical steroids |
| Minor scalp burns Minor scalp rashes | High ambient temperatures | Device usage at cooler temperature Liquefied hydrogel |
| Skin erosion Severe skin rash Ulcers | Allergy to array pads Sensitivity to hydrogel Reaction to tape Mechanical trauma | Treatment interruptions for 2–7 days Transducer array removal Wound dressing with gauze Topical antibiotics |
| Pruritus Dry, itchy, flaky skin | Cold, dry temperature Dehydration Sensitivity to array pads | Fragrance-free shampoo No alcohol-based products Topical corticosteroids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.N.; Huang, J.H.; Qi, X.; Pan, Y.; Wu, E.; Nizamutdinov, D. Tumor Treating Fields and Combination Therapy in Management of Brain Oncology. Cancers 2025, 17, 1211. https://doi.org/10.3390/cancers17071211
Liu RN, Huang JH, Qi X, Pan Y, Wu E, Nizamutdinov D. Tumor Treating Fields and Combination Therapy in Management of Brain Oncology. Cancers. 2025; 17(7):1211. https://doi.org/10.3390/cancers17071211
Chicago/Turabian StyleLiu, Ruisi Nicole, James H. Huang, Xiaoming Qi, Yizhong Pan, Erxi Wu, and Damir Nizamutdinov. 2025. "Tumor Treating Fields and Combination Therapy in Management of Brain Oncology" Cancers 17, no. 7: 1211. https://doi.org/10.3390/cancers17071211
APA StyleLiu, R. N., Huang, J. H., Qi, X., Pan, Y., Wu, E., & Nizamutdinov, D. (2025). Tumor Treating Fields and Combination Therapy in Management of Brain Oncology. Cancers, 17(7), 1211. https://doi.org/10.3390/cancers17071211





