The Clinical Utility of Next-Generation Sequencing in Childhood and Adolescent/Young Adult Solid Tumors: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Pediatric Solid Tumors and Actionable Mutations
2.1. Clinical Utility of Precision Oncology in Pediatric Tumors
2.2. Need for Standardized Protocols
3. Methods
3.1. Study Design and Search Strategy
3.2. Eligibility Criteria
- Population: Pediatric and AYA patients (aged 0–40 years) diagnosed with solid tumors.
- Intervention: Next-generation sequencing (NGS) as part of genomic testing, precision medicine, or targeted therapy approaches.
- Outcome: Reported proportions of actionable mutations and/or decision-making based on NGS findings.
- Study Design: Original studies, including observational studies, clinical trials, and retrospective cohorts.
3.3. Screening and Data Extraction
- Study characteristics: Author, year, journal, trial name, study methods, location, and age.
- NGS metrics: Number of patients, samples, actionable mutation rates, decision-making rates, and germline mutation rates (when reported).
3.4. Data Analysis
3.5. Ethical Considerations
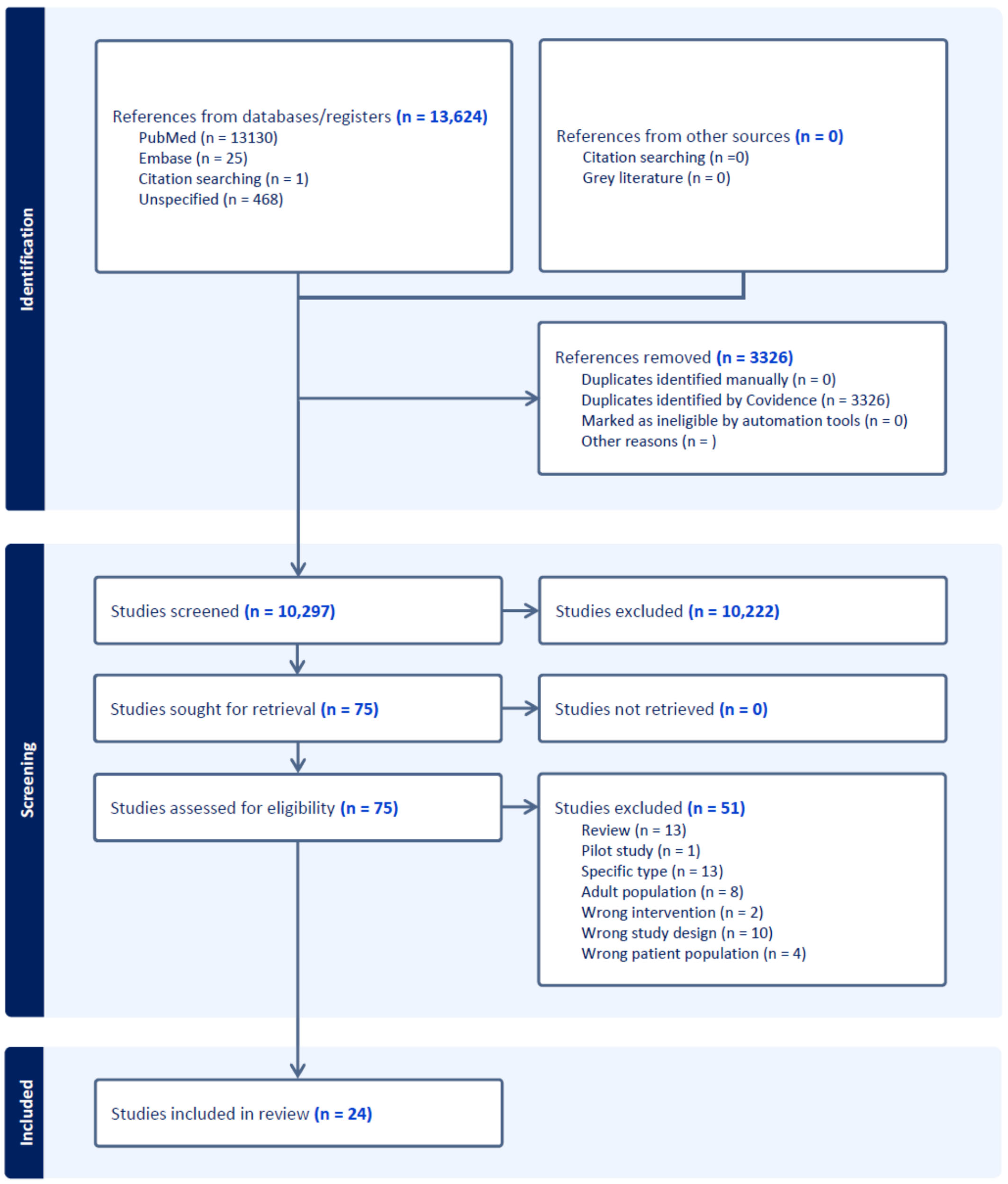
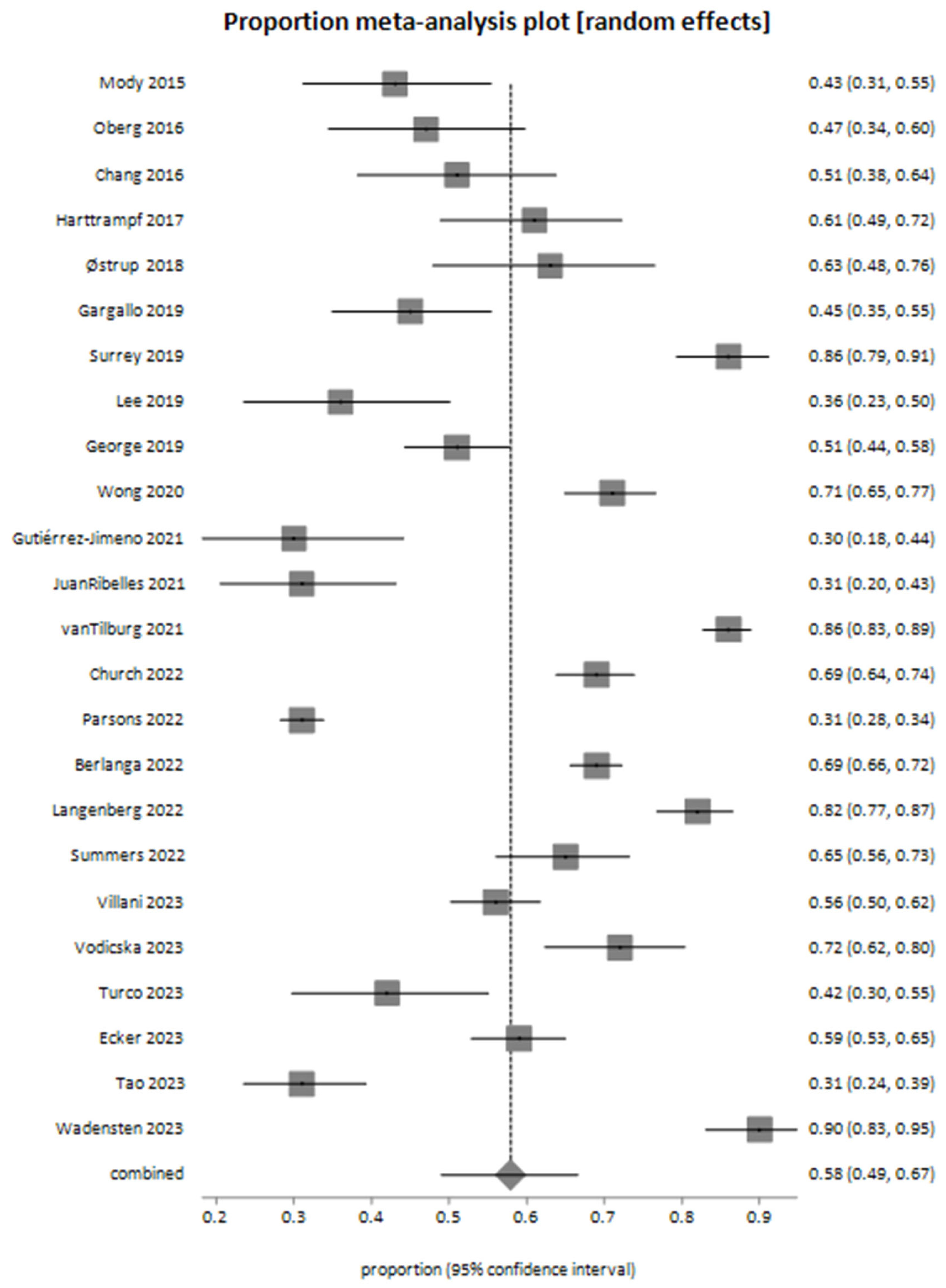
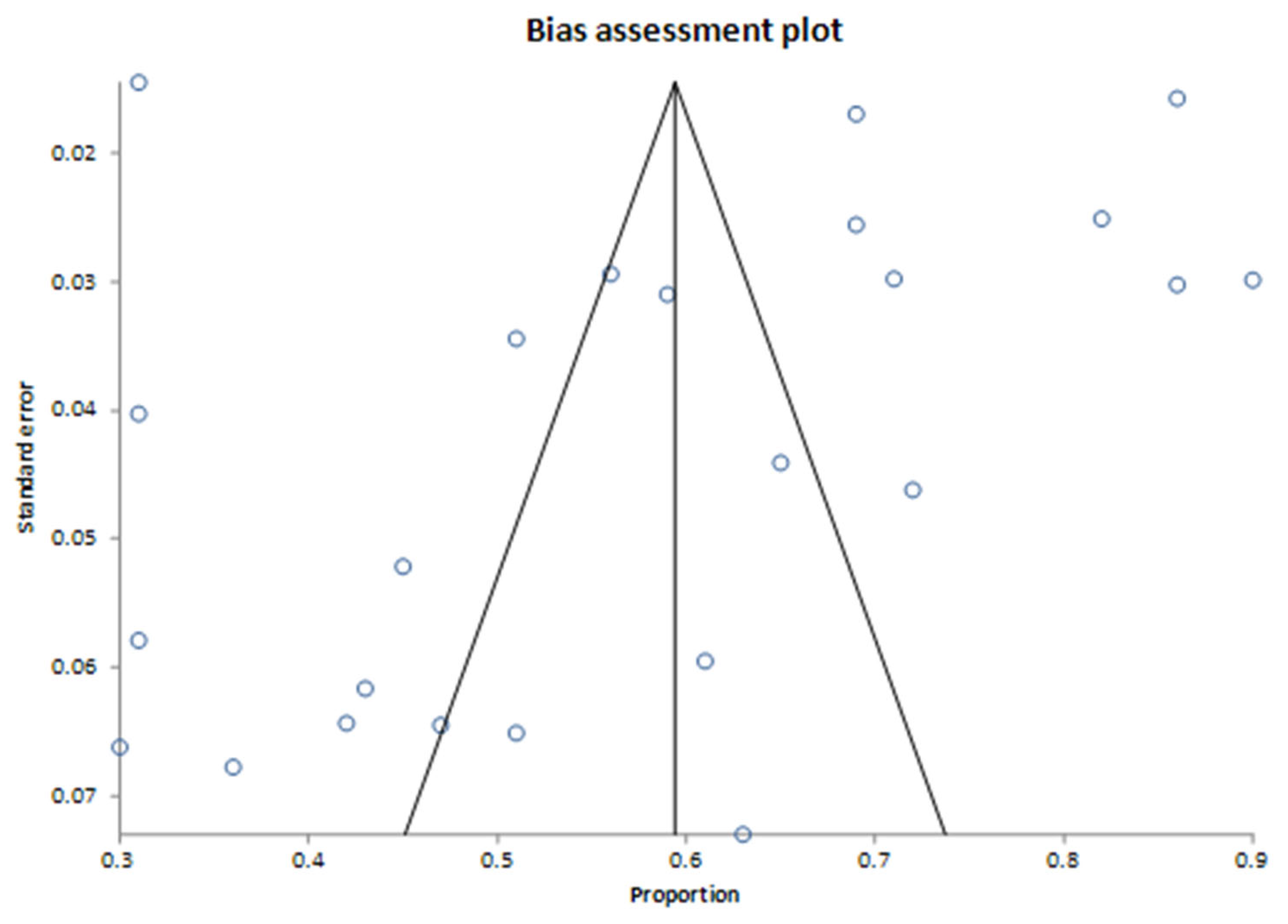
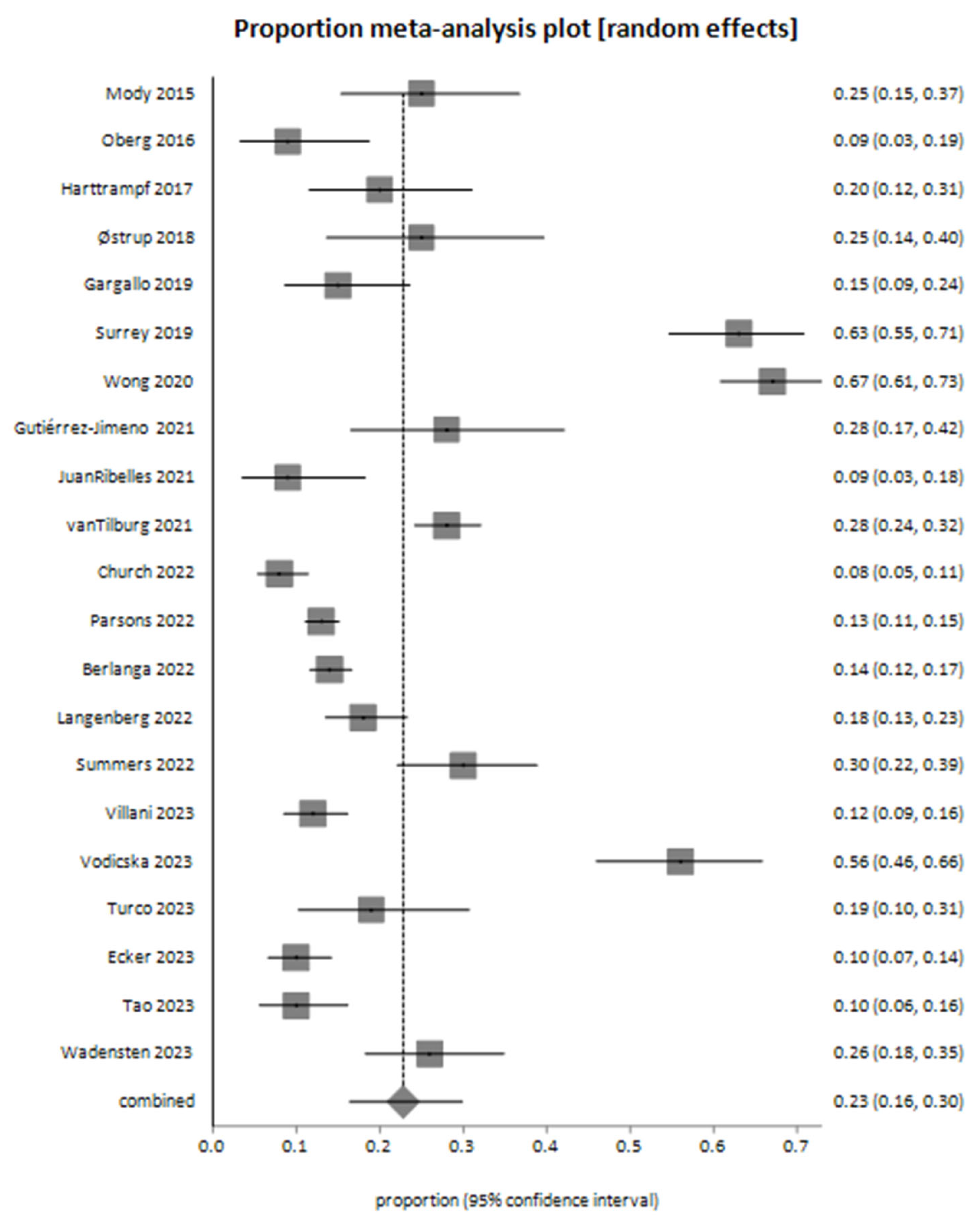
4. Results
Study Selection
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Summary of Studies Included in the Meta-Analysis
| Study ID | Trial Name | Journal | Year | Age Range | Median Age | Countries | Methods |
|---|---|---|---|---|---|---|---|
| Mody 2015 [26] | Peds-MiOncoSeq | JAMA | 2015 | 0–22 | 12 | USA | WES; RNA |
| Chang 2016 [27] | POB of NCI | Clin Cancer Res | 2016 | 0.7–25 | 15 | USA | WES; WTS—whole-transcriptome sequencing; SNP Array |
| Oberg 2016 [28] | PIPseq | Genome Medicine | 2016 | 0.2–26 | 8 | USA | NGS panel; WES; RNA; WTS—whole-transcriptome sequencing |
| Harttrampf 2017 [29] | MOSCATO-01 | Clinical Cancer Research Online | 2017 | 0.8–24.3 | 10.9 | France | NGS panel; WES; RNA |
| Østrup 2018 [30] | Frontiers in Pediatrics | 2018 | 0–17 | 9.5 | Other | WES; RNA; SNP Array | |
| Gargallo 2019 [31] | Spanish initiative | Translational Medicine Communications | 2019 | 0–25 | 9.9 | Spain | NGS panel; SNP Array; IHC |
| George 2019 [32] | SMPAEDS | European Journal of Cancer | 2019 | 0–24 | United Kingdom | NGS panel | |
| Lee 2019 [33] | SMC | PLOS ONE | 2019 | 0.8–20.7 | 10.7 | Other | NGS panel |
| Surrey 2019 [34] | Genome Medicine | 2019 | 0–26 | 8.6 | USA | NGS panel; RNA | |
| Wong 2020 [35] | Zero Childhood Cancer Program | Nature Medicine | 2020 | 0–31 | 10 | Australia | WGS; WTS—whole-transcriptome sequencing; methylation array |
| Gutiérrez-Jimeno 2021 [36] | Cancers | 2021 | 0–30.8 | 11.8 | Spain | NGS panel | |
| JuanRibelles 2021 [37] | Journal of Personalized Medicine | 2021 | 1–18 | 11.5 | Spain | NGS panel | |
| vanTilburg 2021 [38] | INFORM | Cancer Discovery | 2021 | 0–36 | 13 | International | WES; WGS; methylation array |
| Berlanga 2022 [39] | MAPPYACTS | Cancer Discovery | 2022 | 0.5–38.5 | 11.6 | France; Spain; Ireland; Italy | WES; RNA |
| Church 2022 [40] | GAIN/iCAT2 | Nature Medicine | 2022 | 0–27.5 | 12 | USA | NGS panel |
| Langenberg 2022 [41] | iTHER | European Journal of Cancer | 2022 | 0.9–23 | 13.1 | Germany | WES; RNA |
| Parsons 2022 [42] | Pediatric MATCH Trial | Journal of Clinical Oncology | 2022 | 1–21 | 13 | USA | NGS panel; RNA; IHC |
| Summers 2022 [43] | Aflac Precision Medicine Program (APMP) | JCO Precision Oncology | 2022 | 0.2–25.7 | 12.1 | USA | WES; RNA |
| Ecker 2023 [44] | The Pediatric Targeted Therapy 2.0 registry | European Journal of Cancer | 2023 | 0–54 | 10 | International | NGS panel; RNA; methylation array; IHC |
| Tao 2023 [45] | TOP-GEAR Project | JCO Precision Oncology | 2023 | 1–28 | 12 | Japan | NGS panel |
| Turco 2023 [46] | Pediatric Blood & Cancer | 2023 | 0.9–21 | USA | NGS panel; RNA | ||
| Villani 2023 [47] | SickKids Cancer Sequencing (KiCS) program | Nature Cancer | 2023 | 0–38 | 7.1 | Canada | NGS panel; RNA |
| Vodicska 2023 [48] | World Journal of Pediatrics | 2023 | 0–21 | 8 | Other | NGS panel; WES | |
| Wadensten 2023 [49] | JCO Precision Oncology | 2023 | 0–18 | Other | WGS; RNA |
| Study ID | Number of Patients | Samples | Number of Sequencing Analysis Samples | % Failure | % Actionable Alterations | % Decision-Making Relevant to Test Results | % Suspected Germline |
|---|---|---|---|---|---|---|---|
| Mody 2015 [26] | 70 | 70 | 63 | 10 | 42.8 | 25 | 10 |
| Chang 2016 [27] | 64 | 59 | 59 | 8 | 51 | 12 | |
| Oberg 2016 [28] | 65 | 77 | 77 | 47 | 9.2 | 20 | |
| Harttrampf 2017 [29] | 73 | 73 | 69 | 61 | 20.3 | 6 | |
| Østrup 2018 [30] | 48 | 46 | 46 | 63 | 25 | ||
| Gargallo 2019 [31] | 98 | 84 | 84 | 14.3 | 45 | 15 | 4.8 |
| George 2019 [32] | 223 | 255 | 209 | 18 | 51 | ||
| Lee 2019 [33] | 55 | 55 | 55 | 3.6 | 36.4 | ||
| Surrey 2019 [34] | 147 | 154 | 154 | 86.3 | 62.6 | 25.2 | |
| Wong 2020 [35] | 247 | 252 | 252 | 71 | 67 | 16.2 | |
| Gutiérrez-Jimeno 2021 [36] | 53 | 55 | 55 | 30 | 28 | ||
| JuanRibelles 2021 [37] | 70 | 70 | 70 | 31 | 9 | ||
| vanTilburg 2021 [38] | 519 | 519 | 519 | 6 | 85.9 | 28.3 | 7.5 |
| Berlanga 2022 [39] | 774 | 833 | 695 | 16 | 69 | 13.8 | 7.6 |
| Church 2022 [40] | 345 | 345 | 345 | 69 | 8.4 | ||
| Langenberg 2022 [41] | 253 | 302 | 302 | 81.9 | 17.4 | 16 | |
| Parsons 2022 [42] | 1056 | 1000 | 1000 | 5.3 | 31 | 13 | |
| Summers 2022 [43] | 126 | 127 | 127 | 65.1 | 30.6 | ||
| Ecker 2023 [44] | 266 | 266 | 263 | 1.1 | 59 | 10 | 7 |
| Tao 2023 [45] | 142 | 142 | 133 | 5 | 31 | 10 | 9 |
| Turco 2023 [46] | 64 | 64 | 64 | 42 | 18.75 | ||
| Villani 2023 [47] | 300 | 348 | 348 | 56 | 12.33 | ||
| Vodicska 2023 [48] | 103 | 100 | 100 | 72 | 56 | ||
| Wadensten 2023 [49] | 117 | 118 | 118 | 90 | 26.5 | 8.5 |
References
- Sultan, I.; Alfaar, A.S.; Sultan, Y.; Salman, Z.; Qaddoumi, I. Trends in childhood cancer: Incidence and survival analysis over 45 years of SEER data. PLoS ONE 2025, 20, e0314592. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, P.; Ortiz, R.; Fuentes, S.; Gamboa, Y.; Ah Chu-Sanchez, M.S.; Arambú, I.C.; Montero, M.; Báez, F.; Rodríguez-Galindo, C.; Antillón-Klussmann, F.; et al. Barriers to effective treatment of pediatric solid tumors in middle-income countries: Can we make sense of the spectrum of nonbiologic factors that influence outcomes? Cancer 2014, 120, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Spector, L.G. Cancer Progress and Priorities: Childhood Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Selt, F.; Deiß, A.; Korshunov, A.; Capper, D.; Witt, H.; van Tilburg, C.M.; Jones, D.T.; Witt, R.; Sahm, F.; Reuss, D.; et al. Pediatric Targeted Therapy: Clinical Feasibility of Personalized Diagnostics in Children with Relapsed and Progressive Tumors. Brain Pathol. 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Norris, R.E.; Adamson, P.C. Challenges and opportunities in childhood cancer drug development. Nat. Rev. Cancer. 2012, 12, 776–782. [Google Scholar] [CrossRef]
- Suthapot, P.; Chiangjong, W.; Chaiyawat, P.; Khongcharoen, N.; Hongeng, S.; Anurathapan, U.; Surachat, K.; Sangkhathat, S.; Thai Pediatric Cancer Atlas Tpca Consortium. Genomics-Driven Precision Medicine in Pediatric Solid Tumors. Cancers 2023, 15, 1418. [Google Scholar] [CrossRef]
- Langenberg, K.P.S.; Looze, E.J.; Molenaar, J.J. The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development. Cancers 2021, 13, 4324. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Fidler, M.M.; Colombet, M.; Lacour, B.; Kaatsch, P.; Piñeros, M.; Soerjomataram, I.; Bray, F.; Coebergh, J.W.; Peris-Bonet, R.; et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 2018, 19, 1159–1169. [Google Scholar] [CrossRef]
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Atun, R. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019, 20, 483–493. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Pui, C.H.; Gajjar, A.J.; Kane, J.R.; Qaddoumi, I.A.; Pappo, A.S. Challenging issues in pediatric oncology. Nat. Rev. Clin. Oncol. 2011, 8, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Vaske, O.M.; Bjork, I.; Salama, S.R.; Beale, H.; Tayi Shah, A.; Sanders, L.; Pfeil, J.; Lam, D.L.; Learned, K.; Durbin, A.; et al. Comparative Tumor RNA Sequencing Analysis for Difficult-to-Treat Pediatric and Young Adult Patients With Cancer. JAMA Netw. Open 2019, 2, e1913968. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Rahal, Z.; Abdulhai, F.; Kadara, H.; Saab, R. Genomics of adult and pediatric solid tumors. Am. J. Cancer Res. 2018, 8, 1356–1386. [Google Scholar]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Jones, D.T.W.; Banito, A.; Grünewald, T.G.P.; Haber, M.; Jäger, N.; Kool, M.; Milde, T.; Molenaar, J.J.; Nabbi, A.; Pugh, T.J.; et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer 2019, 19, 420–438. [Google Scholar] [CrossRef]
- DuBois, S.G.; Corson, L.B.; Stegmaier, K.; Janeway, K.A. Ushering in the next generation of precision trials for pediatric cancer. Science 2019, 363, 1175–1181. [Google Scholar] [CrossRef]
- Evans, W.E.; Pui, C.H.; Yang, J.J. The Promise and the Reality of Genomics to Guide Precision Medicine in Pediatric Oncology: The Decade Ahead. Clin. Pharmacol. Ther. 2020, 107, 176–180. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Cahaney, C.; Dhir, A.; Ghosh, T. Role of Precision Medicine in Pediatric Oncology. Pediatr. Ann. 2022, 51, e8–e14. [Google Scholar] [CrossRef]
- Schnepp, R.W.; Bosse, K.R.; Maris, J.M. Improving Patient Outcomes with Cancer Genomics: Unique Opportunities and Challenges in Pediatric Oncology. JAMA 2015, 314, 881–883. [Google Scholar] [CrossRef]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr Blood Cancer. 2017, 64, e26288. [Google Scholar] [CrossRef] [PubMed]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 7 April 2025).
- Buchan, I. StatsDirect Statistical Software; StatsDirect Ltd.: Wirral, UK, 2024; Available online: http://www.statsdirect.com (accessed on 7 April 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.J.; Wu, Y.-M.; Lonigro, R.J.; Cao, X.; Roychowdhury, S.; Vats, P.; Frank, K.M.; Prensner, J.R.; Asangani, I.; Palanisamy, N.; et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 2015, 314, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Brohl, A.S.; Patidar, R.; Sindiri, S.; Shern, J.F.; Wei, J.S.; Song, Y.K.; Yohe, M.E.; Gryder, B.; Zhang, S.; et al. MultiDimensional ClinOmics for Precision Therapy of Children and Adolescent Young Adults with Relapsed and Refractory Cancer: A Report from the Center for Cancer Research. Clin. Cancer Res. 2016, 22, 3810–3820. [Google Scholar] [CrossRef]
- Oberg, J.A.; Bender, J.G.; Sulis, M.L.; Pendrick, D.; Sireci, A.N.; Hsiao, S.J.; Turk, A.T.; Cruz, F.S.D.; Hibshoosh, H.; Remotti, H.; et al. Implementation of next Generation Sequencing into Pediatric Hematology-Oncology Practice: Moving beyond Actionable Alterations. Genome Med. 2016, 8, 133. [Google Scholar] [CrossRef]
- Harttrampf, A.C.; Lacroix, L.; Deloger, M.; Deschamps, F.; Puget, S.; Auger, N.; Vielh, P.; Varlet, P.; Balogh, Z.; Abbou, S.; et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in Pediatric Patients: A Single-Institutional Prospective Molecular Stratification Trial. Clin. Cancer Res. 2017, 23, 6101–6112. [Google Scholar] [CrossRef]
- Østrup, O.; Nysom, K.; Scheie, D.; Schmidt, A.Y.; Mathiasen, R.; Hjalgrim, L.L.; Olsen, T.E.; Skjøth-Rasmussen, J.; Henriksen, B.M.; Nielsen, F.C.; et al. Importance of Comprehensive Molecular Profiling for Clinical Outcome in Children with Recurrent Cancer. Front. Pediatr. 2018, 6, 114. [Google Scholar] [CrossRef]
- Gargallo, P.; de Mora, J.F.; Berlanga, P.; Calabria, I.; Llavador, M.; Pedrola, L.; Panadero, J.; Dolz, S.; Zúñiga, Á.; Oltra, J.S.; et al. Precision Medicine in Relapsed or Refractory Pediatric Solid Tumors: A Collaborative Spanish Initiative. Transl. Med. Commun. 2019, 4, 10. [Google Scholar] [CrossRef]
- George, S.L.; Izquierdo, E.; Campbell, J.; Koutroumanidou, E.; Proszek, P.; Jamal, S.; Hughes, D.; Yuan, L.; Marshall, L.V.; Carceller, F.; et al. A Tailored Molecular Profiling Programme for Children with Cancer to Identify Clinically Actionable Genetic Alterations. Eur. J. Cancer 2019, 121, 224–235. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, N.K.D.; Lee, S.H.; Cho, H.W.; Ma, Y.; Ju, H.Y.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Park, W.Y. Discovery of Actionable Genetic Alterations with Targeted Panel Sequencing in Children with Relapsed or Refractory Solid Tumors. PLoS ONE 2019, 14, e0224227. [Google Scholar] [CrossRef] [PubMed]
- Surrey, L.F.; MacFarland, S.P.; Chang, F.; Cao, K.; Rathi, K.S.; Akgumus, G.T.; Gallo, D.; Lin, F.; Gleason, A.; Raman, P.; et al. Clinical Utility of Custom-Designed NGS Panel Testing in Pediatric Tumors. Genome Med. 2019, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.-A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Jimeno, M.; Alba-Pavón, P.; Astigarraga, I.; Imízcoz, T.; Panizo-Morgado, E.; García-Obregón, S.; Catalán-Lambán, A.; San-Julián, M.; Lamo-Espinosa, J.M.; Echebarria-Barona, A.; et al. Clinical Value of NGS Genomic Studies for Clinical Management of Pediatric and Young Adult Bone Sarcomas. Cancers 2021, 13, 5436. [Google Scholar] [CrossRef]
- Juan Ribelles, A.; Gargallo, P.; Berlanga, P.; Segura, V.; Yáñez, Y.; Juan, B.; Salom, M.; Llavador, M.; Font de Mora, J.; Castel, V.; et al. Next-Generation Sequencing Identifies Potential Actionable Targets in Paediatric Sarcomas. J. Pers. Med. 2021, 11, 268. [Google Scholar] [CrossRef]
- van Tilburg, C.M.; Pfaff, E.; Pajtler, K.W.; Langenberg, K.P.; Fiesel, P.; Jones, B.C.; Balasubramanian, G.P.; Stark, S.; Johann, P.D.; Blattner-Johnson, M.; et al. The Pediatric Precision Oncology INFORM Registry: Clinical Outcome and Benefit for Patients with Very High-Evidence Targets. Cancer Discov. 2021, 11, 2764–2779. [Google Scholar] [CrossRef]
- Berlanga, P.; Pierron, G.; Lacroix, L.; Chicard, M.; de Beaumais, T.A.; Marchais, A.; Harttrampf, A.C.; Iddir, Y.; Larive, A.; Fernandez, A.S.; et al. The European MAPPYACTS Trial: Precision Medicine Program in Pediatric and Adolescent Patients with Recurrent Malignancies. Cancer Discov. 2022, 12, 1266–1281. [Google Scholar] [CrossRef]
- Church, A.J.; Corson, L.B.; Kao, P.-C.; Imamovic-Tuco, A.; Reidy, D.; Doan, D.; Kang, W.; Pinto, N.; Maese, L.; Laetsch, T.W.; et al. Molecular Profiling Identifies Targeted Therapy Opportunities in Pediatric Solid Cancer. Nat. Med. 2022, 28, 1581–1589. [Google Scholar] [CrossRef]
- Langenberg, K.P.; Meister, M.T.; Bakhuizen, J.J.; Boer, J.M.; van Eijkelenburg, N.K.; Hulleman, E.; Ilan, U.; Looze, E.J.; Dierselhuis, M.P.; van der Lugt, J.; et al. Implementation of Paediatric Precision Oncology into Clinical Practice: The Individualized Therapies for Children with Cancer Program ‘iTHER’. Eur. J. Cancer 2022, 175, 311–325. [Google Scholar] [CrossRef]
- Parsons, D.W.; Janeway, K.A.; Patton, D.R.; Winter, C.L.; Coffey, B.; Williams, P.M.; Roy-Chowdhuri, S.; Tsongalis, G.J.; Routbort, M.; Ramirez, N.C.; et al. Actionable Tumor Alterations and Treatment Protocol Enrollment of Pediatric and Young Adult Patients With Refractory Cancers in the National Cancer Institute-Children’s Oncology Group Pediatric MATCH Trial. J. Clin. Oncol. 2022, 40, 2224–2234. [Google Scholar] [CrossRef]
- Summers, R.J.; Castellino, S.M.; Porter, C.C.; MacDonald, T.J.; Basu, G.D.; Szelinger, S.; Bhasin, M.K.; Cash, T.; Carter, A.B.; Castellino, R.C.; et al. Comprehensive Genomic Profiling of High-Risk Pediatric Cancer Patients Has a Measurable Impact on Clinical Care. JCO Precis. Oncol. 2022, 6, e2100451. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Selt, F.; Sturm, D.; Sill, M.; Korshunov, A.; Hirsch, S.; Capper, D.; Dikow, N.; Sutter, C.; Müller, C.; et al. Molecular Diagnostics Enables Detection of Actionable Targets: The Pediatric Targeted Therapy 2.0 Registry. Eur. J. Cancer 2023, 180, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Yamazaki, F.; Kubo, T.; Sunami, K.; Kumamoto, T.; Arakawa, A.; Sugiyama, M.; Watanabe, Y.; Nakajima, M.; Shirakawa, N.; et al. Pediatric Precision Medicine at the National Cancer Center Japan: Prospective Genomic Study of Pediatric Patients with Cancer as Part of the TOP-GEAR Project. JCO Precis. Oncol. 2023, 7, e2200266. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.M.; Gupta, A.; Monteleone, P.; Kelly, K.M.; Klein, R.D.; Wiltsie, L.; Barth, M. An Institutional Review of Genomic Sequencing in Pediatric Solid Tumors. Pediatr. Blood Cancer 2023, 70, e30324. [Google Scholar] [CrossRef]
- Villani, A.; Davidson, S.; Kanwar, N.; Lo, W.W.; Li, Y.; Cohen-Gogo, S.; Fuligni, F.; Edward, L.-M.; Light, N.; Layeghifard, M.; et al. The Clinical Utility of Integrative Genomics in Childhood Cancer Extends beyond Targetable Mutations. Nat. Cancer 2023, 4, 203–221. [Google Scholar] [CrossRef]
- Vodicska, B.; Déri, J.; Tihanyi, D.; Várkondi, E.; Kispéter, E.; Dóczi, R.; Lakatos, D.; Dirner, A.; Vidermann, M.; Filotás, P.; et al. Real-World Performance Analysis of a Novel Computational Method in the Precision Oncology of Pediatric Tumors. World J. Pediatr. 2023, 19, 992–1008. [Google Scholar] [CrossRef]
- Wadensten, E.; Wessman, S.; Abel, F.; De Ståhl, T.D.; Tesi, B.; Pietras, C.O.; Arvidsson, L.; Taylan, F.; Fransson, S.; Vogt, H.; et al. Diagnostic Yield From a Nationwide Implementation of Precision Medicine for All Children With Cancer. JCO Precis. Oncol. 2023, 7, e2300039. [Google Scholar] [CrossRef]
- Kratz, C.P. Re-envisioning genetic predisposition to childhood and adolescent cancers. Nat. Rev. Cancer 2025, 25, 109–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katz, L.; Ben-Arush, M.; Blanche, E.; Meir, I.; Mordechai, O. The Clinical Utility of Next-Generation Sequencing in Childhood and Adolescent/Young Adult Solid Tumors: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1292. https://doi.org/10.3390/cancers17081292
Katz L, Ben-Arush M, Blanche E, Meir I, Mordechai O. The Clinical Utility of Next-Generation Sequencing in Childhood and Adolescent/Young Adult Solid Tumors: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(8):1292. https://doi.org/10.3390/cancers17081292
Chicago/Turabian StyleKatz, Lior, Myriam Ben-Arush, Einav Blanche, Inbar Meir, and Oz Mordechai. 2025. "The Clinical Utility of Next-Generation Sequencing in Childhood and Adolescent/Young Adult Solid Tumors: A Systematic Review and Meta-Analysis" Cancers 17, no. 8: 1292. https://doi.org/10.3390/cancers17081292
APA StyleKatz, L., Ben-Arush, M., Blanche, E., Meir, I., & Mordechai, O. (2025). The Clinical Utility of Next-Generation Sequencing in Childhood and Adolescent/Young Adult Solid Tumors: A Systematic Review and Meta-Analysis. Cancers, 17(8), 1292. https://doi.org/10.3390/cancers17081292








