Exploratory Algorithms to Aid in Risk of Malignancy Prediction for Indeterminate Pulmonary Nodules
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Handling, Storage, and Immunoassay/Clinical Chemistry Testing
2.2. Autoantibody Testing
2.3. Statistical Methods
3. Results
3.1. Patient Cohorts
3.2. Classification Algorithm Development
3.3. Classification Performance Characteristics of Algorithms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| AutoAB | autoantibody |
| IACC | immunoassay and clinical chemistry, ARCHITECT® platform |
| IPN | indeterminate pulmonary nodule |
| IQR | interquartile range |
| LASSO | least absolute shrinkage and selection operator |
| LDCT | low-dose helical computed tomography |
| Lung-RADS | Lung Imaging Reporting and Data System |
| Mayo score | solitary pulmonary nodule malignancy risk score from the Mayo Clinic |
| NLST | National Lung Screening Trial |
| NSCLC | non-small cell lung cancer |
| NPV | negative predictive value |
| ROC | receiver operating characteristic |
| RUO | research use only |
| RUMC | Rush University Medical Center |
| PPV | positive predictive value |
| USPSTF | US Preventative Services Task Force |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef] [PubMed]
- Wender, R.; Fontham, E.T.; Barrera, E., Jr.; Colditz, G.A.; Church, T.R.; Ettinger, D.S.; Etzioni, R.; Flowers, C.R.; Gazelle, G.S.; Kelsey, D.K.; et al. American Cancer Society lung cancer screening guidelines. CA Cancer J. Clin. 2013, 63, 107–117. [Google Scholar] [CrossRef]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Massion, P.P.; Walker, R.C. Indeterminate pulmonary nodules: Risk for having or for developing lung cancer? Cancer Prev. Res. 2014, 7, 1173–1178. [Google Scholar] [CrossRef]
- Christensen, J.; Prosper, A.E.; Wu, C.C.; Chung, J.; Lee, E.; Elicker, B.; Hunsaker, A.R.; Petranovic, M.; Sandler, K.L.; Stiles, B.; et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. J. Am. Coll. Radiol. 2023, 21, 473–488. [Google Scholar] [CrossRef]
- Schultz, E.M.; Sanders, G.D.; Trotter, P.R.; Patz, E.F., Jr.; Silvestri, G.A.; Owens, D.K.; Gould, M.K. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax 2008, 63, 335–341. [Google Scholar] [CrossRef]
- Swensen, S.J.; Silverstein, M.D.; Edell, E.S.; Trastek, V.F.; Aughenbaugh, G.L.; Ilstrup, D.M.; Schleck, C.D. Solitary pulmonary nodules: Clinical prediction model versus physicians. Mayo Clin. Proc. 1999, 74, 319–329. [Google Scholar] [CrossRef]
- Swensen, S.J.; Silverstein, M.D.; Ilstrup, D.M.; Schleck, C.D.; Edell, E.S. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch. Intern. Med. 1997, 157, 849–855. [Google Scholar]
- Isbell, J.M.; Deppen, S.; Putnam, J.B., Jr.; Nesbitt, J.C.; Lambright, E.S.; Dawes, A.; Massion, P.P.; Speroff, T.; Jones, D.R.; Grogan, E.L. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann. Thorac. Surg. 2011, 91, 227–233; discussion 233. [Google Scholar] [CrossRef] [PubMed]
- Deppen, S.A.; Blume, J.D.; Aldrich, M.C.; Fletcher, S.A.; Massion, P.P.; Walker, R.C.; Chen, H.C.; Speroff, T.; Degesys, C.A.; Pinkerman, R.; et al. Predicting lung cancer prior to surgical resection in patients with lung nodules. J. Thorac. Oncol. 2014, 9, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.R.; Mazzone, P.J. Biomarkers in Lung Cancer. Clin. Chest Med. 2020, 41, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Cicenia, J. Role of biomarkers in lung nodule evaluation. Curr. Opin. Pulm. Med. 2022, 28, 275–281. [Google Scholar] [CrossRef]
- Quinn, F.A. ARCHITECT i2000 and i2000SR Analyzers. In The Immunoassay Handbook, 3rd ed.; Wild, D., Ed.; Elsevier Science: London, UK, 2005. [Google Scholar]
- Reed, R. Learning Guide: Clinical Chemistry. Available online: https://www.corelaboratory.abbott/int/en/knowledge-center/learning-guides.html#clinical-chemistry (accessed on 9 August 2024).
- Farlow, E.C.; Patel, K.; Basu, S.; Lee, B.S.; Kim, A.W.; Coon, J.S.; Faber, L.P.; Bonomi, P.; Liptay, M.J.; Borgia, J.A. Development of a multiplexed tumor-associated autoantibody-based blood test for the detection of non-small cell lung cancer. Clin. Cancer Res. 2010, 16, 3452–3462. [Google Scholar]
- Auger, C.; Moudgalya, H.; Neely, M.R.; Stephan, J.T.; Tarhoni, I.; Gerard, D.; Basu, S.; Fhied, C.L.; Abdelkader, A.; Vargas, M.; et al. Development of a Novel Circulating Autoantibody Biomarker Panel for the Identification of Patients with ‘Actionable’ Pulmonary Nodules. Cancers 2023, 15, 2259. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Buhlmann, P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Classification and Regression Trees, 1st ed.; Chapman & Hall: Monterey, CA, USA, 1984; p. 368. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 16 October 2024).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U.; Liao, W.; Faris, N.R.; Fehnel, C.; Goss, J.; Shepherd, C.J.; Qureshi, T.; Matthews, A.T.; Smeltzer, M.P.; Pinsky, P.F. Evaluation of Lung Cancer Risk Among Persons Undergoing Screening or Guideline-Concordant Monitoring of Lung Nodules in the Mississippi Delta. JAMA Netw. Open 2023, 6, e230787. [Google Scholar] [CrossRef]
- Rivera, M.P.; Durham, D.D.; Long, J.M.; Perera, P.; Lane, L.; Lamb, D.; Metwally, E.; Henderson, L.M. Receipt of Recommended Follow-up Care After a Positive Lung Cancer Screening Examination. JAMA Netw. Open 2022, 5, e2240403. [Google Scholar] [CrossRef] [PubMed]
- Maiga, A.W.; Deppen, S.A.; Pinkerman, R.; Callaway-Lane, C.; Massion, P.P.; Dittus, R.S.; Lambright, E.S.; Nesbitt, J.C.; Baker, D.; Grogan, E.L. Timeliness of Care and Lung Cancer Tumor-Stage Progression: How Long Can We Wait? Ann. Thorac. Surg. 2017, 104, 1791–1797. [Google Scholar] [CrossRef]
- Writing Group for the CKD Prognosis Consortium; Grams, M.E.; Coresh, J.; Matsushita, K.; Ballew, S.H.; Sang, Y.; Surapaneni, A.; Alencar de Pinho, N.; Anderson, A.; Appel, L.J.; et al. Estimated Glomerular Filtration Rate, Albuminuria, and Adverse Outcomes: An Individual-Participant Data Meta-Analysis. JAMA 2023, 330, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Ling, C.; Sun, Q.; Harb, R.; Ashmaig, M.; Warnick, R.; Sethi, A.; Fleming, J.K.; Otvos, J.D.; Meeusen, J.W.; et al. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia. JAMA Cardiol. 2020, 5, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Rodriguez-Garcia, M.; Fernandez-Galan, E. Clinical usefulness of circulating tumor markers. Clin. Chem. Lab. Med. 2023, 61, 895–905. [Google Scholar] [CrossRef]
- Liu, T.; Ma, Q.; Li, W.; Hu, Y.; Yang, J.; Yao, Q. Ubiquilin 1 suppresses the cancer stem cell-like traits of non-small cell lung cancer cells by regulating reactive oxygen species homeostasis. Bioengineered 2021, 12, 7143–7155. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, N.; Shah, P.P.; Rowbotham, D.A.; Malik, D.; Srivastav, A.; Shankar, J.; Lam, W.L.; Lockwood, W.W.; Beverly, L.J. MIR155 Regulation of Ubiquilin1 and Ubiquilin2: Implications in Cellular Protection and Tumorigenesis. Neoplasia 2017, 19, 321–332. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Y.; Lin, H.; Yao, X. The impacts of ubiquilin 1 (UBQLN1) knockdown on cells viability, proliferation, and apoptosis are mediated by p53 in A549 lung cancer cells. J. Thorac. Dis. 2020, 12, 5887–5895. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, L.; Xu, S.; Fu, B.; Che, Y.; Zaky, M.Y.; Tian, R.; Yao, R.; Guo, D.; Sha, Z.; et al. Small molecule Z363 co-regulates TAF10 and MYC via the E3 ligase TRIP12 to suppress tumour growth. Clin. Transl. Med. 2023, 13, e1153. [Google Scholar] [CrossRef]

| Train Set n = 242 (68.9%) | Test Set n = 109 (31.1%) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Non- Malignant n = 78 (32%) | Malignant n = 164 (68%) | Overall n = 242 | Non- Malignant n = 47 (43%) | Malignant n = 62 (57%) | Overall n = 109 |
| IPN Clinical Classification 1 | ||||||
| Radiographically non-malignant based on follow-up radiography | 27 (35%) | 0 (0%) | 27 (11%) | 18 (38%) | 0 (0%) | 18 (17%) |
| Histologically diagnosed non-malignancies | 51 (65%) | 0 (0%) | 51 (21%) | 29 (62%) | 0 (0%) | 29 (27%) |
| Lung malignancies | 0 (0%) | 164 (100%) | 164 (68%) | 0 (0%) | 62 (100%) | 62 (57%) |
| Age | ||||||
| Median (IQR) | 66 (60, 71) | 72 (66, 76) | 71 (63, 75) | 67 (60, 72) | 71 (64, 77) | 68 (63, 75) |
| Range | 41, 82 | 44, 87 | 41, 87 | 43, 84 | 52, 87 | 43, 87 |
| Sex 1 | ||||||
| Female | 34 (44%) | 95 (58%) | 129 (53%) | 22 (47%) | 33 (53%) | 55 (50%) |
| Male | 44 (56%) | 69 (42%) | 113 (47%) | 25 (53%) | 29 (47%) | 54 (50%) |
| Race 1 | ||||||
| White | 63 (81%) | 144 (88%) | 207 (86%) | 36 (77%) | 54 (89%) | 90 (83%) |
| Black or African American | 11 (14%) | 16 (9.8%) | 27 (11%) | 7 (15%) | 5 (8.2%) | 12 (11%) |
| Asian | 2 (2.6%) | 1 (0.6%) | 3 (1.2%) | 1 (2.1%) | 2 (3.3%) | 3 (2.8%) |
| Native American or Pac. Islander | 1 (1.3%) | 0 (0%) | 1 (0.4%) | 1 (2.1%) | 0 (0%) | 1 (0.9%) |
| Other | 1 (1.3%) | 3 (1.8%) | 4 (1.7%) | 2 (4.3%) | 0 (0%) | 2 (1.9%) |
| Unknown | 0 | 0 | 0 | 0 | 1 | 1 |
| Ethnicity 1 | ||||||
| Hispanic or Latino | 1 (1.3%) | 3 (1.8%) | 4 (1.7%) | 1 (2.4%) | 0 (0%) | 1 (1.0%) |
| Not Hispanic or Latino | 75 (99%) | 161 (98%) | 236 (98%) | 41 (98%) | 61 (100%) | 102 (99%) |
| Unknown | 2 | 0 | 2 | 5 | 1 | 6 |
| Pack-years | ||||||
| Median (IQR) | 25 (5, 43) | 40 (30, 60) | 40 (23, 50) | 31 (3, 40) | 40 (30, 50) | 40 (30, 46) |
| Range | 0, 120 | 15, 120 | 0, 120 | 0, 80 | 20, 150 | 0, 150 |
| Unknown | 1 | 2 | 3 | 0 | 1 | 1 |
| Nodule Size (mm) | ||||||
| Median (IQR) | 12 (8, 18) | 20 (14, 31) | 17 (12, 26) | 12 (8, 16) | 20 (15, 31) | 16 (11, 25) |
| Range | 4, 53 | 7, 110 | 4, 110 | 6, 48 | 8, 95 | 6, 95 |
| Upper Lobe Location 1 | 33 (42%) | 97 (59%) | 130 (54%) | 23 (49%) | 40 (65%) | 63 (58%) |
| Spiculation 1 | 28 (36%) | 94 (57%) | 122 (50%) | 18 (38%) | 39 (63%) | 57 (52%) |
| History of Extrathoracic Cancer ≥ 5 Years Prior 1 | 16 (21%) | 44 (27%) | 60 (25%) | 12 (26%) | 15 (24%) | 27 (25%) |
| Model | Candidate Predictors | Final Model Predictors |
|---|---|---|
| Mayo Score | Age + Lesion Size + History of Smoking + History of Extrathoracic Cancer + Upper Lobe Location + Spiculation | Age + Lesion Size + History of Smoking + History of Extrathoracic Cancer + Upper Lobe Location + Spiculation |
| LASSO Clinical | Clinical variables: Age + Lesion Size + Pack-years + History of Extrathoracic Cancer + Upper Lobe Location + Spiculation | Age + Lesion Size + Pack-years + Upper Lobe Location + Spiculation |
| LASSO AutoAb + Clinical | AutoAb markers + clinical variables * | Age + Lesion Size + Pack-years + Upper Lobe Location + TAF10 AutoAb (log2) |
| LASSO IACC + Clinical | IACC markers + clinical variables * | Age + Lesion Size + Pack-years + History of Extrathoracic Cancer + Upper Lobe Location + Spiculation + IgE ≥25 + IgM (log2) + Spiculation + hs-CRP (log2) + NSE (log2) + Ferritin (log2) + CA-125 (log2) |
| LASSO IACC + AutoAb + Clinical | IACC markers + AutoAb markers + clinical variables * | Age + Lesion Size + Pack-years + History of Extrathoracic Cancer + Upper Lobe Location + Spiculation + IgE ≥25 + IgM (log2) + hs-CRP (log2) + CA-125 (log2) + Ferritin (log2) + NSE (log2) + TAF10 AutoAb (log2) + Ubiquilin-1 AutoAb (log2) |
| Decision Tree | IACC markers + AutoAb markers + clinical variables * | Age + Lesion Size + Pack-years + Spiculation + hs-CRP (log2) + Ubiquilin-1 AutoAb (log2) |
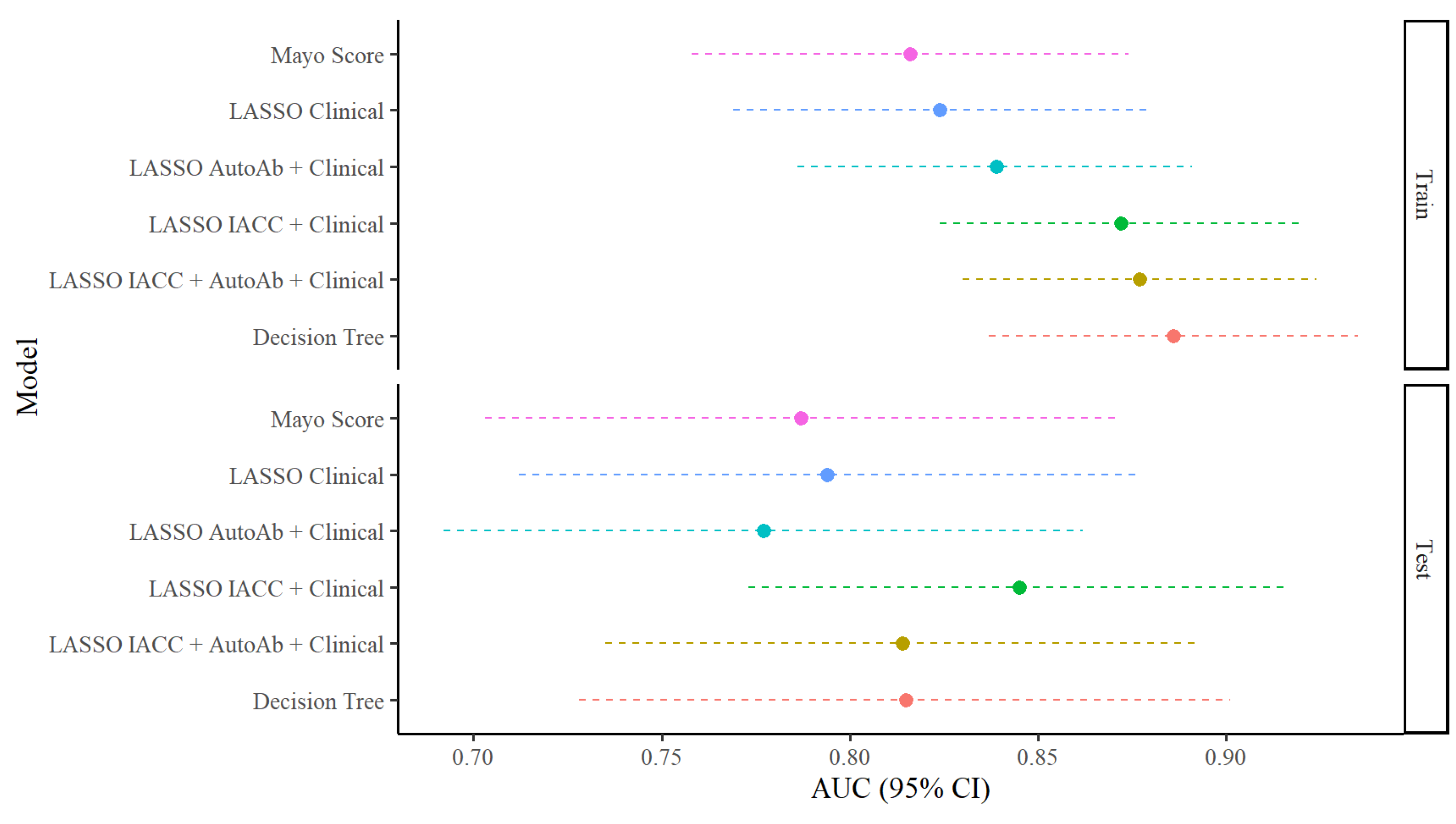
| Model | AUC (95% CI) | SE | SP | PPV * | NPV * | SE (SP = 90%) | SP (SE = 90%) | SP (SE = 75%) |
|---|---|---|---|---|---|---|---|---|
| Train Set Performance (n = 242 with 164 events) | ||||||||
| Mayo Score | 0.816 (0.758, 0.874) | 71.3 | 80.3 | 88.6 | 56.5 | 48.8 | 52.6 | 72.4 |
| LASSO Clinical | 0.824 (0.769, 0.879) | 98.8 | 29.5 | 74.7 | 92.0 | 50.0 | 48.7 | 74.4 |
| LASSO AutoAb + Clinical | 0.839 (0.786, 0.891) | 97.0 | 32.1 | 75.0 | 83.3 | 51.8 | 52.6 | 76.9 |
| LASSO IACC + Clinical | 0.872 (0.824, 0.921) | 94.5 | 56.4 | 82.0 | 83.0 | 56.7 | 66.7 | 78.2 |
| LASSO IACC + AutoAb + Clinical | 0.877 (0.830, 0.924) | 95.7 | 57.7 | 82.6 | 86.5 | 54.9 | 64.1 | 83.3 |
| Decision Tree | 0.886 (0.837, 0.935) | 95.7 | 65.4 | 85.3 | 87.9 | 64.2 | 75.1 | 86.1 |
| Test Set Performance (n = 109 with 62 events) | ||||||||
| Mayo Score | 0.787 (0.703, 0.872) | 72.6 | 69.6 | 76.3 | 65.3 | 45.2 | 41.3 | 69.6 |
| LASSO Clinical | 0.794 (0.712, 0.876) | 96.8 | 27.7 | 63.8 | 86.7 | 53.2 | 46.8 | 59.6 |
| LASSO AutoAb + Clinical | 0.777 (0.692, 0.862) | 91.9 | 25.5 | 62.0 | 70.6 | 53.2 | 36.2 | 59.6 |
| LASSO IACC + Clinical | 0.845 (0.773, 916) | 87.1 | 61.7 | 75.0 | 78.4 | 59.7 | 53.2 | 74.5 |
| LASSO IACC + AutoAb + Clinical | 0.814 (0.735, 0.893) | 90.3 | 53.2 | 71.8 | 80.6 | 56.5 | 53.2 | 68.1 |
| Decision Tree | 0.815 (0.728, 0.901) | 91.9 | 66.0 | 78.1 | 86.1 | 28.8 | 68.1 | 70.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, L.; Auger, C.; Jeanblanc, N.; Jacobson, C.; Pandya, K.; Gawel, S.; Moudgalya, H.; Sharma, A.; Seder, C.W.; Liptay, M.J.; et al. Exploratory Algorithms to Aid in Risk of Malignancy Prediction for Indeterminate Pulmonary Nodules. Cancers 2025, 17, 1231. https://doi.org/10.3390/cancers17071231
Jackson L, Auger C, Jeanblanc N, Jacobson C, Pandya K, Gawel S, Moudgalya H, Sharma A, Seder CW, Liptay MJ, et al. Exploratory Algorithms to Aid in Risk of Malignancy Prediction for Indeterminate Pulmonary Nodules. Cancers. 2025; 17(7):1231. https://doi.org/10.3390/cancers17071231
Chicago/Turabian StyleJackson, Laurel, Claire Auger, Nicolette Jeanblanc, Christopher Jacobson, Kinnari Pandya, Susan Gawel, Hita Moudgalya, Akanksha Sharma, Christopher W. Seder, Michael J. Liptay, and et al. 2025. "Exploratory Algorithms to Aid in Risk of Malignancy Prediction for Indeterminate Pulmonary Nodules" Cancers 17, no. 7: 1231. https://doi.org/10.3390/cancers17071231
APA StyleJackson, L., Auger, C., Jeanblanc, N., Jacobson, C., Pandya, K., Gawel, S., Moudgalya, H., Sharma, A., Seder, C. W., Liptay, M. J., Gaddikeri, R., Geissen, N. M., Shah, P., Borgia, J. A., & Davis, G. J. (2025). Exploratory Algorithms to Aid in Risk of Malignancy Prediction for Indeterminate Pulmonary Nodules. Cancers, 17(7), 1231. https://doi.org/10.3390/cancers17071231







