Histology-Specific Treatment Strategies and Survival Prediction in Lung Cancer Patients with Spinal Metastases: A Nationwide Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Selection
2.2. Patient Demographics and Disease and Treatment Characteristics
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
2.5. Deep Learning Implementation
3. Results
3.1. Cohort Selection, Patient Demographics, and Disease Characteristics
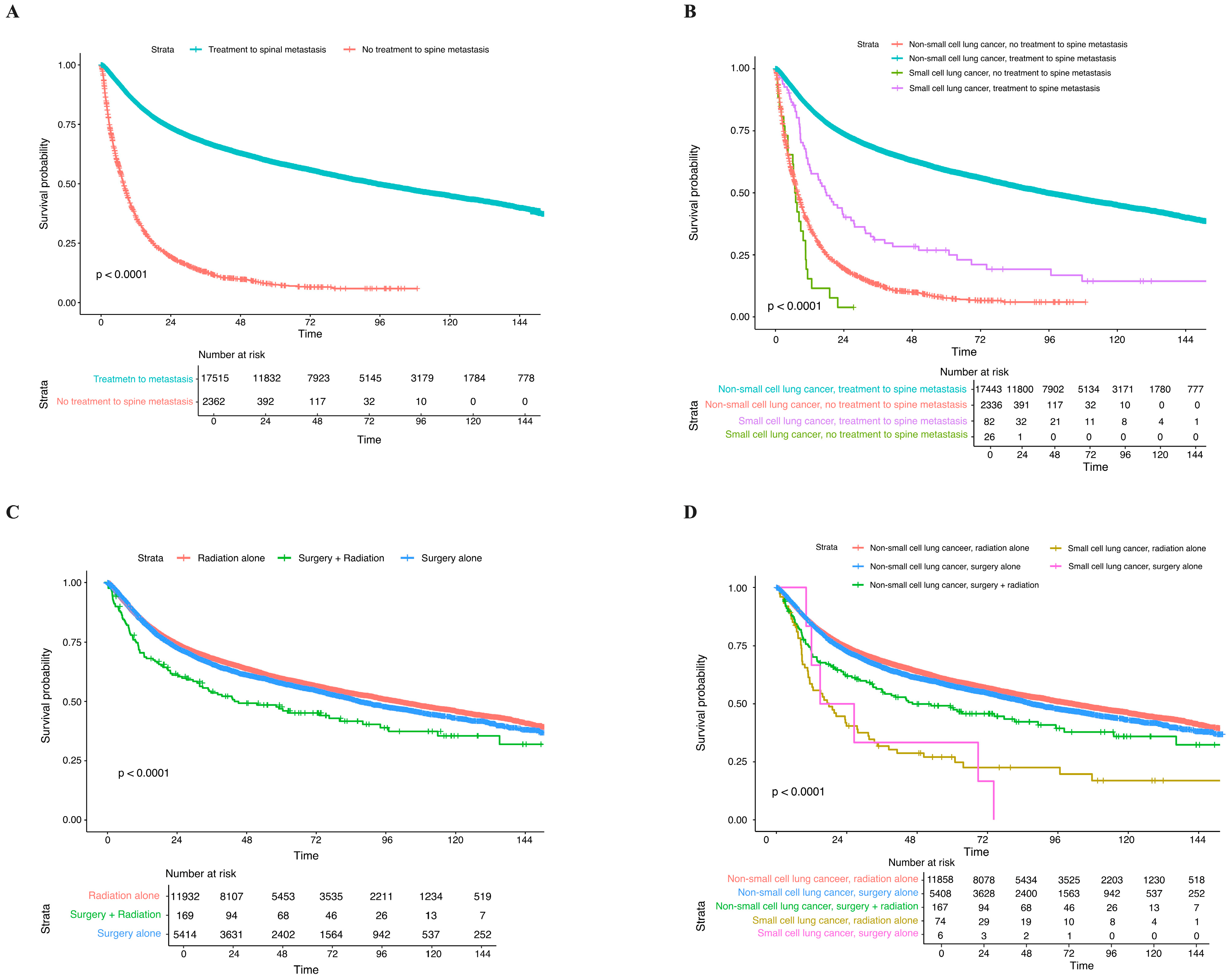
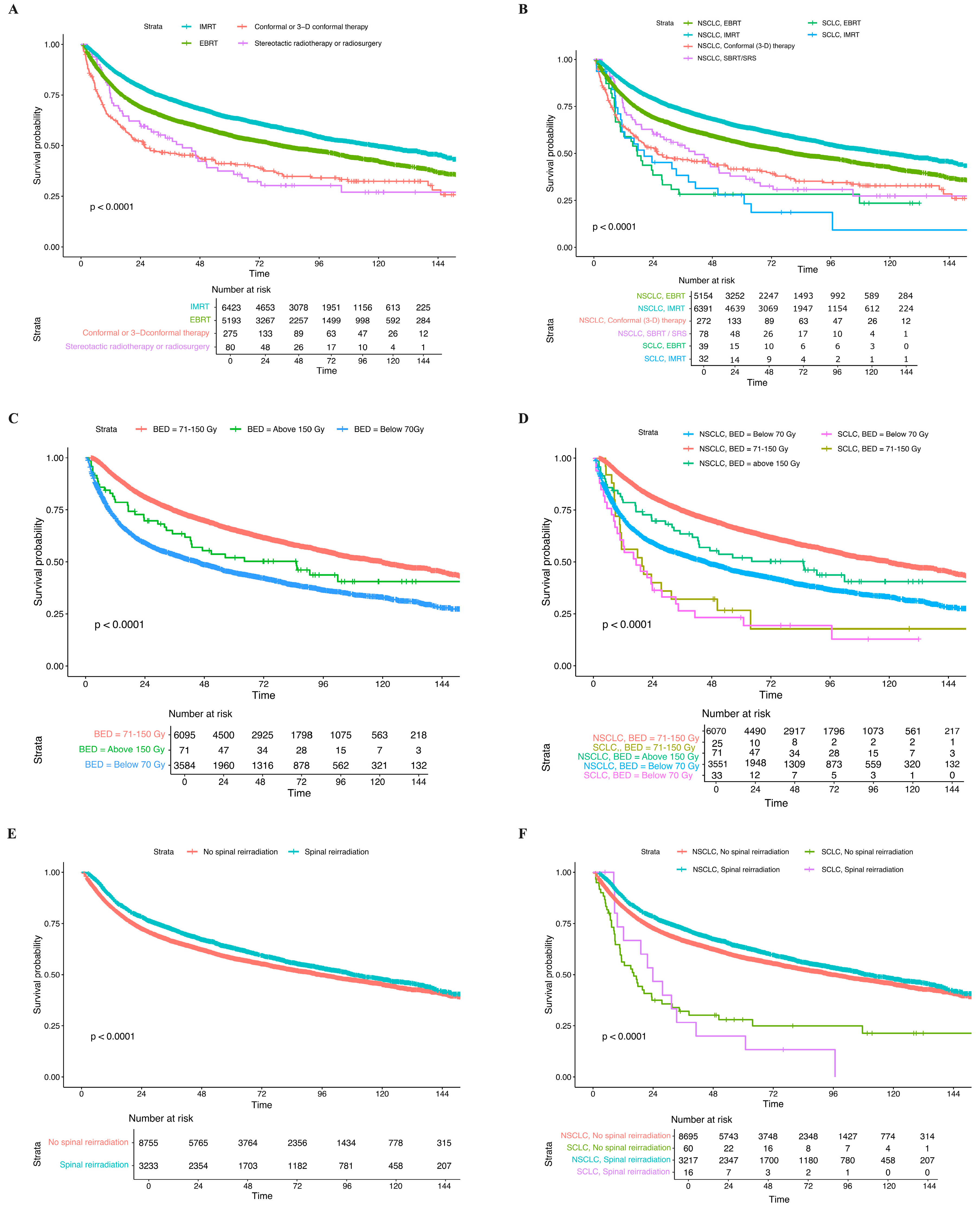
3.2. Treatment Characteristics
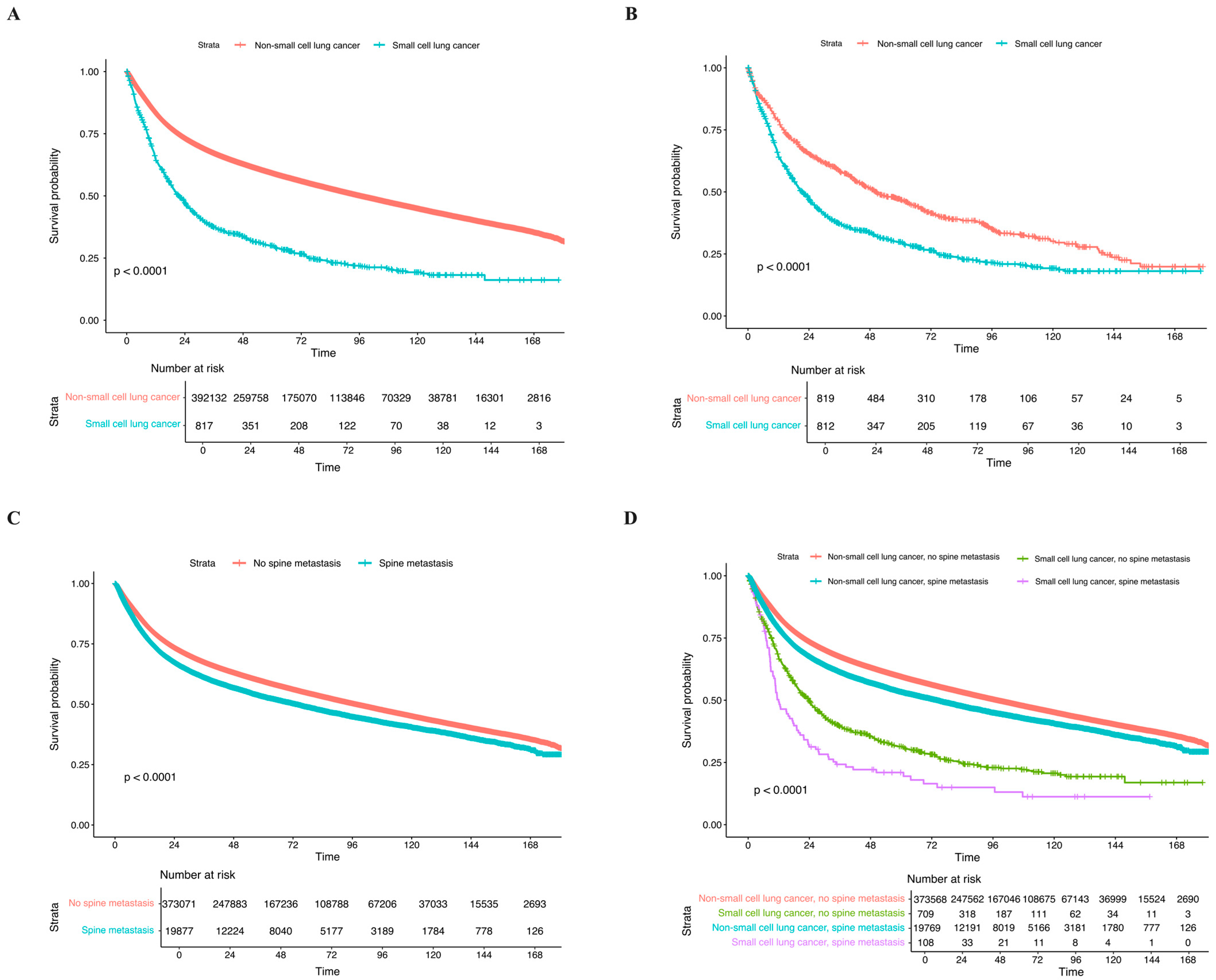
3.3. Clinical Outcomes and Long-Term OS
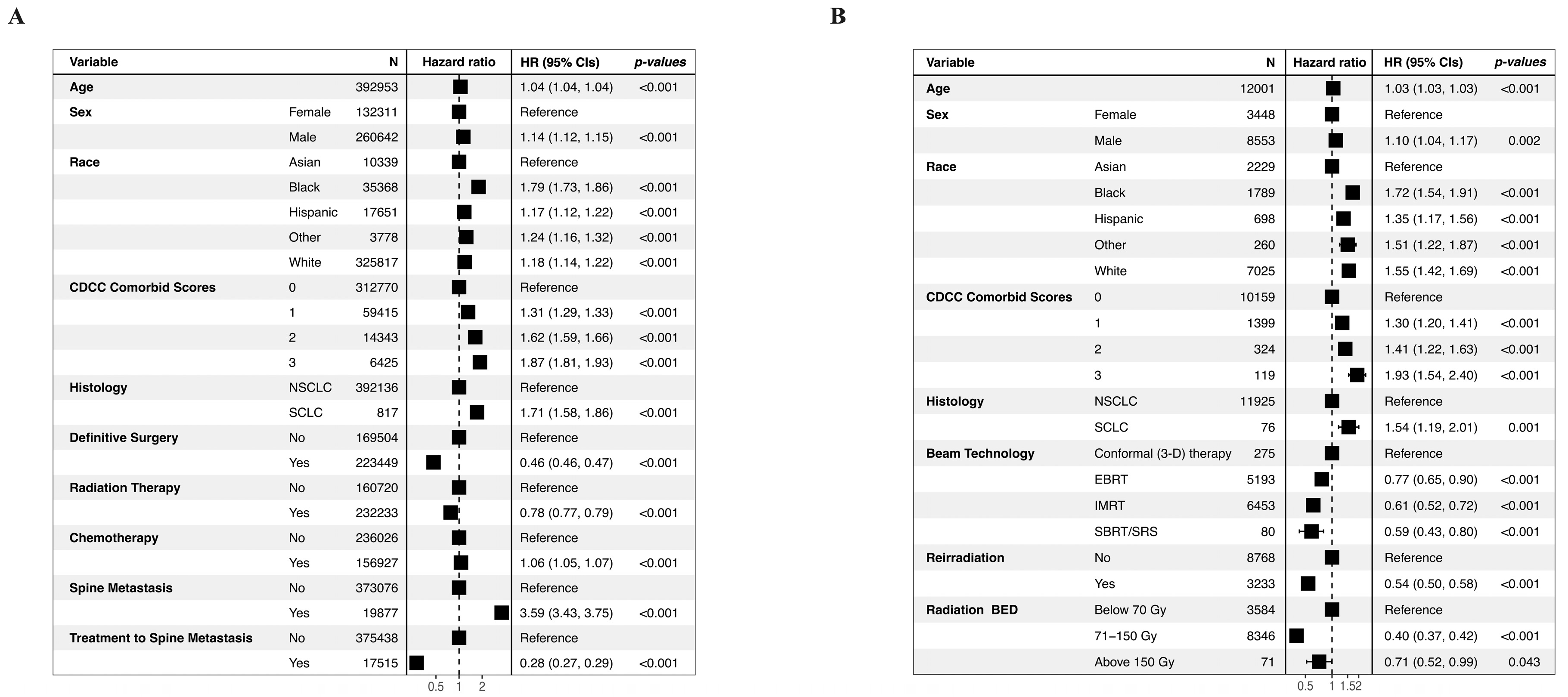
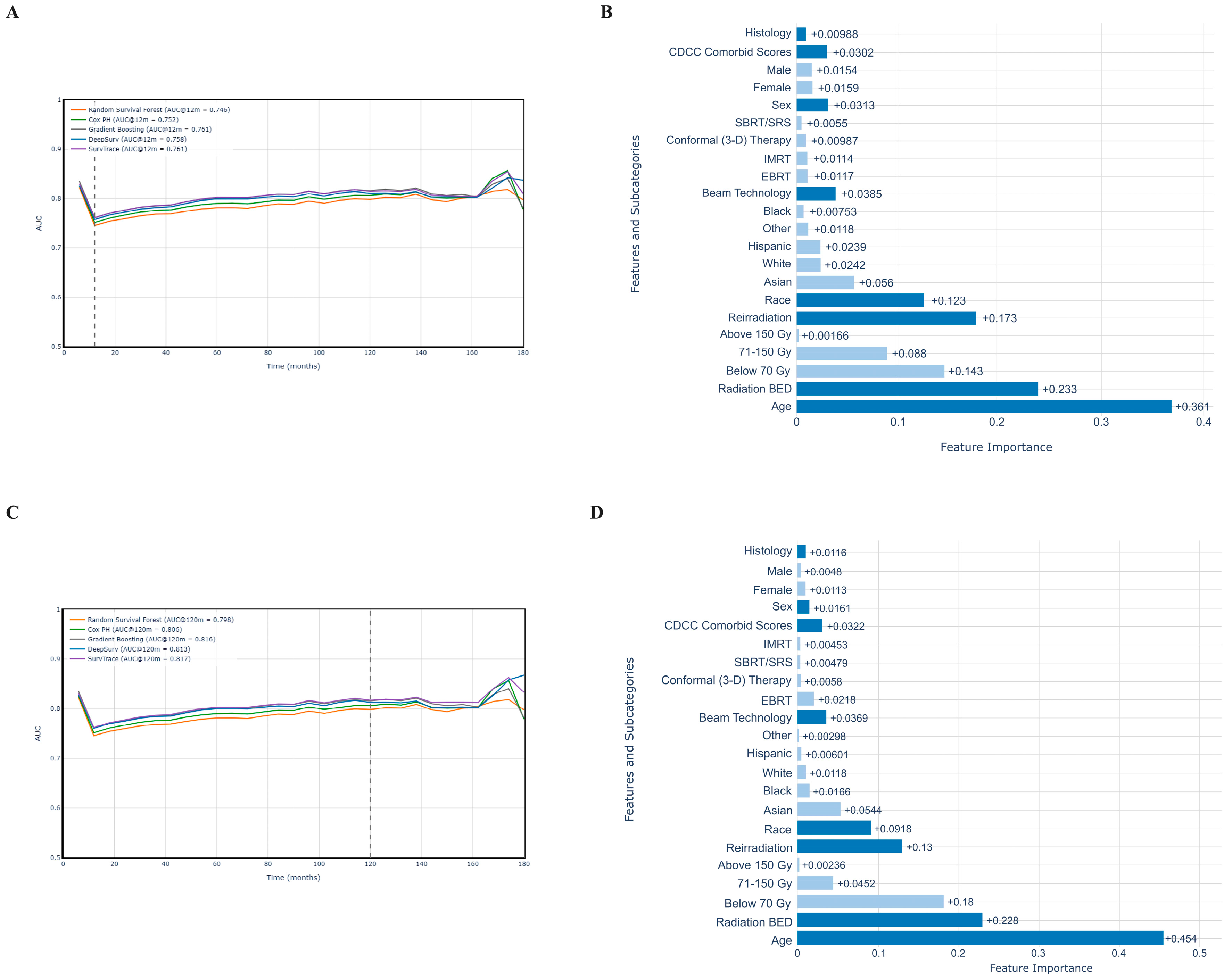
3.4. Mortality Risk Prediction
3.5. Feature Importance Analysis
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCDB | National Cancer Database |
| NSCLC | Non-small cell lung cancer |
| SCLC | Small cell lung cancer |
| OS | Overall survival |
| RT | Radiation therapy |
| SBRT | Stereotactic body radiation therapy |
| SRS | Stereotactic radiosurgery |
| BED | Biologically effective dose |
| CDCC | Charlson–Deyo Comorbidity Classification |
| LOS | Length of stay |
| KM | Kaplan–Meier |
| AUC | Area under the curve |
| HIPAA | Health Insurance Portability and Accountability Act |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| SMOTE | Synthetic Minority Over-sampling Technique |
| SHAP | SHapley Additive exPlanations |
| ECOG | Eastern Cooperative Oncology Group |
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Zhan, Y.; Song, F.; Zhang, W.; Gong, T.; Zhao, S.; Lv, F. Prediction of benign and malignant pulmonary nodules using preoperative CT features: Using PNI-GARS as a predictor. Front. Immunol. 2024, 15, 1446511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bostock, I.C.; Fox, A.H.; Ward, R.C.; Engelhardt, K.E.; Farjah, F.; Jeffrey Yang, C.-F.; Smith, R.A.; Gibney, B.C.; Silvestri, G.A.; American Cancer Society National Lung Cancer Roundtable (NLCRT). Outcomes After Surgical Management of Early-Stage Lung Cancer in Octogenarians: An In-Depth Analysis of a Nationally Representative Cohort. J. Thorac. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Liu, B.; Gu, M.M.; Zou, S.; Xiao, B.B.; Yu, L.; Ding, W.Q.; Zhou, P.K.; Zhou, J.; et al. PIG3 promotes NSCLC cell mitotic progression and is associated with poor prognosis of NSCLC patients. J. Exp. Clin. Cancer Res. 2017, 36, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Improving Timeliness of NSCLC Treatment. Available online: https://www.academia.edu/101194791/Improving_Timeliness_of_NSCLC_Treatment?uc-sb-sw=97571039 (accessed on 4 April 2025).
- Sugiura, H.; Yamada, K.; Sugiura, T.; Hida, T.; Mitsudomi, T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008, 466, 729–736. [Google Scholar] [CrossRef]
- Fry, W.A.; Phillips, J.L.; Menck, H.R. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: A national cancer data base report. Cancer 1999, 86, 1867–1876. [Google Scholar] [CrossRef]
- Tsuang, F.Y.; Jeon, J.P.; Huang, A.P.; Chai, C.L. Overall Survival of Non-Small Cell Lung Cancer With Spinal Metastasis: A Systematic Review and Meta-Analysis. Neurospine 2023, 20, 567–576. [Google Scholar] [CrossRef]
- Kelly, P.D.; Zuckerman, S.L.; Than, K.D.; Attia, A.; Jaboin, J.J. Metastatic spine disease in lung cancer patients: National patterns of radiation and surgical care. J. Spine Surg. 2019, 5, 320–328. [Google Scholar] [CrossRef]
- Liu, W.; Wu, J. Lung cancer with bone metastases in the United States: An analysis from the Surveillance, Epidemiologic, and End Results database. Clin. Exp. Metastasis 2018, 35, 753–761. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Mendel, E.; Yamada, Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine 2009, 34 (Suppl. S22), S78–S92. [Google Scholar] [CrossRef]
- Barzilai, O.; McLaughlin, L.; Amato, M.K.; Reiner, A.S.; Ogilvie, S.Q.; Lis, E.; Yamada, Y.; Bilsky, M.H.; Laufer, I. Predictors of quality of life improvement after surgery for metastatic tumors of the spine: Prospective cohort study. Spine J. 2018, 18, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Bilsky, M.H. Advances in the treatment of metastatic spine tumors: The future is not what it used to be. J. Neurosurg. Spine 2019, 30, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Versteeg, A.L.; Goodwin, C.R.; Sahgal, A.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; Fehlings, M.G.; et al. Association of neurologic deficits with surgical outcomes and health-related quality of life after treatment for metastatic epidural spinal cord compression. Cancer 2019, 125, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Boyce-Fappiano, D.; Elibe, E.; Zhao, B.; Siddiqui, M.S.; Lee, I.; Rock, J.; Ryu, S.; Siddiqui, F. Reirradiation of the spine with stereotactic radiosurgery: Efficacy and toxicity. Pract. Radiat. Oncol. 2017, 7, e409–e417. [Google Scholar] [CrossRef]
- Bunkhumpornpat, C.; Boonchieng, E.; Chouvatut, V.; Lipsky, D. FLEX-SMOTE: Synthetic over-sampling technique that flexibly adjusts to different minority class distributions. Patterns 2024, 5, 101073. [Google Scholar] [CrossRef]
- Karachaliou, N.; Pilotto, S.; Lazzari, C.; Bria, E.; de Marinis, F.; Rosell, R. Cellular and molecular biology of small cell lung cancer: An overview. Transl. Lung Cancer Res. 2016, 5, 2–15. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.; Simms, N.; Polanski, R.; et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwendenwein, A.; Megyesfalvi, Z.; Barany, N.; Valko, Z.; Bugyik, E.; Lang, C.; Ferencz, B.; Paku, S.; Lantos, A.; Fillinger, J.; et al. Molecular profiles of small cell lung cancer subtypes: Therapeutic implications. Mol. Ther. Oncolytics 2021, 20, 470–483. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes. Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.L.; Dyson, N.J. RB: Mitotic implications of a tumour suppressor. Nat. Rev. Cancer 2012, 12, 220–226. [Google Scholar] [CrossRef]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 2021, 13, e13122. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Hyun, S.J.; Kim, K.J. Surgical Impacts of Metastatic Non-small Cell Lung Cancer to the Thoracic and Lumbar Spine. J. Korean Med. Sci. 2021, 36, e52. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Ricciardi, F.; Arts, M.; Buchowski, J.M.; Chung, C.K.; Coppes, M.; Crockard, A.; Depreitere, B.; Fehlings, M.; Kawahara, N.; et al. Metastatic Spine Tumor Epidemiology: Comparison of Trends in Surgery Across Two Decades and Three Continents. World Neurosurg. 2018, 114, e809–e817. [Google Scholar] [CrossRef]
- Amelot, A.; Terrier, L.M.; Cristini, J.; Buffenoir, K.; Pascal-Moussellard, H.; Carpentier, A.; Bonaccorsi, R.; Le Nail, L.R.; Mathon, B. Spinal metastases from lung cancer: Survival depends only on genotype, neurological and personal status, scarcely of surgical resection. Surg. Oncol. 2020, 34, 51–56. [Google Scholar] [CrossRef]
- Anguiano, N.; Nestler, J.; Ayala, K.; Sampene, E.; Sonetti, D.; Ferguson, J. Improving Timeliness of Nsclc Treatment. Chest 2018, 154 (Suppl. S4), 552A–553A. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Y.; Mao, Y.; Chen, Y.; He, Y. Current progress and mechanisms of bone metastasis in lung cancer: A narrative review. Transl. Lung Cancer Res. 2021, 10, 439–451. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Burton, S.A.; Belani, C.P.; Ramalingam, S.; Friedland, D.M.; Ozhasoglu, C.; Quinn, A.E.; McCue, K.J.; Welch, W.C. Radiosurgery for the treatment of spinal lung metastases. Cancer 2006, 107, 2653–2661. [Google Scholar] [CrossRef]
- Kuo, J.V.; Cabebe, E.; Al-Ghazi, M.; Yakoob, I.; Ramsinghani, N.S.; Sanford, R. Intensity-modulated radiation therapy for the spine at the University of California, Irvine. Med. Dosim. 2002, 27, 137–145. [Google Scholar] [CrossRef]
- Kawashiro, S.; Harada, H.; Katagiri, H.; Asakura, H.; Ogawa, H.; Onoe, T.; Sumita, K.; Murayama, S.; Murata, H.; Nemoto, K.; et al. Reirradiation of spinal metastases with intensity-modulated radiation therapy: An analysis of 23 patients. J. Radiat. Res. 2016, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.S.; Hoskin, P.J. Stereotactic body radiotherapy for spinal and bone metastases. Clin. Oncol. (R Coll. Radiol.) 2015, 27, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, A.; Iatì, G.; Cacciola, A.; Conti, A.; Brogna, A.; Siragusa, C.; Ferini, G.; Davì, V.; Tamburella, C.; Molino, L.; et al. Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost in Patients With Spinal Metastases. Technol. Cancer Res. Treat. 2020, 19, 1533033820904447. [Google Scholar] [CrossRef] [PubMed]
- Moussazadeh, N.; Lis, E.; Katsoulakis, E.; Kahn, S.; Svoboda, M.; DiStefano, N.M.; McLaughlin, L.; Bilsky, M.H.; Yamada, Y.; Laufer, I. Five-Year Outcomes of High-Dose Single-Fraction Spinal Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 361–367. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H. Selecting the Appropriate Radiation Therapy Technique for Malignant Spinal Cord Compression Syndrome. Front. Oncol. 2019, 9, 65. [Google Scholar] [CrossRef]
- Esperança-Martins, M.; Roque, D.; Barroso, T.; Abrunhosa-Branquinho, A.; Belo, D.; Simas, N.; Costa, L. Multidisciplinary Approach to Spinal Metastases and Metastatic Spinal Cord Compression-A New Integrative Flowchart for Patient Management. Cancers 2023, 15, 1796. [Google Scholar] [CrossRef]
- Sasamura, K.; Suzuki, R.; Kozuka, T.; Yoshimura, R.; Yoshioka, Y.; Oguchi, M. Outcomes after reirradiation of spinal metastasis with stereotactic body radiation therapy (SBRT): A retrospective single institutional study. J. Radiat. Res. 2020, 61, 929–934. [Google Scholar] [CrossRef]
- Gong, L.; Xu, L.; Yuan, Z.; Wang, Z.; Zhao, L.; Wang, P. Clinical outcome for small cell lung cancer patients with bone metastases at the time of diagnosis. J. Bone Oncol. 2019, 19, 100265. [Google Scholar] [CrossRef]
- Rades, D.; Haus, R.; Janssen, S.; Schild, S.E. An easy-to-use scoring system to estimate the survival of patients irradiated for bone metastases from lung cancer. Transl. Lung Cancer Res. 2020, 9, 1067–1073. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006, Erratum in Nat. Commun. 2014, 5, 4644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Variable | Total (N = 428,915) | NSCLC (N = 428,040) | SCLC (N = 875) | p-Value | Total (N = 1750) | NSCLC (N = 875) | SCLC (N = 875) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Pre-Matching | 1:1 KNN Matched | |||||||
| Age | <0.001 | 0.148 | ||||||
| Mean ± SD (years) | 61.2 ± 13.7 | 61.2 ± 13.7 | 67.2 ± 13.8 | 66.7 ± 13.8 | 66.2 ± 13.9 | 67.2 ± 13.8 | ||
| Sex | 0.003 | 1.000 | ||||||
| Male | 285,673 (66.6%) | 285,049 (66.6%) | 624 (71.3%) | 1248 (71.3%) | 624 (71.3%) | 624 (71.3%) | ||
| Female | 143,242 (33.4%) | 142,991 (33.4%) | 251 (28.7%) | 502 (28.7%) | 251 (28.7%) | 251 (28.7%) | ||
| Race | <0.001 | 0.002 | ||||||
| White | 350,881 (82.7%) | 350,106 (82.7%) | 775 (89.1%) | 1496 (86.1%) | 721 (83.1%) | 775 (89.1%) | ||
| Black | 38,210 (9.0%) | 38,159 (9.0%) | 51 (5.9%) | 126 (7.2%) | 75 (8.6%) | 51 (5.9%) | ||
| Asian | 11,448 (2.7%) | 11,437 (2.7%) | 11 (1.3%) | 30 (1.7%) | 19 (2.2%) | 11 (1.3%) | ||
| Hispanic | 19,400 (4.6%) | 19,370 (4.6%) | 30 (3.4%) | 69 (4.0%) | 39 (4.5%) | 30 (3.4%) | ||
| Other | 4221 (1.0%) | 4218 (1.0%) | 3 (0.3%) | 17 (0.3%) | 14 (0.3%) | 3 (0.3%) | ||
| N-Miss | 4755 | 4750 | 5 | 12 | 7 | 5 | ||
| CDCC Comorbidity Scores | <0.001 | 1.000 | ||||||
| 0 | 340,059 (79.3%) | 339,405 (79.3%) | 654 (74.7%) | 1308 (74.7%) | 654 (74.7%) | 654 (74.7%) | ||
| 1 | 64,904 (15.1%) | 64,761 (15.1%) | 143 (16.3%) | 286 (16.3%) | 143 (16.3%) | 143 (16.3%) | ||
| 2 | 16,064 (3.7%) | 16,019 (3.7%) | 45 (5.1%) | 90 (5.1%) | 45 (5.1%) | 45 (5.1%) | ||
| 3 | 7888 (1.8%) | 7855 (1.8%) | 33 (3.8%) | 66 (3.8%) | 33 (3.8%) | 33 (3.8%) | ||
| Tumor Size | 0.520 | 0.268 | ||||||
| Mean ± SD (mm) | 391.25 ± 468.93 | 391.23 ± 468.93 | 401.45 ± 466.72 | 389.14 ± 464.98 | 376.83 ± 463.18 | 401.45 ± 466.71 | ||
| Primary Surgery | <0.001 | <0.001 | ||||||
| Yes | 241,533 (56.5%) | 241,162 (56.6%) | 371 (42.6%) | 854 (49.0%) | 483 (55.4%) | 371 (42.6%) | ||
| No | 185,765 (43.5%) | 185,266 (43.4%) | 499 (57.4%) | 888 (51.0%) | 389 (44.6%) | 499 (57.4%) | ||
| N-Miss | 1617 | 1612 | 5 | 8 | 3 | 5 | ||
| Radiation Therapy | <0.001 | 0.728 | ||||||
| Yes | 247,156 (58.5%) | 246,605 (58.5%) | 551 (64.5%) | 1080 (63.0%) | 540 (62.6%) | 540 (63.5%) | ||
| No | 175,256 (41.5%) | 174,953 (41.5%) | 303 (35.5%) | 633 (37.0%) | 322 (37.4%) | 311 (36.%) | ||
| N-Miss | 6503 | 6482 | 21 | 37 | 13 | 24 | ||
| Chemotherapy | <0.001 | <0.001 | ||||||
| Yes | 171,275 (41.0%) | 170,674 (41.0%) | 601 (69.8%) | 920 (53.8%) | 319 (37.6%) | 601 (69.8%) | ||
| NOS | 15,497 (3.7%) | 15,458 (3.7%) | 39 (4.5%) | 75 (4.4%) | 36 (4.2%) | 39 (4.5%) | ||
| Single-agent | 96,375 (23.1%) | 96,333 (23.1%) | 42 (4.9%) | 639 (37.7%) | 166 (19.6%) | 42 (4.9%) | ||
| Multi-agent | 59,403 (14.2%) | 58,883 (14.1%) | 520 (60.4%) | 208 (12.2%) | 117 (13.8%) | 520 (60.4%) | ||
| No | 246,023 (59.0%) | 245,763 (59.0%) | 260 (30.2%) | 790 (46.2%) | 530 (62.4%) | 260 (30.2%) | ||
| N-Miss | 11,617 | 11,603 | 14 | 40 | 26 | 14 | ||
| Immunotherapy | <0.001 | 0.002 | ||||||
| Yes | 9699 (2.3%) | 9694 (2.3%) | 5 (0.6%) | 26 (1.5%) | 21 (2.4%) | 5 (0.6%) | ||
| No | 413,885 (97.7%) | 413,019 (97.7%) | 866 (99.4%) | 1712 (98.5%) | 846 (97.6%) | 866 (99.4%) | ||
| N-Miss | 5331 | 5327 | 4 | 12 | 6 | 4 | ||
| Clinical Outcome | ||||||||
| Length of Stay | 0.036 | 0.407 | ||||||
| Mean ± SD (days) | 4.1 ± 8.9 | 4.1 ± 8.9 | 3.1 ± 7.3 | 3.4 ± 7.7 | 3.6 ± 8.0 | 3.1 ± 7.3 | ||
| 30-Day Readmission | 12,843 (3.1%) | 12,819 (3.1%) | 24 (2.8%) | 0.693 | 52 (3.1%) | 28 (3.3%) | 24 (2.8%) | 0.582 |
| Palliative Care | 9180 (2.2%) | 9133 (2.2%) | 47 (5.4%) | < 0.001 | 73 (4.2%) | 26 (3.0%) | 47 (5.4%) | 0.012 |
| Spine Metastasis | 21,984 (5.1%) | 21,865 (5.1%) | 119 (13.6%) | < 0.001 | 216 (12.3%) | 97 (11.1%) | 119 (13.6%) | 0.110 |
| Mortality Rates | ||||||||
| 1-year | 64,312 (15.0%) | 64,026 (15.0%) | 286 (32.7%) | <0.001 | 458 (26.2%) | 172 (19.7%) | 286 (32.7%) | <0.001 |
| 5-year | 144,678 (33.7%) | 144,144 (33.7%) | 534 (61.0%) | <0.001 | 933 (53.3%) | 399 (45.6%) | 534 (61.0%) | <0.001 |
| 10-year | 167,718 (39.1%) | 167,141 (39.0%) | 577 (65.9%) | <0.001 | 1039 (59.4%) | 462 (52.8%) | 577 (65.9%) | <0.001 |
| 15-year | 171,950 (40.1%) | 171,370 (40.0%) | 580 (66.3%) | <0.001 | 1053 (59.8%) | 473 (54.1%) | 580 (66.3%) | <0.001 |
| LFU | 171,972 (43.8%) | 171,392 (43.7%) | 580 (71.0%) | <0.001 | 1053 (59.8%) | 473 (54.1%) | 580 (66.3%) | <0.001 |
| Total (N = 21,984) | NSCLC (N = 21,865) | SCLC (N = 119) | p-Value | Total (N = 238) | NSCLC (N = 119) | SCLC (N = 119) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Pre-Matching | 1:1 KNN Matched | |||||||
| Age | <0.001 | 0.187 | ||||||
| Mean ± SD | 57.7 ± 14.4 | 57.6 ± 14.4 | 62.7 ± 11.3 | 61.7 ± 11.4 | 60.8 ± 11.5 | 62.7 ± 11.3 | ||
| Sex | 0.120 | 1.000 | ||||||
| Male | 15,772 (71.7%) | 15,679 (71.7%) | 93 (78.2%) | 186 (78.2%) | 93 (78.2%) | 93 (78.2%) | ||
| Female | 6212 (28.3%) | 6186 (28.3%) | 26 (21.8%) | 52 (21.8%) | 26 (21.8%) | 26 (21.8%) | ||
| Race | <0.001 | 0.007 | ||||||
| White | 14,669 (67.4%) | 14,566 (67.3%) | 103 (87.3%) | 183 (77.2%) | 80 (67.2%) | 103 (87.3%) | ||
| Black | 2831 (13.0%) | 2823 (13.0%) | 8 (6.8%) | 27 (11.4%) | 19 (16.0%) | 8 (6.8%) | ||
| Asian | 2724 (12.5%) | 2723 (12.6%) | 1 (0.8%) | 7 (3.0%) | 6 (5.0%) | 1 (0.8%) | ||
| Hispanic | 1162 (5.3%) | 1157 (5.3%) | 5 (4.2%) | 17 (7.2%) | 12 (10.1%) | 5 (4.2%) | ||
| Other | 387 (1.8%) | 386 (1.8%) | 1 (0.8%) | 3 (1.3%) | 2 (1.7%) | 1 (0.8%) | ||
| N-Miss | 211 | 210 | 1 | 1 | 0 | 1 | ||
| CDCC Comorbidity Scores | 0.002 | 1.000 | ||||||
| 0 | 17,793 (80.9%) | 17,707 (81.0%) | 86 (72.3%) | 172 (72.3%) | 86 (72.3%) | 86 (72.3%) | ||
| 1 | 3059 (13.9%) | 3039 (13.9%) | 20 (16.8%) | 40 (16.8%) | 20 (16.8%) | 20 (16.8%) | ||
| 2 | 763 (3.5%) | 757 (3.5%) | 6 (5.0%) | 12 (5.0%) | 6 (5.0%) | 6 (5.0%) | ||
| 3 | 369 (1.7%) | 362 (1.7%) | 7 (5.9%) | 14 (5.9%) | 7 (5.9%) | 7 (5.9%) | ||
| Tumor Size | 0.134 | 0.487 | ||||||
| Mean ± SD (mm) | 514.68 ± 481.04 | 514.32 ± 481.05 | 580.59 ± 477.05 | 558.92 ± 479.81 | 537.24 ± 483.60 | 580.59 ± 477.05 | ||
| Treatment Status of Spine Metastasis | <0.001 | 0.008 | ||||||
| Treated | 19,194 (87.3%) | 19,110 (87.4%) | 84 (70.6%) | 185 (77.7%) | 101 (84.9%) | 84 (70.6%) | ||
| Conservative | 2790 (12.7%) | 2755 (12.6%) | 35 (29.4%) | 53 (22.3%) | 18 (15.1%) | 35 (29.4%) | ||
| Treatment to Spine Metastases | <0.001 | <0.001 | ||||||
| Surgery alone | 5993 (31.2%) | 5987 (31.3%) | 6 (7.1%) | 35 (18.9%) | 29 (28.7%) | 6 (7.1%) | ||
| Radiation alone | 13,011 (67.8%) | 12,935 (67.7%) | 76 (90.5%) | 145 (78.4%) | 69 (68.3%) | 76 (90.5%) | ||
| Surgery + radiation | 190 (1.0%) | 188 (1.0%) | 2 (2.4%) | 5 (2.7%) | 3 (3.0%) | 2 (2.4%) | ||
| N-Miss | 2790 | 2755 | 35 | 53 | 18 | 35 | ||
| Reirradiation Status | 0.252 | 0.798 | ||||||
| Yes | 3425 (26.2%) | 3409 (26.2%) | 16 (20.5%) | 32 (21.3%) | 16 (22.2%) | 16 (20.5%) | ||
| No | 9645 (73.8%) | 9583 (73.8%) | 62 (79.5%) | 118 (78.7%) | 56 (77.8%) | 62 (79.5%) | ||
| N-Miss | 8914 | 8873 | 41 | 88 | 47 | 41 | ||
| Beam Technology of Spinal Radiation | 0.023 | 0.064 | ||||||
| EBRT | 5555 (42.5%) | 5514 (42.5%) | 41 (52.6%) | 72 (48.0%) | 31 (43.1%) | 41 (52.6%) | ||
| IMRT | 7110 (54.4%) | 7078 (54.5%) | 32 (41.0%) | 73 (48.7%) | 41 (56.9%) | 32 (41.0%) | ||
| Conformal (3-D) therapy | 309 (2.4%) | 306 (2.4%) | 3 (3.8%) | 3 (2.0%) | 0 (0.0%) | 3 (3.8%) | ||
| SBRT/SRS | 86 (0.7%) | 84 (0.6%) | 2 (2.6%) | 2 (1.3%) | 0 (0.0%) | 2 (2.6%) | ||
| N-Miss | 8924 | 8883 | 41 | 88 | 47 | 41 | ||
| Biologically Effective Dose (BED) | 0.019 | 0.083 | ||||||
| Mean ± SD (Gy) | 70.6 ± 44.8 | 70.7 ± 44.9 | 57.9 ± 25.5 | 62.433 (29.548) | 66.350 (32.268) | 57.882 (25.521) | ||
| BED strata | <0.001 | 0.243 | ||||||
| Below 70 Gy | 5601 (38.1%) | 5560 (38.0%) | 41 (60.3%) | 79 (53.7%) | 38 (48.1%) | 41 (60.3%) | ||
| 71–150 Gy | 8990 (61.1%) | 8963 (61.2%) | 27 (39.7%) | 67 (45.6%) | 40 (50.6%) | 27 (39.7%) | ||
| Above 150 Gy | 114 (0.8%) | 114 (0.8%) | 0 (0.0%) | 1 (0.7%) | 1 (1.3%) | 0 (0.0%) | ||
| N-Miss | 7279 | 7228 | 51 | 91 | 40 | 51 | ||
| Chemotherapy | <0.001 | 0.026 | ||||||
| Yes | 15,690 (72.7%) | 15,587 (72.6%) | 103 (87.3%) | 192 (81.7%) | 89 (76.1%) | 103 (87.3%) | ||
| No | 5905 (27.3%) | 5890 (27.4%) | 15 (12.7%) | 43 (18.3%) | 28 (23.9%) | 15 (12.7%) | ||
| N-Miss | 389 | 388 | 1 | 3 | 2 | 1 | ||
| Clinical Outcome | ||||||||
| Length of Stay | 0.438 | 0.728 | ||||||
| Mean ± SD (days) | 5.7 ± 11.1 | 5.7 ± 11.1 | 3.8 ± 10.1 | 3.4 ± 8.1 | 2.9 ± 5.1 | 3.8 ± 10.1 | ||
| 30-Day Readmission Rate | 554 (2.6%) | 551 (2.6%) | 3 (2.6%) | 0.992 | 4 (1.7%) | 1 (0.9%) | 3 (2.6%) | 0.322 |
| Palliative Care Rate | 1503 (6.8%) | 1488 (6.8%) | 15 (12.6%) | 0.012 | 22 (9.2%) | 7 (5.9%) | 15 (12.6%) | 0.073 |
| Mortality Rates | ||||||||
| 1-year | 4198 (19.1%) | 4144 (19.0%) | 54 (45.4%) | <0.001 | 87 (36.6%) | 33 (27.7%) | 54 (45.4%) | 0.005 |
| 5-year | 8520 (38.8%) | 8437 (38.6%) | 83 (69.7%) | <0.001 | 136 (57.1%) | 53 (44.5%) | 83 (69.7%) | <0.001 |
| 10-year | 9566 (43.5%) | 9477 (43.3%) | 89 (74.8%) | <0.001 | 154 (64.7%) | 65 (54.6%) | 89 (74.8%) | 0.001 |
| 15-year | 9771 (44.4%) | 9682 (44.3%) | 89 (74.8%) | <0.001 | 157 (66.0%) | 68 (57.1%) | 89 (74.8%) | 0.004 |
| LFU | 9771 (49.2%) | 9682 (49.0%) | 89 (82.4%) | <0.001 | 157 (71.0%) | 68 (60.2%) | 89 (82.4%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaith, A.K.; Yang, X.; Khalilullah, T.; Wang, X.; Alfonzo Horowitz, M.; Khalifeh, J.; Ahmed, A.K.; Azad, T.; Weinberg, J.; Al-Mistarehi, A.-H.; et al. Histology-Specific Treatment Strategies and Survival Prediction in Lung Cancer Patients with Spinal Metastases: A Nationwide Analysis. Cancers 2025, 17, 1374. https://doi.org/10.3390/cancers17081374
Ghaith AK, Yang X, Khalilullah T, Wang X, Alfonzo Horowitz M, Khalifeh J, Ahmed AK, Azad T, Weinberg J, Al-Mistarehi A-H, et al. Histology-Specific Treatment Strategies and Survival Prediction in Lung Cancer Patients with Spinal Metastases: A Nationwide Analysis. Cancers. 2025; 17(8):1374. https://doi.org/10.3390/cancers17081374
Chicago/Turabian StyleGhaith, Abdul Karim, Xinlan Yang, Taha Khalilullah, Xihang Wang, Melanie Alfonzo Horowitz, Jawad Khalifeh, A. Karim Ahmed, Tej Azad, Joshua Weinberg, Abdel-Hameed Al-Mistarehi, and et al. 2025. "Histology-Specific Treatment Strategies and Survival Prediction in Lung Cancer Patients with Spinal Metastases: A Nationwide Analysis" Cancers 17, no. 8: 1374. https://doi.org/10.3390/cancers17081374
APA StyleGhaith, A. K., Yang, X., Khalilullah, T., Wang, X., Alfonzo Horowitz, M., Khalifeh, J., Ahmed, A. K., Azad, T., Weinberg, J., Al-Mistarehi, A.-H., Foster, C., Bhimreddy, M., Menta, A. K., Redmond, K. J., Theodore, N., & Lubelski, D. (2025). Histology-Specific Treatment Strategies and Survival Prediction in Lung Cancer Patients with Spinal Metastases: A Nationwide Analysis. Cancers, 17(8), 1374. https://doi.org/10.3390/cancers17081374








