Development of a miRNA-Based Model for Lung Cancer Detection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. miRNA Selection
2.3. Sample Processing and miRNA Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
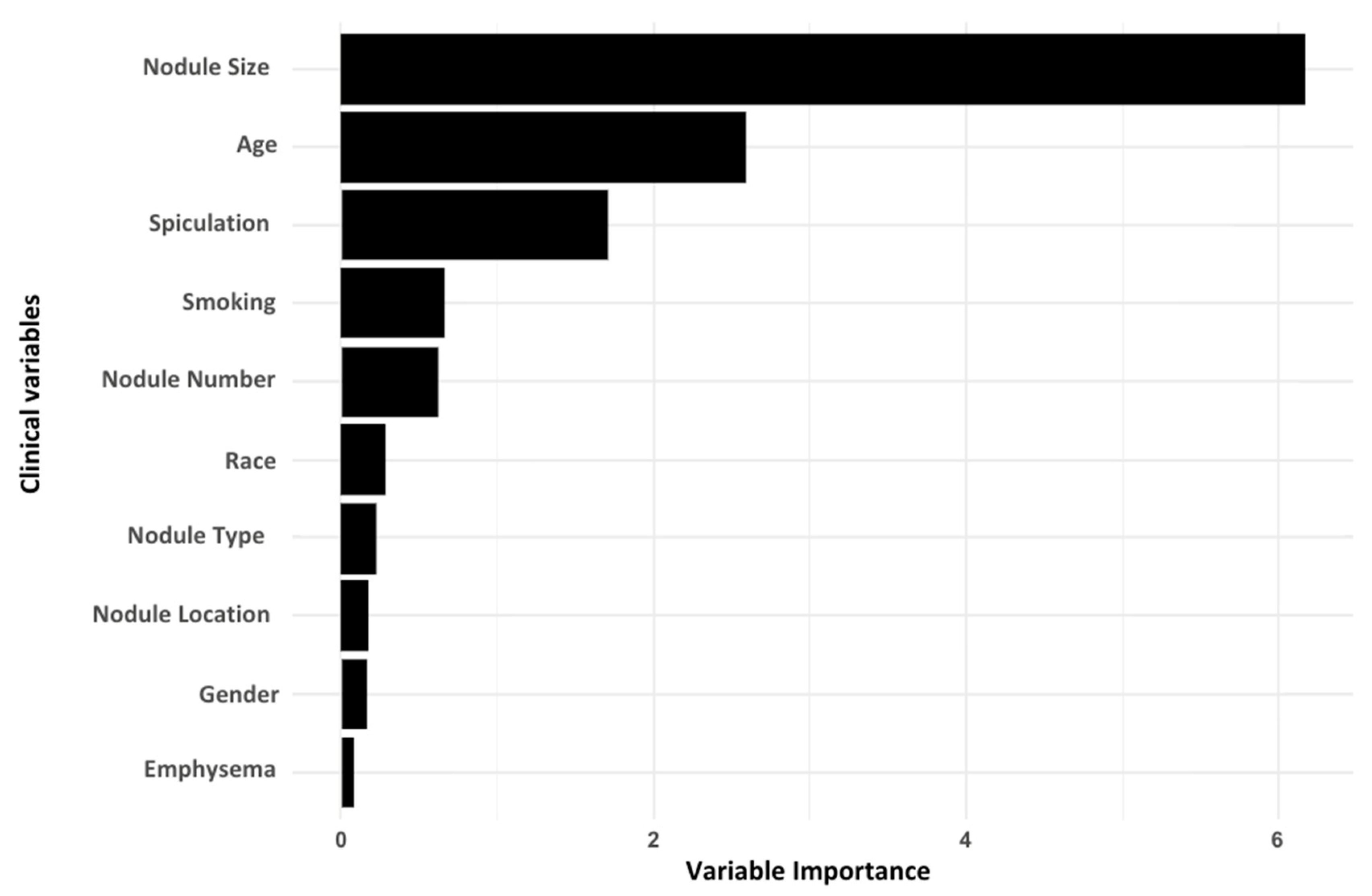
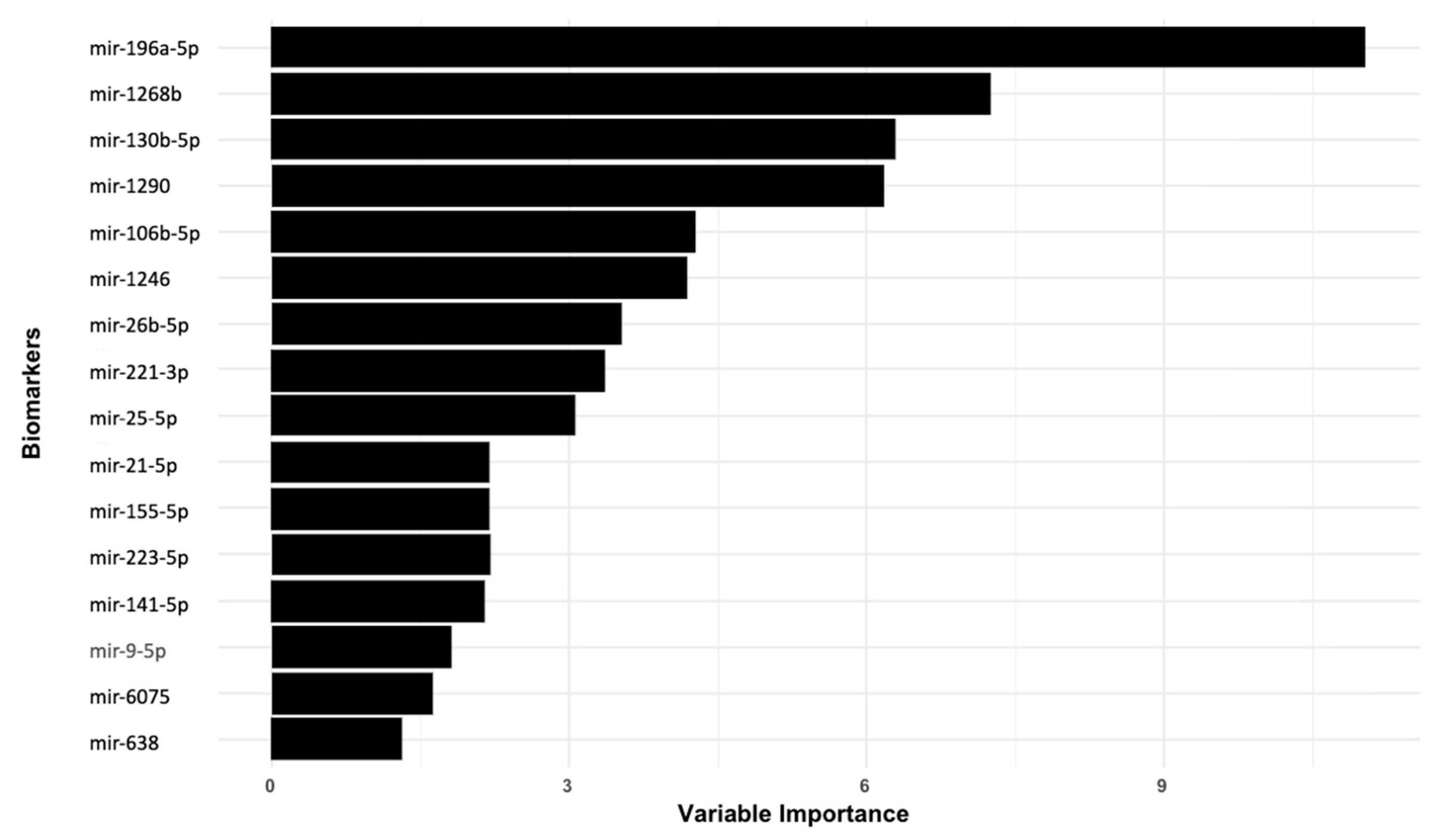
3.2. Identifying Top Clinical Features and miRNA Biomarkers for Lung Cancer Detection
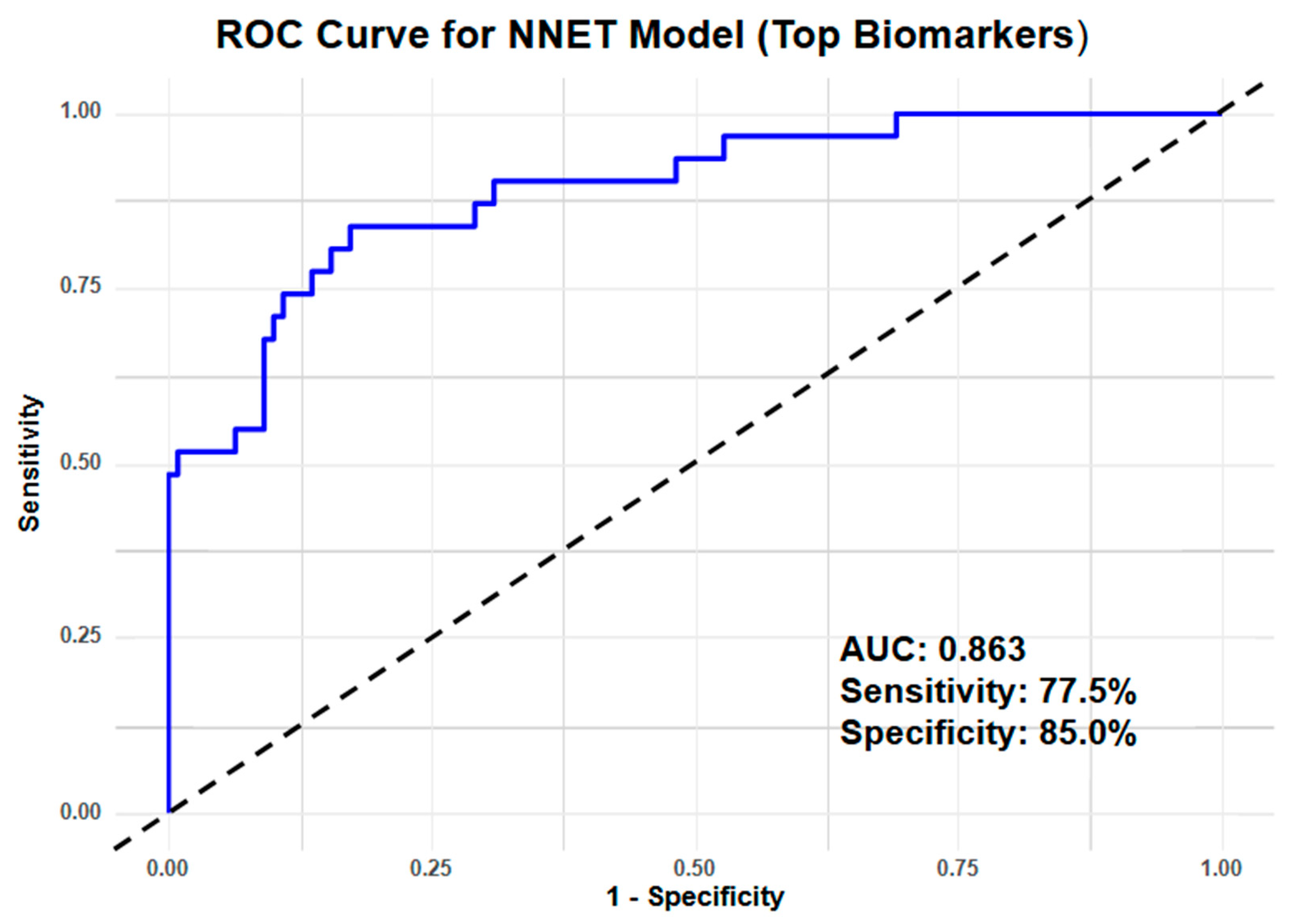
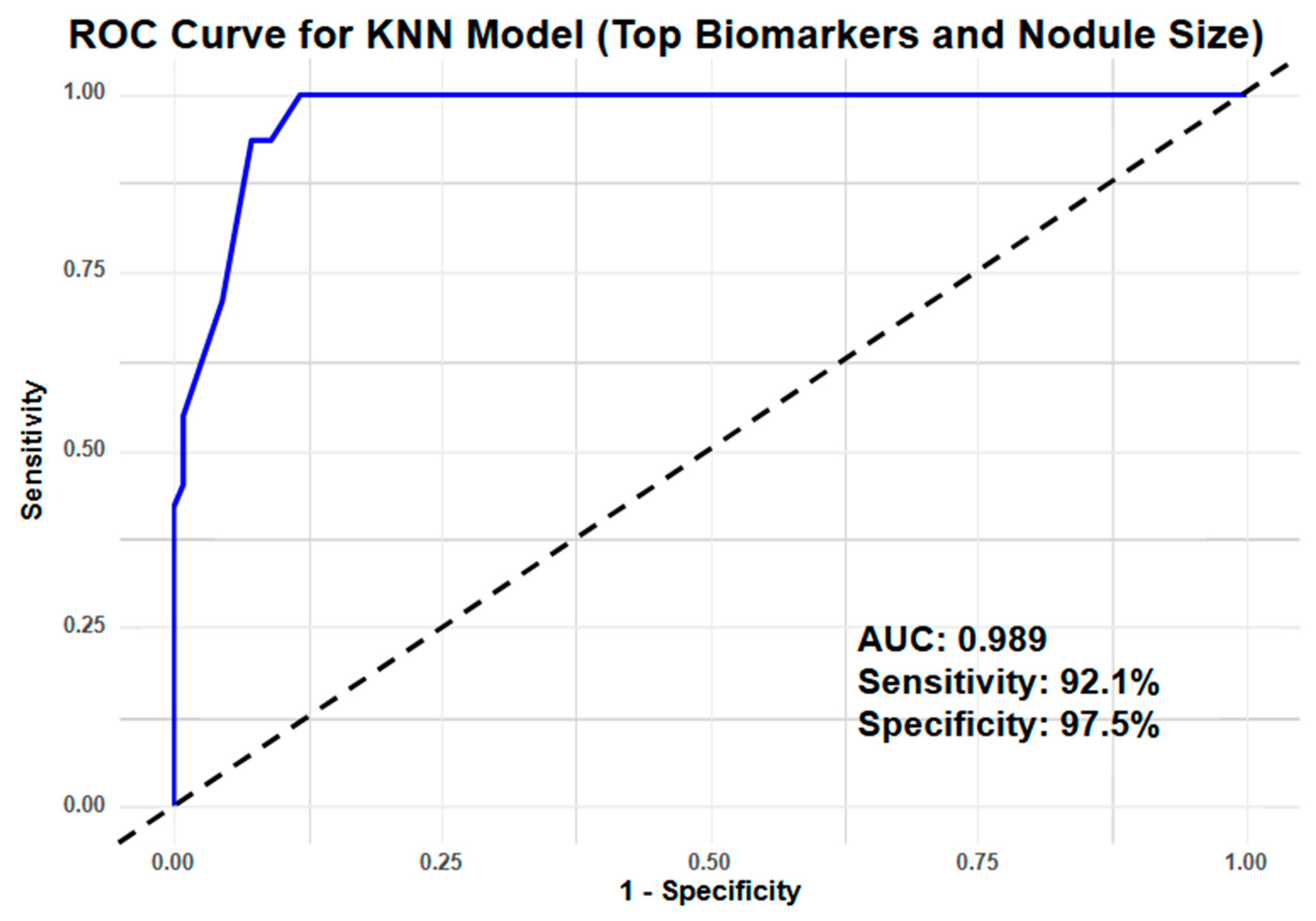
3.3. Clinical Performance Index
4. Discussion
4.1. Synergistic Role of a miRNA Panel in Lung Cancer Detection
4.2. Limitations of Existing Lung Cancer Screening Strategy: Identifying Risk Amongst Non-Smokers
4.3. Challenges in Management of Screen-Detected Lung Nodules
4.4. Improving Lung Cancer Screening with miRNA-Based Prediction Models
4.5. Study Strength and Limitations
4.6. Challenges and Future Directions for miRNA-Based Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1—Positive, Advanced Non—Small-Cell Lung Cancer in the KEYNOTE-010 Study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest. Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance Epidemiology, and End Results Program (SEER). Cancer Stat Facts: Lung and Bronchus Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 11 April 2023).
- Chang, G.C.; Chiu, C.H.; Yu, C.J.; Chang, Y.C.; Chang, Y.H.; Hsu, K.H.; Wu, Y.C.; Chen, C.Y.; Hsu, H.H.; Wu, M.T.; et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: A prospective cohort study. Lancet Respir. Med. 2024, 12, 141–152. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lin, Y.T.; Lin, L.J.; Chang, Y.H.; Chen, H.Y.; Wang, Y.P.; Shih, J.Y.; Yu, C.J.; Yang, P.C. Stage Shift Improves Lung Cancer Survival: Real-World Evidence. J. Thorac. Oncol. 2023, 18, 47–56. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.-L.; Liam, C.-K.; Andarini, S.; Park, S.; Tan, D.S.W.; Singh, N.; Jang, S.H.; Vardhanabhuti, V.; Ramos, A.B.; Nakayama, T.; et al. Lung Cancer Screening in Asia: An Expert Consensus Report. J. Thorac. Oncol. 2023, 18, 1303–1322. [Google Scholar] [CrossRef]
- Wait, S.; Alvarez-Rosete, A.; Osama, T.; Bancroft, D.; Cornelissen, R.; Marusic, A.; Garrido, P.; Adamek, M.; van Meerbeeck, J.; Snoeckx, A.; et al. Implementing Lung Cancer Screening in Europe: Taking a Systems Approach. JTO Clin. Res. Rep. 2022, 3, 100329. [Google Scholar] [CrossRef]
- van Meerbeeck, J.P.; Franck, C. Lung cancer screening in Europe: Where are we in 2021? Transl Lung Cancer Res. 2021, 10, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; Wilsdon, T.; Sarwar, I.; Roediger, A.; Yuan, M. Why is the screening rate in lung cancer still low? A seven-country analysis of the factors affecting adoption. Front. Public Health 2023, 11, 1264342. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Behar Harpaz, S.; Weber, M.F.; Wade, S.; Ngo, P.J.; Vaneckova, P.; Sarich, P.E.A.; Cressman, S.; Tammemagi, M.C.; Fong, K.; Marshall, H.; et al. Updated cost-effectiveness analysis of lung cancer screening for Australia, capturing differences in the health economic impact of NELSON and NLST outcomes. Br. J. Cancer 2023, 128, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Watson, T.R.; Criss, S.D.; Eckel, A.; Palazzo, L.; Sheehan, D.F.; Kong, C.Y. A simulation study of the effect of lung cancer screening in China, Japan, Singapore, and South Korea. PLoS ONE 2019, 14, e0220610. [Google Scholar] [CrossRef]
- Freitas, C.; Sousa, C.; Machado, F.; Serino, M.; Santos, V.; Cruz-Martins, N.; Teixeira, A.; Cunha, A.; Pereira, T.; Oliveira, H.P.; et al. The Role of Liquid Biopsy in Early Diagnosis of Lung Cancer. Front. Oncol. 2021, 11, 634316. [Google Scholar] [CrossRef] [PubMed]
- Bestvina, C.M.; Garassino, M.C.; Neal, J.W.; Wakelee, H.A.; Diehn, M.; Vokes, E.E. Early-Stage Lung Cancer: Using Circulating Tumor DNA to Get Personal. J. Clin. Oncol. 2023, 41, 4093–4096. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H., 3rd; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-based tests for multicancer early detection (PATHFINDER): A prospective cohort study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Poh, J.; Ngeow, K.C.; Pek, M.; Tan, K.H.; Lim, J.S.; Chen, H.; Ong, C.K.; Lim, J.Q.; Lim, S.T.; Lim, C.M.; et al. Analytical and clinical validation of an amplicon-based next generation sequencing assay for ultrasensitive detection of circulating tumor DNA. PLoS ONE 2022, 17, e0267389. [Google Scholar] [CrossRef]
- Marinello, A.; Tagliamento, M.; Pagliaro, A.; Conci, N.; Cella, E.; Vasseur, D.; Remon, J.; Levy, A.; Dall′Olio, F.G.; Besse, B. Circulating tumor DNA to guide diagnosis and treatment of localized and locally advanced non-small cell lung cancer. Cancer Treat. Rev. 2024, 129, 102791. [Google Scholar] [CrossRef]
- Bittla, P.; Kaur, S.; Sojitra, V.; Zahra, A.; Hutchinson, J.; Folawemi, O.; Khan, S. Exploring Circulating Tumor DNA (CtDNA) and Its Role in Early Detection of Cancer: A Systematic Review. Cureus 2023, 15, e45784. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.F.K.; Unis, G.D.; Li, L.Z.; Gunn, S.; Li, L.; Soyer, H.P.; Stark, M.S. Circulating Biomarkers for Early Stage Non-Small Cell Lung Carcinoma Detection: Supplementation to Low-Dose Computed Tomography. Front. Oncol. 2021, 11, 555331. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef]
- Li, R.Y.; Liang, Z.Y. Circulating tumor DNA in lung cancer: Real-time monitoring of disease evolution and treatment response. Chin. Med. J. 2020, 133, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. MicroRNAs in cancer: Small molecules with a huge impact. J. Clin. Oncol. 2009, 27, 5848–5856. [Google Scholar] [CrossRef]
- Nana-Sinkam, S.P.; Croce, C.M. MicroRNAs as therapeutic targets in cancer. Transl. Res. 2011, 157, 216–225. [Google Scholar] [CrossRef]
- Wu, K.-L.; Tsai, Y.-M.; Lien, C.-T.; Kuo, P.-L.; Hung, J.-Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [PubMed]
- Carrà, G.; Petiti, J.; Tolino, F.; Vacca, R.; Orso, F. MicroRNAs in metabolism for precision treatment of lung cancer. Cell. Mol. Biol. Lett. 2024, 29, 121. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liang, D.; Deubler, E.L.; Sarnat, J.A.; Chow, S.S.; Diver, W.R.; Wang, Y. Lung cancer metabolomics: A pooled analysis in the Cancer Prevention Studies. BMC Med. 2024, 22, 262. [Google Scholar] [CrossRef]
- Madama, D.; Martins, R.; Pires, A.S.; Botelho, M.F.; Alves, M.G.; Abrantes, A.M.; Cordeiro, C.R. Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabolites 2021, 11, 630. [Google Scholar] [CrossRef]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Zhong, J.; Lin, J.; Reddy, R.M.; Ramnath, N.; Orringer, M.B.; Chang, A.C.; Beer, D.G.; Chen, G. A MicroRNA Cluster at 14q32 Drives Aggressive Lung Adenocarcinoma. Clin. Cancer Res. 2014, 20, 3107–3117. [Google Scholar] [CrossRef]
- Bianchi, F.; Nicassio, F.; Marzi, M.; Belloni, E.; Dall′olio, V.; Bernard, L.; Pelosi, G.; Maisonneuve, P.; Veronesi, G.; Di Fiore, P.P. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol. Med. 2011, 3, 495–503. [Google Scholar] [CrossRef]
- Ying, L.; Du, L.; Zou, R.; Shi, L.; Zhang, N.; Jin, J.; Xu, C.; Zhang, F.; Zhu, C.; Wu, J.; et al. Development of a serum miRNA panel for detection of early stage non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Montani, F.; Marzi, M.J.; Dezi, F.; Dama, E.; Carletti, R.M.; Bonizzi, G.; Bertolotti, R.; Bellomi, M.; Rampinelli, C.; Maisonneuve, P.; et al. miR-Test: A Blood Test for Lung Cancer Early Detection. J. Natl. Cancer Inst. 2015, 107, djv063. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Lu, K.H.; Wang, K.M.; Sun, M.; Zhang, E.B.; Yang, J.S.; Yin, D.D.; Liu, Z.L.; Zhou, J.; Liu, Z.J.; et al. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer 2012, 12, 348. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, W.; Huang, F.; Tang, J.; Lin, X. Downregulation of microRNA-196a inhibits stem cell self-renewal ability and stemness in non-small-cell lung cancer through upregulating GPX3 expression. Int. J. Biochem. Cell Biol. 2019, 115, 105571. [Google Scholar] [CrossRef]
- Guerriero, I.; D′Angelo, D.; Pallante, P.; Santos, M.; Scrima, M.; Malanga, D.; De Marco, C.; Ravo, M.; Weisz, A.; Laudanna, C.; et al. Correction: Analysis of miRNA profiles identified miR-196a as a crucial mediator of aberrant PI3K/AKT signaling in lung cancer cells. Oncotarget 2022, 13, 755. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Ghule, S.S.; Liaw, C.-C.; Achudhan, D.; Fang, S.-Y.; Liu, P.-I.; Huang, C.-L.; Hsieh, C.-L.; Tang, C.-H. Ugonin P inhibits lung cancer motility by suppressing DPP-4 expression via promoting the synthesis of miR-130b-5p. Biomed. Pharmacother. 2023, 167, 115483. [Google Scholar] [CrossRef]
- Zhang, W.C.; Chin, T.M.; Yang, H.; Nga, M.E.; Lunny, D.P.; Lim, E.K.H.; Sun, L.L.; Pang, Y.H.; Leow, Y.N.; Malusay, S.R.Y.; et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat. Commun. 2016, 7, 11702. [Google Scholar] [CrossRef]
- Wei, K.; Pan, C.; Yao, G.; Liu, B.; Ma, T.; Xia, Y.; Jiang, W.; Chen, L.; Chen, Y. MiR-106b-5p Promotes Proliferation and Inhibits Apoptosis by Regulating BTG3 in Non-Small Cell Lung Cancer. Cell Physiol. Biochem. 2017, 44, 1545–1558. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Bang, S.; Jee, S.; Jang, K. MicroRNA-130b functions as an oncogene and is a predictive marker of poor prognosis in lung adenocarcinoma. Lab. Investig. 2021, 101, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; An, H.J.; Lee, M.J.; Song, J.Y.; Jeong, J.Y.; Lee, J.H.; Jeong, H.C. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer 2016, 91, 15–22. [Google Scholar] [CrossRef] [PubMed]
- El-Aal, A.E.A.; Elshafei, A.; Ismail, M.Y.; El-Shafey, M.M. Identification of miR-106b-5p, miR-601, and miR-760 Expression and Their Clinical Values in Non-Small Cell Lung Cancer (NSCLC) Patients’ Serum. Pathol. Res. Pract. 2023, 248, 154663. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, L.; Wang, F.; Huang, Y.; Wang, J.; Zhao, S.; Qi, L.; Liu, L.; Liang, M.; Hou, D.; et al. Assessing the efficiency of eligibility criteria for low-dose computed tomography lung screening in China according to current guidelines. BMC Med. 2024, 22, 267. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.H.; Koh, P.W.; Ang, D.J.M.; Lee, W.C.; Chew, W.M.; Koh, J.M.K. Characteristics of Singapore lung cancer patients who miss out on lung cancer screening recommendations. Singap. Med. J. 2024, 65, 279–287. [Google Scholar] [CrossRef]
- Kakinuma, R.; Muramatsu, Y.; Asamura, H.; Watanabe, S.-i.; Kusumoto, M.; Tsuchida, T.; Kaneko, M.; Tsuta, K.; Maeshima, A.M.; Ishii, G.; et al. Low-dose CT lung cancer screening in never-smokers and smokers: Results of an eight-year observational study. Transl. Lung Cancer Res. 2020, 9, 10–22. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Lv, L.; Wang, X.; Kang, R.; Guo, X.; Wang, H.; Zheng, L.; Liu, H.; Guo, L.; et al. Risk-based lung cancer screening in heavy smokers: A benefit–harm and cost-effectiveness modeling study. BMC Med. 2024, 22, 73. [Google Scholar] [CrossRef]
- Ten Haaf, K.; Bastani, M.; Cao, P.; Jeon, J.; Toumazis, I.; Han, S.S.; Plevritis, S.K.; Blom, E.F.; Kong, C.Y.; Tammemagi, M.C.; et al. A Comparative Modeling Analysis of Risk-Based Lung Cancer Screening Strategies. J. Natl. Cancer Inst. 2020, 112, 466–479. [Google Scholar] [CrossRef]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef]
- Kovalchik, S.A.; Tammemagi, M.; Berg, C.D.; Caporaso, N.E.; Riley, T.L.; Korch, M.; Silvestri, G.A.; Chaturvedi, A.K.; Katki, H.A. Targeting of low-dose CT screening according to the risk of lung-cancer death. N. Engl. J. Med. 2013, 369, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.A.; Cheung, L.C.; Chaturvedi, A.K.; Baldwin, D.R.; Berg, C.D.; Katki, H.A. Management of Lung Cancer Screening Results Based on Individual Prediction of Current and Future Lung Cancer Risks. J. Thorac. Oncol. 2022, 17, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, T.; Bittoni, M.A.; Dann, R.A.; D′Souza, A.O.; Johnson, M.; Nagy, R.J.; Lanman, R.B.; Merritt, R.E.; Carbone, D.P. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin. Lung Cancer 2017, 18, e27–e34. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of Individuals with Pulmonary Nodules: When Is It Lung Cancer?: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143 (Suppl. S5), e93S–e120S. [Google Scholar] [CrossRef]
- Callister, M.E.J.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: Accredited by NICE. Thorax 2015, 70 (Suppl. S2), ii1. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Baldwin, D.R.; Brain, K.; Quaife, S. Participation in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1091–1098. [Google Scholar] [CrossRef]
- Heideman, B.E.; Kammer, M.N.; Paez, R.; Swanson, T.; Godfrey, C.M.; Low, S.-W.; Xiao, D.; Li, T.Z.; Richardson, J.R.; Knight, M.A.; et al. The Lung Cancer Prediction Model “Stress Test”: Assessment of Models’ Performance in a High-Risk Prospective Pulmonary Nodule Cohort. CHEST Pulm. 2024, 2, 100033. [Google Scholar] [CrossRef]
- Bai, C.; Choi, C.-M.; Chu, C.M.; Anantham, D.; Chung-man Ho, J.; Khan, A.Z.; Lee, J.-M.; Li, S.Y.; Saenghirunvattana, S.; Yim, A. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016, 150, 877–893. [Google Scholar] [CrossRef]
- Pastorino, U.; Boeri, M.; Sestini, S.; Sabia, F.; Milanese, G.; Silva, M.; Suatoni, P.; Verri, C.; Cantarutti, A.; Sverzellati, N.; et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann. Oncol. 2022, 33, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Zhu, W.Y.; He, J.Y.; Chen, D.D.; Huang, Y.Y.; Le, H.B.; Liu, X.G. miRNAs expression profiling to distinguish lung squamous-cell carcinoma from adenocarcinoma subtypes. J. Cancer Res. Clin. Oncol. 2012, 138, 1641–1650. [Google Scholar] [CrossRef]
- Geng, Q.; Fan, T.; Zhang, B.; Wang, W.; Xu, Y.; Hu, H. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir. Res. 2014, 15, 149. [Google Scholar] [CrossRef]
- Siddika, T.; Heinemann, I.U. Bringing MicroRNAs to Light: Methods for MicroRNA Quantification and Visualization in Live Cells. Front. Bioeng. Biotechnol. 2021, 8, 619583. [Google Scholar] [CrossRef]
- Karabegović, I.; Maas, S.C.E.; Shuai, Y.; Ikram, M.A.; Stricker, B.; Aerts, J.; Brusselle, G.; Lahousse, L.; Voortman, T.; Ghanbari, M. Smoking-related dysregulation of plasma circulating microRNAs: The Rotterdam study. Hum. Genom. 2023, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Qu, D. Early diagnostic and prognostic value of serum exosomal miR-1246 in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 1601–1607. [Google Scholar] [PubMed]
- Cordoba-Lanus, E.; Dominguez de-Barros, A.; Oliva, A.; Mayato, D.; Gonzalvo, F.; Remirez-Sanz, A.; Zulueta, J.J.; Celli, B.; Casanova, C. Circulating miR-206 and miR-1246 as Markers in the Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 12437. [Google Scholar] [CrossRef]
- Mo, D.; Gu, B.; Gong, X.; Wu, L.; Wang, H.; Jiang, Y.; Zhang, B.; Zhang, M.; Zhang, Y.; Xu, J.; et al. miR-1290 is a potential prognostic biomarker in non-small cell lung cancer. J. Thorac. Dis. 2015, 7, 1570–1579. [Google Scholar] [CrossRef]
- Sozzi, G.; Boeri, M.; Rossi, M.; Verri, C.; Suatoni, P.; Bravi, F.; Roz, L.; Conte, D.; Grassi, M.; Sverzellati, N.; et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: A correlative MILD trial study. J. Clin. Oncol. 2014, 32, 768–773. [Google Scholar] [CrossRef]
- Yin, G.; Zhang, B.; Li, J. miR-221-3p promotes the cell growth of non-small cell lung cancer by targeting p27. Mol. Med. Rep. 2019, 20, 604–612. [Google Scholar] [CrossRef]
- Dou, L.; Han, K.; Xiao, M.; Lv, F. miR-223-5p Suppresses Tumor Growth and Metastasis in Non-Small Cell Lung Cancer by Targeting E2F8. Oncol. Res. 2019, 27, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, F.; Shen, T.; Luo, Q.; Ding, Z.; Qian, L.; Huang, J. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncol. Lett. 2017, 13, 669–676. [Google Scholar] [CrossRef] [PubMed]
- D′Antona, P.; Cattoni, M.; Dominioni, L.; Poli, A.; Moretti, F.; Cinquetti, R.; Gini, E.; Daffrè, E.; Noonan, D.M.; Imperatori, A.; et al. Serum miR-223: A Validated Biomarker for Detection of Early-Stage Non–Small Cell Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1926–1933. [Google Scholar] [CrossRef]
- Asakura, K.; Kadota, T.; Matsuzaki, J.; Yoshida, Y.; Yamamoto, Y.; Nakagawa, K.; Takizawa, S.; Aoki, Y.; Nakamura, E.; Miura, J.; et al. A miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun. Biol. 2020, 3, 134. [Google Scholar] [CrossRef]
- Foss, K.M.; Sima, C.; Ugolini, D.; Neri, M.; Allen, K.E.; Weiss, G.J. miR-1254 and miR-574-5p: Serum-Based microRNA Biomarkers for Early-Stage Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Liao, X.; Tang, Q.; Ye, G.; Bin, X.; Wang, J.; Pang, Y.; Qi, G. MicroRNA-106b-5p inhibits growth and progression of lung adenocarcinoma cells by downregulating IGSF10. Aging 2021, 13, 18740–18756. [Google Scholar] [CrossRef]
- Wu, T.; Chen, W.; Liu, S.; Lu, H.; Wang, H.; Kong, D.; Huang, X.; Kong, Q.; Ning, Y.; Lu, Z. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett. 2014, 588, 2107–2114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Chen, H.; Wang, H. Silencing lncRNA DUXAP8 inhibits lung adenocarcinoma progression by targeting miR-26b-5p. Biosci. Rep. 2021, 41, BSR20200884. [Google Scholar] [CrossRef]
- Li, D.; Wei, Y.; Wang, D.; Gao, H.; Liu, K. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-κB/MMP-9/VEGF pathways. Biochem. Biophys. Res. Commun. 2016, 472, 465–470. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.; Wang, Y.; Meng, M.; Wang, K.; Zang, X.; Zhao, S.; Sun, X.; Cui, L.; Pan, L. MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget 2016, 7, 84508. [Google Scholar] [CrossRef]
- Lv, D.; Bi, Q.; Li, Y.; Deng, J.; Wu, N.; Hao, S.; Zhao, M. Long non-coding RNA MEG3 inhibits cell migration and invasion of non-small cell lung cancer cells by regulating the miR-21-5p/PTEN axis. Mol. Med. Rep. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Hirono, T.; Jingushi, K.; Nagata, T.; Sato, M.; Minami, K.; Aoki, M.; Takeda, A.H.; Umehara, T.; Egawa, H.; Nakatsuji, Y.; et al. MicroRNA-130b functions as an oncomiRNA in non-small cell lung cancer by targeting tissue inhibitor of metalloproteinase-2. Sci. Rep. 2019, 9, 6956. [Google Scholar] [CrossRef]
- Shao, C.; Yang, F.; Qin, Z.; Jing, X.; Shu, Y.; Shen, H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: A systematic review with meta-analysis. BMC Cancer 2019, 19, 1103. [Google Scholar] [CrossRef]
- Hetta, H.F.; Zahran, A.M.; Shafik, E.A.; El-Mahdy, R.I.; Mohamed, N.A.; Nabil, E.E.; Esmaeel, H.M.; Alkady, O.A.; Elkady, A.; Mohareb, D.A.; et al. Circulating miRNA-21 and miRNA-23a Expression Signature as Potential Biomarkers for Early Detection of Non-Small-Cell Lung Cancer. Microrna 2019, 8, 206–215. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Chang, W.A.; Lin, Y.S.; Pan, Y.C.; Tsai, P.H.; Wu, C.Y.; Kuo, P.L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.P.; Drigo, S.A.; Carvalho, R.F.; Lopez Lapa, R.M.; Felix, T.F.; Patel, D.; Cheng, D.; Pintilie, M.; Liu, G.; Tsao, M.S. Circulating miR-16-5p, miR-92a-3p, and miR-451a in Plasma from Lung Cancer Patients: Potential Application in Early Detection and a Regulatory Role in Tumorigenesis Pathways. Cancers 2020, 12, 2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.; Wang, W.; Ba, Y.; Ma, L.; Zhang, C.; Wang, C.; Ren, Z.; Zhao, Y.; Wu, S.; et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int. J. Cancer 2012, 130, 1620–1628. [Google Scholar] [CrossRef]
- Chen, W.; Li, X. MiR-222-3p Promotes Cell Proliferation and Inhibits Apoptosis by Targeting PUMA (BBC3) in Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820922558. [Google Scholar] [CrossRef]
- Kanaoka, R.; Iinuma, H.; Dejima, H.; Sakai, T.; Uehara, H.; Matsutani, N.; Kawamura, M. Usefulness of Plasma Exosomal MicroRNA-451a as a Noninvasive Biomarker for Early Prediction of Recurrence and Prognosis of Non-Small Cell Lung Cancer. Oncology 2018, 94, 311–323. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Ouyang, T.; Lu, N.; Lu, J.; Shen, Y.; Bai, Y.; Xie, X.; Ge, Q. MiR-486-5p Serves as a Good Biomarker in Nonsmall Cell Lung Cancer and Suppresses Cell Growth With the Involvement of a Target PIK3R1. Front. Genet. 2019, 10, 688. [Google Scholar] [CrossRef]
- Wu, T.; Hu, H.; Zhang, T.; Jiang, L.; Li, X.; Liu, S.; Zheng, C.; Yan, G.; Chen, W.; Ning, Y.; et al. miR-25 Promotes Cell Proliferation, Migration, and Invasion of Non-Small-Cell Lung Cancer by Targeting the LATS2/YAP Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 9719723. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Liu, C.; Zhang, X.; Wu, Y.; Diao, M.; Tan, S.; Huang, S.; Cheng, Y.; You, T. MicroRNA-21 as a diagnostic and prognostic biomarker of lung cancer: A systematic review and meta-analysis. Biosci. Rep. 2022, 42, BSR20211653. [Google Scholar] [CrossRef]
- Ma, F.; Xie, Y.; Lei, Y.; Kuang, Z.; Liu, X. The microRNA-130a-5p/RUNX2/STK32A network modulates tumor invasive and metastatic potential in non-small cell lung cancer. BMC Cancer 2020, 20, 580. [Google Scholar] [CrossRef]
- Chen, S.; Li, P.; Yang, R.; Cheng, R.; Zhang, F.; Wang, Y.; Chen, X.; Sun, Q.; Zang, W.; Du, Y.; et al. microRNA-30b inhibits cell invasion and migration through targeting collagen triple helix repeat containing 1 in non-small cell lung cancer. Cancer Cell Int. 2015, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, B.; He, M.; Kang, Y.; Zhang, G. MicroRNA biomarkers and their use in evaluating the prognosis of lung cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 16753–16761. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, X.; Li, X.; Zhang, H.; Wang, M.; Sun, W.; Han, X.; Sun, D. Transcriptional identification of potential biomarkers of lung adenocarcinoma. J. Shanghai Jiaotong Univ. (Med. Sci.) 2020, 40, 1598–1606. [Google Scholar]
- Yong-Ming, H.; Ai-Jun, J.; Xiao-Yue, X.; Jian-Wei, L.; Chen, Y.; Ye, C. miR-449a: A potential therapeutic agent for cancer. Anti-Cancer Drugs 2017, 28, 1067–1078. [Google Scholar] [CrossRef]
- Wang, F.; Lou, J.F.; Cao, Y.; Shi, X.H.; Wang, P.; Xu, J.; Xie, E.F.; Xu, T.; Sun, R.H.; Rao, J.Y.; et al. miR-638 is a new biomarker for outcome prediction of non-small cell lung cancer patients receiving chemotherapy. Exp. Mol. Med. 2015, 47, e162. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, Y.; Liu, B.; Wang, P.; Chen, Y. Downregulation of miR-638 promotes invasion and proliferation by regulating SOX2 and induces EMT in NSCLC. FEBS Lett. 2014, 588, 2238–2245. [Google Scholar] [CrossRef]
- Izzotti, A.; Coronel Vargas, G.; Pulliero, A.; Coco, S.; Vanni, I.; Colarossi, C.; Blanco, G.; Agodi, A.; Barchitta, M.; Maugeri, A.; et al. Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments. J. Pers. Med. 2021, 11, 182. [Google Scholar] [CrossRef]
- Dutkowska, A.; Szmyd, B.; Kaszkowiak, M.; Domańska-Senderowska, D.; Pastuszak-Lewandoska, D.; Brzeziańska-Lasota, E.; Kordiak, J.; Antczak, A. Expression of inflammatory interleukins and selected miRNAs in non-small cell lung cancer. Sci. Rep. 2021, 11, 5092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.X.; Duan, X.C.; Cui, Y.; Zhang, Y.; Gu, M.; Wang, Z.Y.; Li, W.Y. Clinical significance of miR-9-5p in NSCLC and its relationship with smoking. Front. Oncol. 2024, 14, 1376502. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Controls (N = 123) | Cases (N = 82) | p Value |
|---|---|---|---|
| Patient demographics | |||
| Age (mean ± SD) | 57.0 ± 14.2 | 66.5 ± 10.7 | <0.005 |
| Gender (%) | |||
| Male | 72 (58.5) | 54 (65.9) | 0.17 |
| Female | 50 (40.7) | 28 (34.1) | |
| Race (%) | |||
| Chinese | 95 (77.2) | 59 (72.0) | 0.23 |
| Malay | 14 (11.4) | 10 (12.2) | |
| Indian | 8 (6.5) | 4 (4.9) | |
| Others | 5 (4.1) | 9 (11.0) | |
| Missing values | |||
| Smoking history (%) | |||
| Never smoker | 89 (72.4) | 32 (39.0) | |
| Smoker/ex-smoker | 29 (23.6) | 42 (51.2) | <0.005 |
| Missing values | 4 (3.3) | 8 (9.8) | |
| Emphysema (%) | |||
| Yes | 9 (7.4) | 13 (15.9) | 0.018 |
| No | 110 (89.4) | 61 (74.4) | |
| Missing values | 4 (3.3) | 8 (9.8) | |
| Cancer stage at diagnosis (%) | |||
| 1 | N.A. | 22 (26.8) | N.A. |
| 2 | N.A. | 5 (6.1) | |
| 3 | N.A. | 8 (9.8) | |
| 4 | N.A. | 40 (48.8) | |
| Limited (for SCLC) | N.A. | 2 (2.4) | |
| Extensive (for SCLC) | N.A. | 5 (6.1) | |
| Nodule characteristics | |||
| Number of nodules | <0.005 | ||
| None | 75 (61.0) | 0 (0.0) | |
| Single | 29 (23.6) | 48 (58.5) | |
| Multiple (>1) | 19 (15.4) | 34 (41.5) | |
| Size (of the most suspicious/malignant nodule) in mm | 14.7 ± 24.9 | 39.7 ± 27.6 | <0.005 |
| Nodule type (%) | |||
| Ground glass opacity | 5 (4.1) | 4 (4.9) | <0.005 |
| Solid | 40 (32.5) | 64 (78.0) | |
| Part solid | 2 (1.6) | 6 (7.3) | |
| No nodule | 76 (61.8) | 0 (0.0) | |
| Spiculation (%) | |||
| Spiculated/lobulated | 3 (2.4) | 26 (31.7) | <0.005 |
| Not spiculated | 44 (35.8) | 48 (58.5) | |
| No nodule | 76 (61.8) | 0 (0.0) | |
| Missing values | 0 (0.0) | 8 (9.8) | |
| Location (%) | |||
| Upper lobe | 24 (19.5) | 40 (48.8) | <0.005 |
| Non-upper lobe | 23 (18.7) | 34 (41.5) | |
| No nodule | 76 (61.8) | 0 (0.0) | |
| Missing values | 0 (0.0) | 8 (9.8) | |
| Histology (%) | |||
| Adenocarcinoma | N.A. | 57 (69.5) | N.A. |
| Squamous cell carcinoma | N.A. | 11 (13.4) | |
| Small cell lung cancer | N.A. | 7 (8.5) | |
| Other malignancies | N.A. | 6 (7.3) | |
| Nodule biopsied, benign | 9 (7.3) | N.A. | |
| Nodule not biopsied | 38 (30.9) | N.A. | |
| No nodule | 75 (61.0) | 0 (0.0) |
| ML Method | Model 1 | Model 2 | |
|---|---|---|---|
| KNN | AUC | 0.781 | 0.989 |
| Sensitivity | 0.700 | 0.921 | |
| Specificity | 0.732 | 0.975 | |
| NNET | AUC | 0.863 | 0.961 |
| Sensitivity | 0.775 | 0.937 | |
| Specificity | 0.850 | 0.929 | |
| SVM | AUC | 0.802 | 0.983 |
| Sensitivity | 0.762 | 0.975 | |
| Specificity | 0.732 | 0.937 | |
| NB | AUC | 0.790 | 0.978 |
| Sensitivity | 0.712 | 0.912 | |
| Specificity | 0.787 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poh, K.C.; Ren, T.M.; Ling, G.L.; Goh, J.S.Y.; Rose, S.; Wong, A.; Mehta, S.S.; Goh, A.; Chong, P.-Y.; Cheng, S.W.; et al. Development of a miRNA-Based Model for Lung Cancer Detection. Cancers 2025, 17, 942. https://doi.org/10.3390/cancers17060942
Poh KC, Ren TM, Ling GL, Goh JSY, Rose S, Wong A, Mehta SS, Goh A, Chong P-Y, Cheng SW, et al. Development of a miRNA-Based Model for Lung Cancer Detection. Cancers. 2025; 17(6):942. https://doi.org/10.3390/cancers17060942
Chicago/Turabian StylePoh, Kai Chin, Toh Ming Ren, Goh Liuh Ling, John S Y Goh, Sarrah Rose, Alexa Wong, Sanhita S. Mehta, Amelia Goh, Pei-Yu Chong, Sim Wey Cheng, and et al. 2025. "Development of a miRNA-Based Model for Lung Cancer Detection" Cancers 17, no. 6: 942. https://doi.org/10.3390/cancers17060942
APA StylePoh, K. C., Ren, T. M., Ling, G. L., Goh, J. S. Y., Rose, S., Wong, A., Mehta, S. S., Goh, A., Chong, P.-Y., Cheng, S. W., Wang, S. S. Y., Saffari, S. E., Lim, D. W.-T., & Chia, N.-Y. (2025). Development of a miRNA-Based Model for Lung Cancer Detection. Cancers, 17(6), 942. https://doi.org/10.3390/cancers17060942






