5-Aminolevulinic Acid: A Novel Approach to Improving Radioresistance in Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Reagents and Equipment
2.3. Cytotoxicity of 5-ALA
2.4. Assessment of Radiosensitivity
2.5. Establishment of Radioresistant PCa Cells
2.6. Apoptosis Analysis
2.7. Western Blot Analysis
2.8. Evaluation of Mitochondrial ROS
2.9. Quantification of Intracellular PpIX Expression
2.10. Transfection of Small Interfering RNA (siRNA) ABCG2
2.11. Syngeneic Mouse Model with Radioresistant PCa
2.12. Immunohistochemistry
2.13. Patients
2.14. Statistical Analysis
3. Results
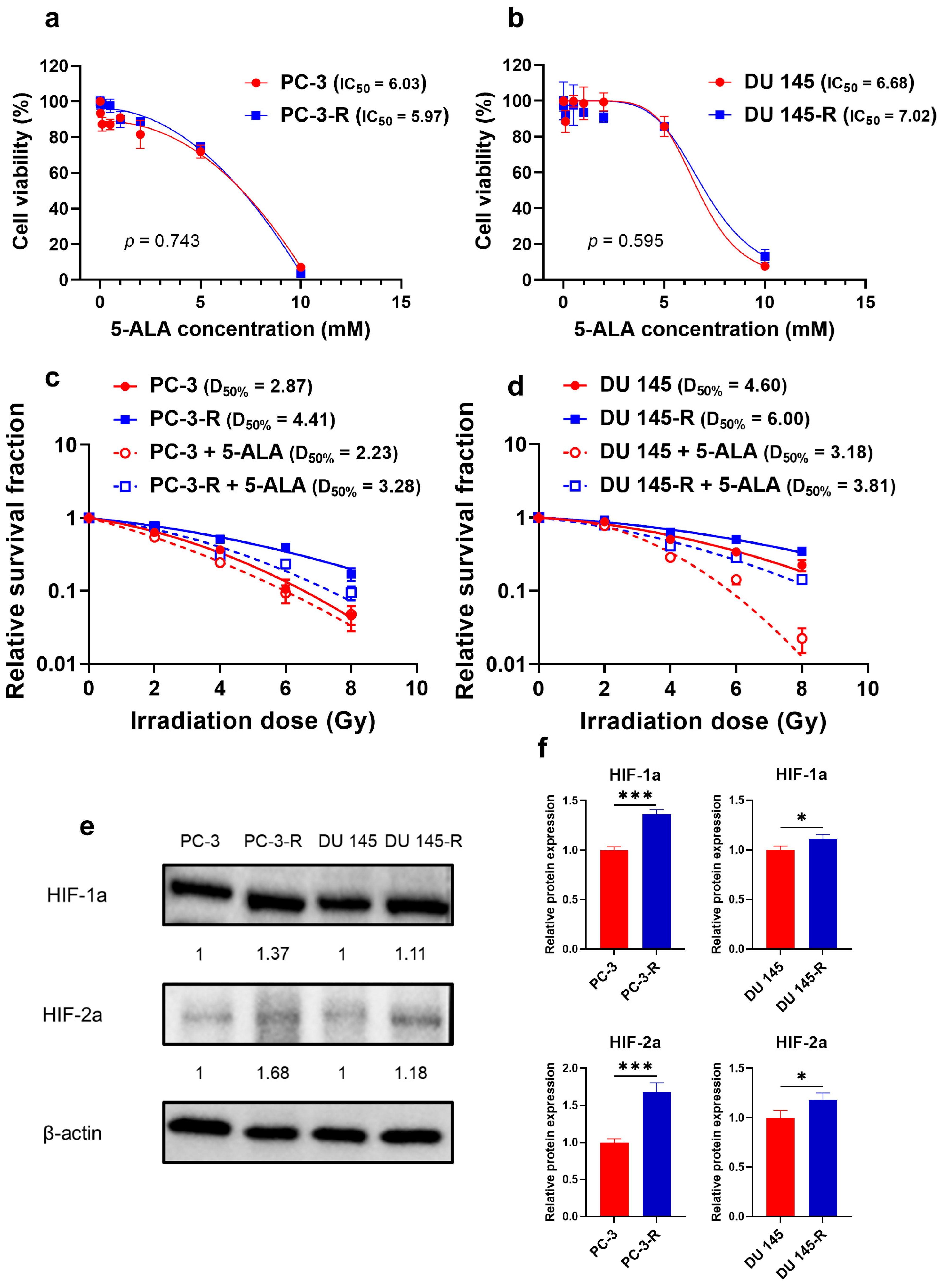
3.1. Cytotoxicity of 5-ALA Against Parental and Radioresistant PCa Cells
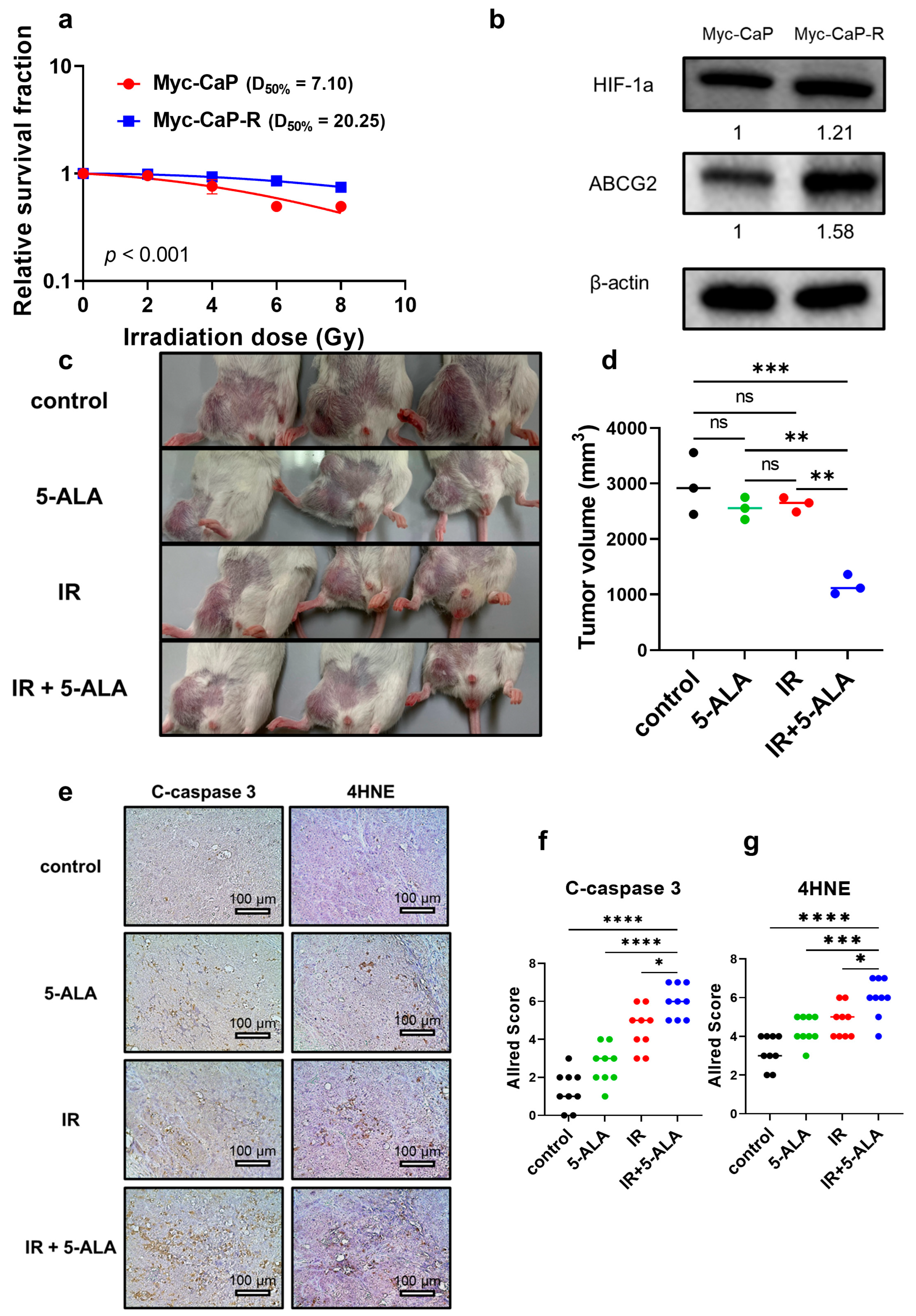
3.2. Establishment of Radioresistant PCa Cells
3.3. Radiosensitizing Effect of 5-ALA on Parental and Radioresistant PCa Cells
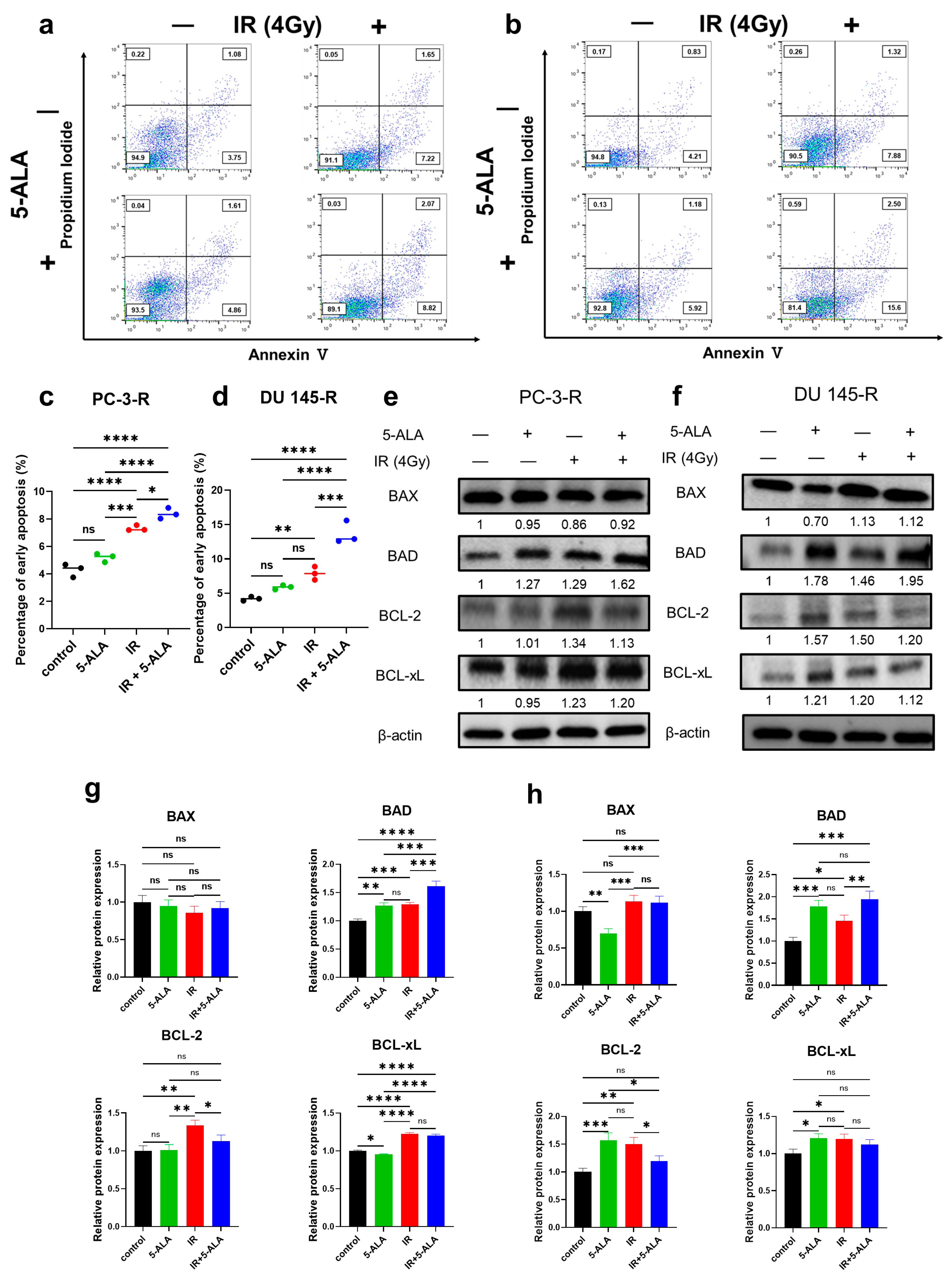
3.4. Effects of 5-ALA on Apoptosis After IR in Parental and Radioresistant PCa Cells
3.5. Effects of 5-ALA on the Expression of Apoptosis-Related Proteins After IR in Parental and Radioresistant PCa Cells
3.6. Difference in the Radiosensitizing Effect of 5-ALA Between Radioresistant PCa Cells and Parental PCa Cells
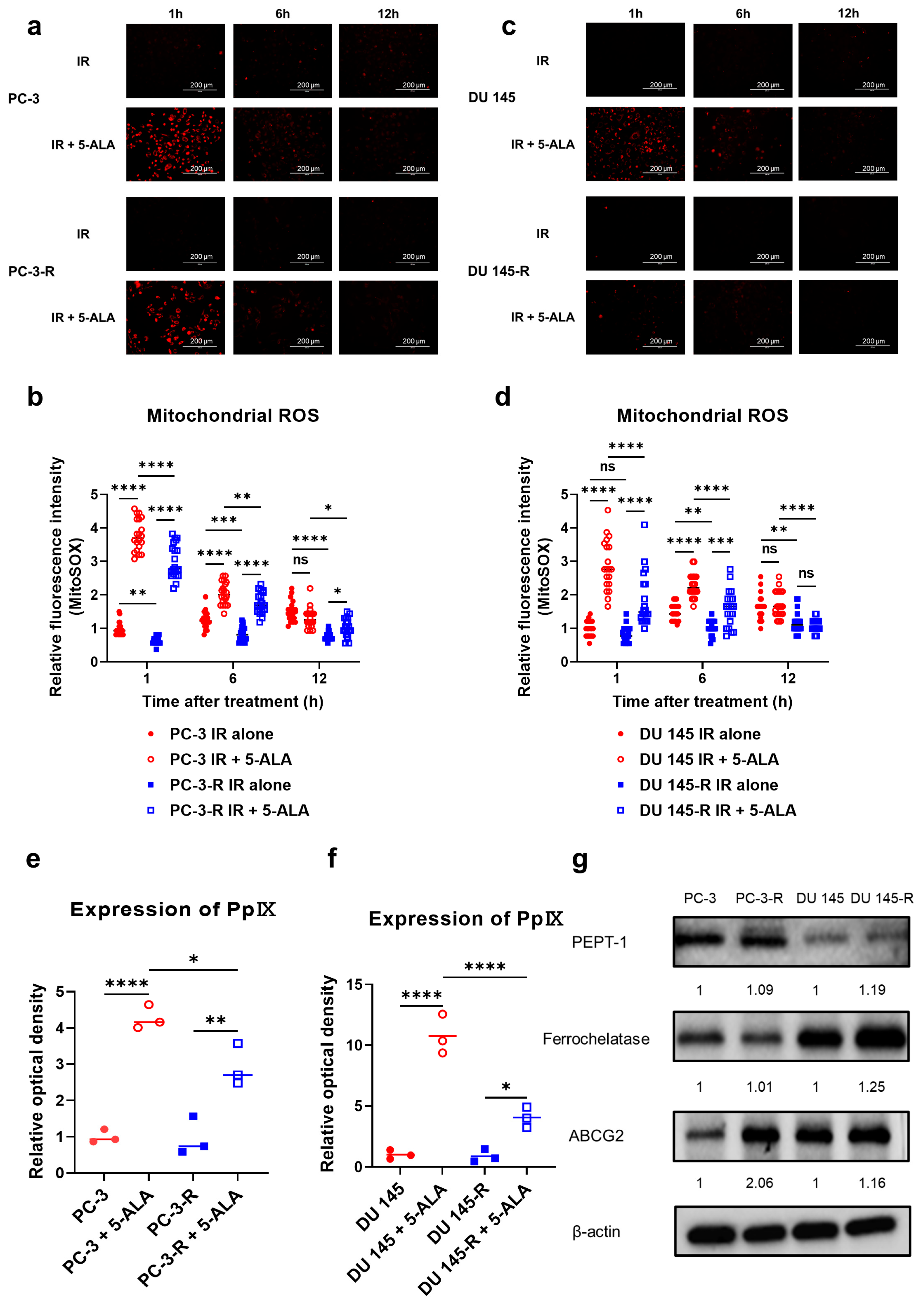
3.7. Effects of 5-ALA on Mitochondrial ROS in Parental and Radioresistant PCa Cells
3.8. Intracellular PpIX Accumulation After Administration of 5-ALA in Parental and Radioresistant PCa Cells
3.9. Expression of Proteins Metabolizing 5-ALA in Parental and Radioresistant PCa Cells
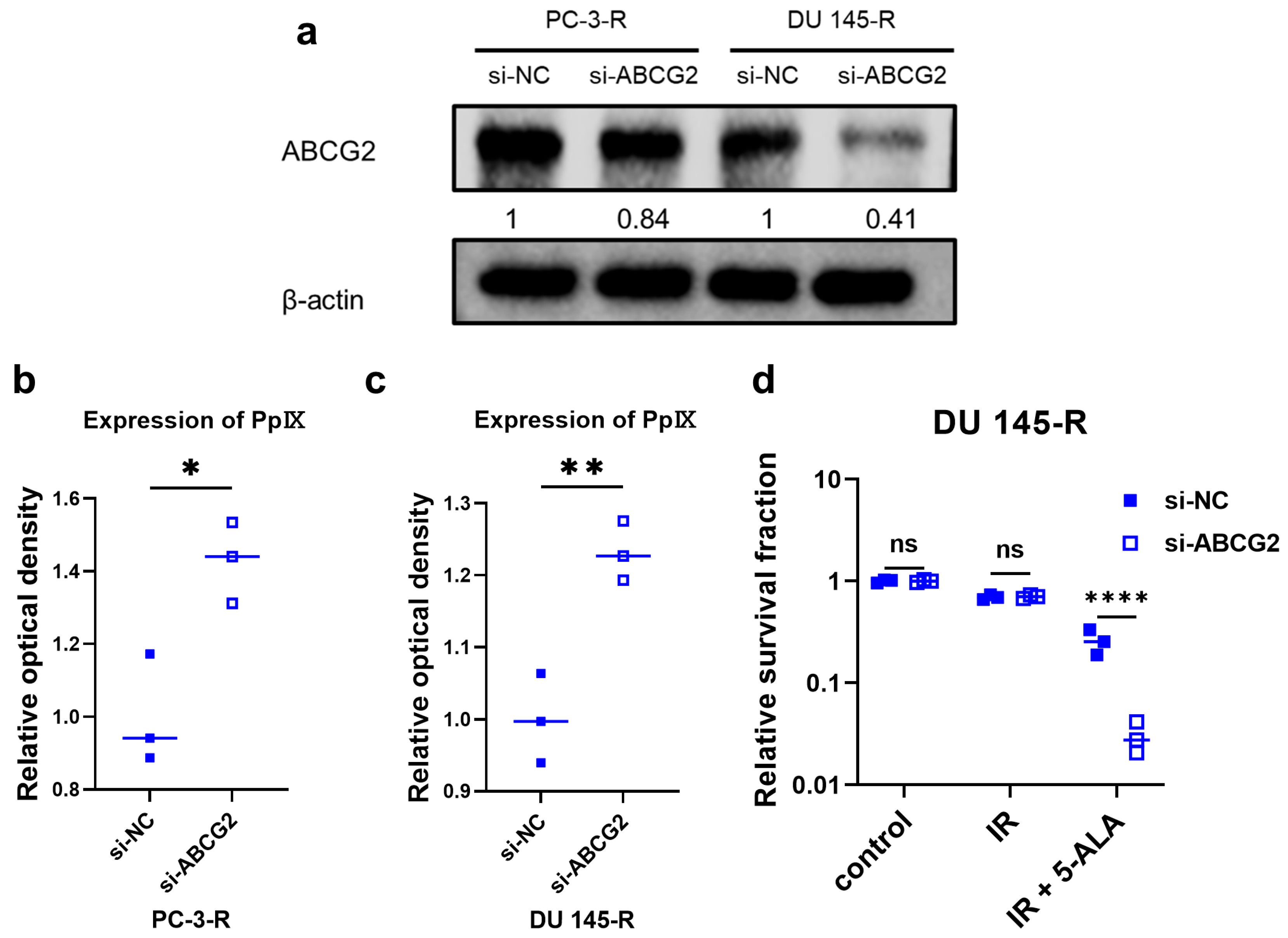
3.10. ABCG2 Knockdown Using siRNA in Radioresistant PCa Cells
3.11. Establishment of Radioresistant PCa Cells from Mice and Radiosensitizing Effects of 5-ALA in Syngeneic Mouse Models
3.12. HIF-1a and ABCG2 Expression in Human PCa Specimens with Recurrence After Primary RT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| 5-ALA | 5-Aminolevulinic acid |
| ABCG2 | ATP-binding cassette transporter subfamily G2 |
| BAD | BCL-2-associated agonist of cell death |
| BAX | BCL-2-associated X protein |
| BCL-xL | BCL extra-large |
| BCL-2 | B-cell/CLL lymphoma 2 |
| C-caspase 3 | Cleaved caspase 3 |
| D50% | Radiation dose required to achieve a 50% survival rate |
| HIF-1a | Hypoxia-inducible-factor 1a |
| IC50 | Half-maximal inhibitory concentration |
| IR | Irradiation |
| PCa | Prostate cancer |
| PDT | Photodynamic therapy |
| PpIX | Protoporphyrin IX |
| ROS | Reactive oxygen species |
| RT | Radiation therapy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Cennamo, M.; Bruzzese, D.; Crocetto, F.; Velotta, R.; Terracciano, D. A Combinatorial Neural Network Analysis Reveals a Synergistic Behaviour of Multiparametric Magnetic Resonance and Prostate Health Index in the Identification of Clinically Significant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, e406–e410. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Buonerba, L.; Ingenito, C.; Crocetto, F.; Buonerba, C.; Libroia, A.; Sciarra, A.; Ragone, G.; Sanseverino, R.; Iaccarino, S.; et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected with COVID-19 in South Italy. Oncology 2020, 98, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012, 109 (Suppl. S1), 22–29. [Google Scholar] [CrossRef]
- Merrick, G.S.; Butler, W.M.; Galbreath, R.W.; Lief, J.; Bittner, N.; Wallner, K.E.; Adamovich, E. Prostate cancer death is unlikely in high-risk patients following quality permanent interstitial brachytherapy. BJU Int. 2011, 107, 226–232. [Google Scholar] [CrossRef]
- Kuban, D.A.; Levy, L.B.; Cheung, M.R.; Lee, A.K.; Choi, S.; Frank, S.; Pollack, A. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1310–1317. [Google Scholar] [CrossRef]
- Deek, M.; Lilleby, W.; Vaage, V.; Hole, K.H.; DeWeese, T.; Stensvold, A.; Tran, P.; Seierstad, T. Impact of radiation dose on recurrence in high-risk prostate cancer patients. Prostate 2020, 80, 1322–1327. [Google Scholar] [CrossRef]
- Miyake, M.; Tanaka, N.; Asakawa, I.; Owari, T.; Hori, S.; Morizawa, Y.; Nakai, Y.; Inoue, T.; Anai, S.; Torimoto, K.; et al. The impact of the definition of biochemical recurrence following salvage radiotherapy on outcomes and prognostication in patients with recurrent prostate cancer after radical prostatectomy: A comparative study of three definitions. Prostate Int. 2019, 7, 47–53. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Spratt, D.E.; Romesser, P.B.; Pei, X.; Zhang, Z.; Kollmeier, M.; McBride, S.; Yamada, Y.; Zelefsky, M.J. Anatomical Patterns of Recurrence Following Biochemical Relapse in the Dose Escalation Era of External Beam Radiotherapy for Prostate Cancer. J. Urol. 2015, 194, 1624–1630. [Google Scholar] [CrossRef]
- Henderson, R.H.; Bryant, C.; Nichols, R.C., Jr.; Mendenhall, W.M.; Mendenhall, N.P. Local salvage of radiorecurrent prostate cancer. Prostate 2023, 83, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Macedo-Silva, C.; Benedetti, R.; Ciardiello, F.; Cappabianca, S.; Jeronimo, C.; Altucci, L. Epigenetic mechanisms underlying prostate cancer radioresistance. Clin. Epigenet. 2021, 13, 125. [Google Scholar] [CrossRef]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116. [Google Scholar] [CrossRef]
- Lee, D.E.; Alhallak, K.; Jenkins, S.V.; Vargas, I.; Greene, N.P.; Quinn, K.P.; Griffin, R.J.; Dings, R.P.M.; Rajaram, N. A Radiosensitizing Inhibitor of HIF-1 alters the Optical Redox State of Human Lung Cancer Cells In Vitro. Sci. Rep. 2018, 8, 8815. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, K.; Gao, L.; Zhang, B.; Li, W.; Yan, W.; Song, X.; Yu, H.; Wang, S.; Yu, N.; et al. Radiation induces the generation of cancer stem cells: A novel mechanism for cancer radioresistance. Oncol. Lett. 2016, 12, 3059–3065. [Google Scholar] [CrossRef]
- Keam, S.P.; Caramia, F.; Gamell, C.; Paul, P.J.; Arnau, G.M.; Neeson, P.J.; Williams, S.G.; Haupt, Y. The Transcriptional Landscape of Radiation-Treated Human Prostate Cancer: Analysis of a Prospective Tissue Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 188–198. [Google Scholar] [CrossRef]
- Gravina, G.L.; Marampon, F.; Sherris, D.; Vittorini, F.; Di Cesare, E.; Tombolini, V.; Lenzi, A.; Jannini, E.A.; Festuccia, C. Torc1/Torc2 inhibitor, Palomid 529, enhances radiation response modulating CRM1-mediated survivin function and delaying DNA repair in prostate cancer models. Prostate 2014, 74, 852–868. [Google Scholar] [CrossRef]
- Miyake, M.; Ishii, M.; Kawashima, K.; Kodama, T.; Sugano, K.; Fujimoto, K.; Hirao, Y. siRNA-mediated knockdown of the heme synthesis and degradation pathways: Modulation of treatment effect of 5-aminolevulinic acid-based photodynamic therapy in urothelial cancer cell lines. Photochem. Photobiol. 2009, 85, 1020–1027. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 1992, 14, 275–292. [Google Scholar] [CrossRef]
- Chan, K.M.; Gleadle, J.; Vasilev, K.; MacGregor, M. Probing Hexaminolevulinate Mediated PpIX Fluorescence in Cancer Cell Suspensions in the Presence of Chemical Adjuvants. Int. J. Mol. Sci. 2020, 21, 2963. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamamoto, J.; Toh, K.; Miyaoka, R. 5-aminiolevulinic acid induces a radiodynamic effect with enhanced delayed reactive oxygen species production under hypoxic conditions in lymphoma cells: An in vitro study. Exp. Ther. Med. 2023, 26, 360. [Google Scholar] [CrossRef] [PubMed]
- Pepper, N.B.; Eich, H.T.; Müther, M.; Oertel, M.; Rehn, S.; Spille, D.C.; Stummer, W. ALA-RDT in GBM: Protocol of the phase I/II dose escalation trial of radiodynamic therapy with 5-Aminolevulinic acid in patients with recurrent glioblastoma. Radiat. Oncol. 2024, 19, 11. [Google Scholar] [CrossRef]
- Owari, T.; Tanaka, N.; Nakai, Y.; Miyake, M.; Anai, S.; Kishi, S.; Mori, S.; Fujiwara-Tani, R.; Hojo, Y.; Mori, T.; et al. 5-Aminolevulinic acid overcomes hypoxia-induced radiation resistance by enhancing mitochondrial reactive oxygen species production in prostate cancer cells. Br. J. Cancer 2022, 127, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Tanaka, N.; Hori, S.; Ohnishi, S.; Takahashi, H.; Fujii, T.; Owari, T.; Ohnishi, K.; Iida, K.; Morizawa, Y.; et al. Dual benefit of supplementary oral 5-aminolevulinic acid to pelvic radiotherapy in a syngenic prostate cancer model. Prostate 2019, 79, 340–351. [Google Scholar] [CrossRef]
- Ikeda, T.; Kurokawa, H.; Ito, H.; Tsuchiya, K.; Matsui, H. Enhancement of cytotoxic effects with ALA-PDT on treatment of radioresistant cancer cells. J. Clin. Biochem. Nutr. 2024, 74, 17–21. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef]
- Watson, P.A.; Ellwood-Yen, K.; King, J.C.; Wongvipat, J.; Lebeau, M.M.; Sawyers, C.L. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005, 65, 11565–11571. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int. J. Mol. Sci. 2015, 16, 25865–25880. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525; discussion 525–526. [Google Scholar] [CrossRef]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Tomita, K.; Urushihara, Y.; Sato, T.; Kurimasa, A.; Fukumoto, M. Association between radiation-induced cell death and clinically relevant radioresistance. Histochem. Cell Biol. 2018, 150, 649–659. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Roudkenar, M.H.; Urushihara, Y.; Saito, Y.; Tomita, K.; Roushandeh, A.M.; Sato, T.; Kurimasa, A.; Fukumoto, M. Clinically relevant radioresistant cell line: A simple model to understand cancer radioresistance. Med. Mol. Morphol. 2017, 50, 195–204. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Li, L.; Baba, T.; Nakagawa, H.; Shimura, T.; Yamamoto, Y.; Ohkubo, Y.; Fukumoto, M. Clinically relevant radioresistant cells efficiently repair DNA double-strand breaks induced by X-rays. Cancer Sci. 2009, 100, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Tatsumi, Y.; Hori, S.; Morizawa, Y.; Iida, K.; Onishi, K.; Miyake, M.; Oda, Y.; Owari, T.; Fujii, T.; et al. 5-Aminolevurinic acid inhibits the proliferation of bladder cancer cells by activating heme synthesis. Oncol. Rep. 2022, 48, 186. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Nakai, Y.; Anai, S.; Tatsumi, Y.; Kuwada, M.; Onishi, S.; Chihara, Y.; Tanaka, N.; Hirao, Y.; Fujimoto, K. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014, 105, 616–622. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Yamamoto, D.; Sasaki, K.; Kosaka, T.; Oya, M.; Sato, T. Functional analysis of GCNT3 for cell migration and EMT of castration-resistant prostate cancer cells. Glycobiology 2022, 32, 897–908. [Google Scholar] [CrossRef]
- Geng, H.; Xue, C.; Mendonca, J.; Sun, X.X.; Liu, Q.; Reardon, P.N.; Chen, Y.; Qian, K.; Hua, V.; Chen, A.; et al. Interplay between hypoxia and androgen controls a metabolic switch conferring resistance to androgen/AR-targeted therapy. Nat. Commun. 2018, 9, 4972. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Lee, W.C.; Ong, L.T.; Lee, P.L.; Jiang, Z.; Oguz, G.; Niu, Z.; Liu, M.; Goh, J.Y.; et al. Hypoxia induces HIF1α-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat. Commun. 2022, 13, 4118. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, C.; Silva, P.; Torres, V.A. Role of glycosylation in hypoxia-driven cell migration and invasion. Cell Adhes. Migr. 2019, 13, 13–22. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Wilkins, A.; Gusterson, B.; Tovey, H.; Griffin, C.; Stuttle, C.; Daley, F.; Corbishley, C.M.; Dearnaley, D.; Hall, E.; Somaiah, N. Multi-candidate immunohistochemical markers to assess radiation response and prognosis in prostate cancer: Results from the CHHiP trial of radiotherapy fractionation. EBioMedicine 2023, 88, 104436. [Google Scholar] [CrossRef]
- Terada, Y.; Inoue, K.; Matsumoto, T.; Ishihara, M.; Hamada, K.; Shimamura, Y.; Ogata, K.; Inoue, K.; Taniguchi, Y.; Horino, T.; et al. 5-Aminolevulinic acid protects against cisplatin-induced nephrotoxicity without compromising the anticancer efficiency of cisplatin in rats in vitro and in vivo. PLoS ONE 2013, 8, e80850. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Li, F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J. Exp. Clin. Cancer Res. 2015, 34, 159. [Google Scholar] [CrossRef]
- Zhou, S.; Zong, Y.; Ney, P.A.; Nair, G.; Stewart, C.F.; Sorrentino, B.P. Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood 2005, 105, 2571–2576. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukuhara, H.; Seki, H.; Kawada, C.; Nakayama, T.; Karashima, T.; Ogura, S.I.; Inoue, K. Predictors of therapeutic efficacy of 5-aminolevulinic acid-based photodynamic therapy in human prostate cancer. Photodiagn. Photodyn. Ther. 2021, 35, 102452. [Google Scholar] [CrossRef]
- Mozner, O.; Bartos, Z.; Zambo, B.; Homolya, L.; Hegedus, T.; Sarkadi, B. Cellular Processing of the ABCG2 Transporter-Potential Effects on Gout and Drug Metabolism. Cells 2019, 8, 1215. [Google Scholar] [CrossRef] [PubMed]
- Samant, M.D.; Jackson, C.M.; Felix, C.L.; Jones, A.J.; Goodrich, D.W.; Foster, B.A.; Huss, W.J. Multi-Drug Resistance ABC Transporter Inhibition Enhances Murine Ventral Prostate Stem/Progenitor Cell Differentiation. Stem Cells Dev. 2015, 24, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Jin, S.; Pabon, K.; Scotto, K.W. A role for ABCG2 beyond drug transport: Regulation of autophagy. Autophagy 2016, 12, 737–751. [Google Scholar] [CrossRef]
- Ingram, W.J.; Crowther, L.M.; Little, E.B.; Freeman, R.; Harliwong, I.; Veleva, D.; Hassall, T.E.; Remke, M.; Taylor, M.D.; Hallahan, A.R. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp. Hematol. Oncol. 2013, 2, 26. [Google Scholar] [CrossRef]
- Gardner, E.R.; Smith, N.F.; Figg, W.D.; Sparreboom, A. Influence of the dual ABCB1 and ABCG2 inhibitor tariquidar on the disposition of oral imatinib in mice. J. Exp. Clin. Cancer Res. 2009, 28, 99. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, P.; Di Serafino, A.; Sorino, L.; Ballerini, P.; Marchisio, M.; Pierdomenico, L.; Stuppia, L.; Antonucci, I. Genetic and epigenetic modifications induced by chemotherapeutic drugs: Human amniotic fluid stem cells as an in-vitro model. BMC Med. Genom. 2019, 12, 146. [Google Scholar] [CrossRef]
- Tomita, K.; Nagasawa, T.; Kuwahara, Y.; Torii, S.; Igarashi, K.; Roudkenar, M.H.; Roushandeh, A.M.; Kurimasa, A.; Sato, T. MiR-7-5p Is Involved in Ferroptosis Signaling and Radioresistance Thru the Generation of ROS in Radioresistant HeLa and SAS Cell Lines. Int. J. Mol. Sci. 2021, 22, 8300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maesaka, F.; Nakai, Y.; Yoshida, T.; Tomizawa, M.; Shimizu, T.; Owari, T.; Onishi, K.; Miyake, M.; Kuniyasu, H.; Fujimoto, K.; et al. 5-Aminolevulinic Acid: A Novel Approach to Improving Radioresistance in Prostate Cancer. Cancers 2025, 17, 1286. https://doi.org/10.3390/cancers17081286
Maesaka F, Nakai Y, Yoshida T, Tomizawa M, Shimizu T, Owari T, Onishi K, Miyake M, Kuniyasu H, Fujimoto K, et al. 5-Aminolevulinic Acid: A Novel Approach to Improving Radioresistance in Prostate Cancer. Cancers. 2025; 17(8):1286. https://doi.org/10.3390/cancers17081286
Chicago/Turabian StyleMaesaka, Fumisato, Yasushi Nakai, Takanori Yoshida, Mitsuru Tomizawa, Takuto Shimizu, Takuya Owari, Kenta Onishi, Makito Miyake, Hiroki Kuniyasu, Kiyohide Fujimoto, and et al. 2025. "5-Aminolevulinic Acid: A Novel Approach to Improving Radioresistance in Prostate Cancer" Cancers 17, no. 8: 1286. https://doi.org/10.3390/cancers17081286
APA StyleMaesaka, F., Nakai, Y., Yoshida, T., Tomizawa, M., Shimizu, T., Owari, T., Onishi, K., Miyake, M., Kuniyasu, H., Fujimoto, K., & Tanaka, N. (2025). 5-Aminolevulinic Acid: A Novel Approach to Improving Radioresistance in Prostate Cancer. Cancers, 17(8), 1286. https://doi.org/10.3390/cancers17081286






