Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies
Simple Summary
Abstract
1. Introduction
1.1. Materials and Methods
1.2. Immunotherapy in Oncology
2. Role of Immune Checkpoint Inhibitors in Selected Cancers
2.1. Non-Small Cell Lung Cancer (NSCLC)
2.2. Hepatocellular Carcinoma
2.3. Melanoma
2.4. Triple-Negative Breast Cancer (TNBC)
3. Key Strategies to Enhance ICI Efficacy
3.1. Anti-Angiogenesis
3.2. Biomarker-Guided Therapy
3.3. Oncolytic Viruses
3.4. Cancer Vaccines
3.5. Radiotherapy and ICI Synergy
3.6. ICIs with JAK Inhibitors
3.7. Microbiome Modulation
| Key Findings | Mechanism of Action | Effect on ICI Therapy | Reference |
|---|---|---|---|
| Gut microbiota modulates GM-CSF via gut–brain axis | ↑ ROS in immature myeloid cells; ↑ MDSC suppression of T cells | Enhances ICI response by weakening immunosuppressive barriers | [77,123] |
| Antigenic similarity between Enterococcus hirae proteins and tumor antigens | Stimulates CD8+ T cells via β-type 4 antigens | Boosts PD-1 blockade efficacy through cross-reactivity | [124] |
| Gut microbiota synergizes with anti-CTLA-4 therapy in GBM | ↑ IFN-γ production; ↑ microglial phagocytosis via CD4+ T cell modulation | Improves ICI response and tumor clearance in glioblastoma | [78] |
| Microbiota modulates FMT and PD-1 therapy response | ↑ MAIT and CD56+CD8+ T cells; CD74 + GZMK → ↑ HLA-II expression | Enhances ICI through improved immune cell infiltration and activation | [79] |
| Gut-driven bile acid metabolism attracts CXCR6+ NKT cells in HCC | CXCL16 from liver sinusoidal endothelial cells → NKT recruitment | Increases ICI efficacy in HCC; probiotics aid liver recovery and reduce toxicity | [125] |
3.8. Checkpoints Inhibitors: LAG3, TIM3, and TIGIT
3.9. Metabolic Reprogramming in Tumors
3.10. Glutamine Metabolism (GM)
4. Immune-Related Adverse Events (irAEs)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—New opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Jhawar, S.; Wang, S.; Thandoni, A. Correction: Combination oncolytic virus, radiation therapy, and immune checkpoint inhibitor treatment in anti-PD-1-refractory cancer. J. Immunother 2024, 12, e006780corr1. [Google Scholar]
- Hu, X.; Zhao, M.; Yue, J. The role of radiotherapy in reshaping tumor immune microenvironment. Front. Immunol. 2023, 14, 1340844. [Google Scholar] [CrossRef]
- Lelliott, E.J.; McArthur, G.A.; Oliaro, J.; Sheppard, K.E. Immunomodulatory effects of BRAF, MEK, and CDK4/6 inhibitors: Implications for combining targeted therapy and immune checkpoint blockade for the treatment of melanoma. Front. Immunol. 2021, 12, 661737. [Google Scholar] [CrossRef]
- Deken, M.A.; Gadiot, J.; Jordanova, E.S.; Lacroix, R.; van Gool, M.; Kroon, P.; Pineda, C.; Geukes Foppen, M.H.; Scolyer, R.; Song, J.-Y.; et al. Targeting the MAPK and PI3K pathways in combination with PD1 blockade in melanoma. Oncoimmunology 2016, 5, e1238557. [Google Scholar] [CrossRef]
- Homet Moreno, B.; Mok, S.; Comin-Anduix, B.; Hu-Lieskovan, S.; Ribas, A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology 2016, 5, e1052212. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Whitaker, K. Earlier diagnosis: The importance of cancer symptoms. Lancet Oncol. 2020, 21, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B.C. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Dobosz, P.; Dzieciątkowski, T. The intriguing history of cancer immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, D.; Li, X.; Peng, Z.; Zhu, L.; Xu, Y. Optimal immunotherapy duration in advanced NSCLC: Defining the ideal treatment window. Cancer Biol. Med. 2025, 22, 284–294. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Pu, X.; Li, J.; Wang, Q.; Liu, L.; Xu, Y.; Xu, L.; Kong, Y.; Li, K.; et al. The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: A retrospective comparative cohort study. Transl. Lung Cancer Res. 2022, 11, 2111. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Wang, S.; Wei, H.; Yu, J. Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review. J. Cancer 2021, 12, 1318. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y. Efficacy and safety of immune checkpoint inhibitors in heavily pretreated patients with microsatellite stable metastatic colorectal cancer: A real-world retrospective study. Am. J. Cancer Res. 2024, 14, 5378. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, M.; Nie, J.; Dai, L.; Hu, W.; Zhang, J.; Wu, D.; Chen, X.; Ma, X.; Tian, G.; et al. Real-world data on the first-line immune checkpoint inhibitors or in combination with chemotherapy in older patients (aged ≥ 75 years) with advanced non-small cell lung cancer. Heliyon 2024, 10, e26026. [Google Scholar] [CrossRef]
- Qian, X.; Tao, Y.; Chen, H.; Li, X.; Wang, Y.; Xu, X.; Li, S.; Chen, H.; Cang, S.; Liu, Y. Real-world evaluation of the efficacy of immune checkpoint inhibitors in the treatment of metastatic breast cancer. Oncol. Lett. 2024, 29, 29. [Google Scholar] [CrossRef]
- Abu Hejleh, T.; AlSawalha, K.; Abdel Hafiz, S.; Al-Batsh, T.; Abu Hejleh, R.; Yaser, S.; Abu Jazar, H.; Khader, J.; Alnsour, A.; Mohamad, I.; et al. Beyond clinical trials: Real-world impact of immunotherapy on NSCLC in Jordan. Front. Oncol. 2024, 14, 1369126. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, W.; Zhang, P.; Guo, F.; Liu, M. Current trends in sensitizing immune checkpoint inhibitors for cancer treatment. Mol. Cancer 2024, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Cuppens, K.; Plönes, T.; Wiesweg, M.; Du Pont, B.; Hegedus, B.; Köster, J.; Mairinger, F.; Darwiche, K.; Paschen, A.; et al. Neoadjuvant nivolumab with or without relatlimab in resectable non-small-cell lung cancer: A randomized phase 2 trial. Nat. Med. 2024, 30, 1602–1611. [Google Scholar] [CrossRef]
- Bell, H.N.; Zou, W. Beyond the barrier: Unraveling the mechanisms of immunotherapy resistance. Annu. Rev. Immunol. 2024, 42, 521–550. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune checkpoint inhibitors in cancer therapy—How to overcome drug resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef]
- Xiao, T.; Verma, S.; Boldt, G.; Rajeh, A.; Breadner, D.; Raphael, J. Efficacy and Safety of Immune Checkpoint Inhibitors (ICI) in Resectable Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, e6. [Google Scholar] [CrossRef]
- Miao, K.; Zhang, X.; Wang, H.; Si, X.; Ni, J.; Zhong, W.; Zhao, J.; Xu, Y.; Chen, M.; Pan, R.; et al. Real-world data of different immune checkpoint inhibitors for non-small cell lung cancer in China. Front. Oncol. 2022, 12, 859938. [Google Scholar] [CrossRef]
- Lee, C.K.; Yoo, C.; Hong, J.Y.; Park, S.J.; Kim, J.W.; Tai, D.W.M.; Kim, H.; Korphaisarn, K.; Tanasanvimon, S.; Chen, S.C.; et al. Real-world study of systemic treatment after first-line atezolizumab plus Bevacizumab for Hepatocellular Carcinoma in Asia-Pacific Countries. Liver Cancer 2024, 14, 127–141. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, E.J.; Kim, H.D.; Kim, K.P.; Ryu, M.H.; Park, S.R.; Choi, W.M.; Lee, D.; Choi, J.; Shim, J.H.; et al. Clinical outcomes of immune checkpoint inhibitors in unresectable or metastatic combined hepatocellular–cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 7547–7555. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoo, C.; Kang, H.J.; Kim, K.P.; Ryu, M.H.; Park, S.R.; Lee, D.; Choi, J.; Shim, J.H.; Kim, K.M.; et al. Clinical outcomes of systemic therapy in patients with unresectable or metastatic combined hepatocellular-cholangiocarcinoma. Liver Int. 2021, 41, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Ono, A.; Yano, S.; Amioka, K.; Naruto, K.; Yamaoka, K.; Fujii, Y.; Uchikawa, S.; Fujino, H.; Nakahara, T.; et al. Real-world efficacy and safety of durvalumab–tremelimumab as second-line systemic therapy after atezolizumab–bevacizumab in unresectable hepatocellular carcinoma. Medicine 2024, 103, e39289. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Ito, T.; Yoshikawa, S.; Yoshino, K.; Kiniwa, Y.; Ohe, S.; Isei, T.; Takenouchi, T.; Kato, H.; Mizuhashi, S.; et al. Systemic therapy for Asian patients with advanced BRAF V600-mutant melanoma in a real-world setting: A multi-center retrospective study in Japan (B-CHECK-RWD study). Cancer Med. 2023, 12, 17967–17980. [Google Scholar] [CrossRef] [PubMed]
- Zaemes, J.; Ari, S.L.; Pascual, L.; Jegede, O.A.; Arias-Orozco, N.; Sinclaire, B.; DellaPia, A.; Zemel, R.; Zhang, Y.; Gupta, S.; et al. 1418 Treatment-free survival in patients with advanced melanoma and non-small cell lung cancer receiving immune checkpoint inhibitors: Real-world outcomes over a 3-year timespan. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Lee, S.J.; Na, J.; Lee, I.H.; Lee, J.; Lee, S.-J. Enhanced dacarbazine efficacy in advanced melanoma patients previously treated with immune checkpoint inhibitors in Korea. Am. Soc. Clin. Oncol. 2024, 42, e21528. [Google Scholar] [CrossRef]
- Nardin, C.; Hennemann, A.; Diallo, K.; Funck-Brentano, E.; Puzenat, E.; Heidelberger, V.; Jeudy, G.; Samimi, M.; Lesage, C.; Boussemart, L.; et al. Efficacy of immune checkpoint inhibitor (ICI) rechallenge in advanced melanoma patients’ Responders to a first course of ICI: A multicenter national retrospective study of the french group of skin cancers (Groupe de cancérologie cutanée, GCC). Cancers 2023, 15, 3564. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Guo, Y.; Guo, W.; Wu, X. Addition of PD-1/PD-L1 inhibitors to chemotherapy for triple-negative breast cancer: A meta-analysis. Front. Oncol. 2024, 14, 1309677. [Google Scholar] [CrossRef]
- Villacampa, G.; Tolosa, P.; Salvador, F.; Sánchez-Bayona, R.; Villanueva, L.; Dienstmann, R.; Ciruelos, E.; Pascual, T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102352. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Tang, Y.; Dai, W.; Si, Y.; Mao, F.; Xu, J.; Yu, C.; Sun, X. A meta-analysis of application of PD-1/PD-L1 inhibitor-based immunotherapy in unresectable locally advanced triple-negative breast cancer. Immunotherapy 2023, 15, 1073–1088. [Google Scholar] [CrossRef]
- Liang, X.; Chen, X.; Li, H.; Li, Y. Immune checkpoint inhibitors in first-line therapies of metastatic or early triple-negative breast cancer: A systematic review and network meta-analysis. Front. Endocrinol. 2023, 14, 1137464. [Google Scholar] [CrossRef]
- Grossi, F.; Crinò, L.; Logroscino, A.; Canova, S.; Delmonte, A.; Melotti, B.; Proto, C.; Gelibter, A.; Cappuzzo, F.; Turci, D.; et al. Use of nivolumab in elderly patients with advanced squamous non–small-cell lung cancer: Results from the Italian cohort of an expanded access programme. Eur. J. Cancer 2018, 100, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; Sayed, I.E.T.E.; Ali, M.M.; Wang, Y.; Baz, A.E.; Khalil, A.A.; Elarabany, N. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt. Liver J. 2024, 14, 2. [Google Scholar] [CrossRef]
- Association, K.L.C. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J. Radiol. 2022, 23, 1126. [Google Scholar]
- Yoon, J.H.; Choi, S.K. Management of early-stage hepatocellular carcinoma: Challenges and strategies for optimal outcomes. J. Liver Cancer 2023, 23, 300–315. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Chen, S.; Xu, B.; Wu, Z.; Wang, P.; Yu, W.; Liu, Z.; Huang, X.; Wu, Y.; Li, T.; Guo, W. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: A multicenter retrospective study. BMC Cancer 2021, 21, 1126. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Di Donato, M.; Cristiani, C.M.; Capone, M.; Garofalo, C.; Madonna, G.; Passacatini, L.C.; Ottaviano, M.; Ascierto, P.A.; Auricchio, F.; Carbone, E.; et al. Role of the androgen receptor in melanoma aggressiveness. Cell Death Dis. 2025, 16, 34. [Google Scholar] [CrossRef]
- Dousset, L.; Poizeau, F.; Robert, C.; Mansard, S.; Mortier, L.; Caumont, C.; Routier, É.; Dupuy, A.; Rouanet, J.; Battistella, M.; et al. Positive association between location of melanoma, ultraviolet signature, tumor mutational burden, and response to anti–PD-1 therapy. JCO Precis. Oncol. 2021, 5, 1821–1829. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demido, L.; Hassel, J.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F.; et al. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.M. Triple-negative breast cancer: From none to multiple therapeutic targets in two decades. Front. Oncol. 2023, 13, 1244781. [Google Scholar] [CrossRef]
- Loi, S.; Salgado, R.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 2022, 8, 3. [Google Scholar] [CrossRef]
- Karn, T.; Denkert, C.; Weber, K.E.; Holtrich, U.; Hanusch, C.; Sinn, B.V.; Higgs, B.W.; Jank, P.; Sinn, H.P.; Huober, J.; et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. 2020, 31, 1216–1222. [Google Scholar] [CrossRef]
- Cortés, J.; Lipatov, O.; Im, S.-A.; Gonçalves, A.; Lee, K.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; Ohtani, S.; et al. KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann. Oncol. 2019, 30, v859–v860. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Heater, N.K.; Warrior, S.; Lu, J. Current and future immunotherapy for breast cancer. J. Hematol. Oncol. 2024, 17, 131. [Google Scholar] [CrossRef]
- Cheng, W.; Kang, K.; Zhao, A.; Wu, Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J. Hematol. Oncol. 2024, 17, 54. [Google Scholar] [CrossRef]
- Ahuja, S.; Zaheer, S. The evolution of cancer immunotherapy: A comprehensive review of its history and current perspectives. Korean J. Clin. Oncol. 2024, 20, 51. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical advances of mRNA vaccines for cancer immunotherapy. Med 2025, 6, 100562. [Google Scholar] [CrossRef] [PubMed]
- Chowaniec, H.; Ślubowska, A.; Mroczek, M.; Borowczyk, M.; Braszka, M.; Dworacki, G.; Dobosz, P.; Wichtowski, M. New hopes for the breast cancer treatment: Perspectives on the oncolytic virus therapy. Front. Immunol. 2024, 15, 1375433. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Na, J.; Zhong, L.; Zhang, P. Advances in preclinical and clinical studies of oncolytic virus combination therapy. Front. Oncol. 2025, 15, 1545542. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, K.H.; Park, E.Y.; Kim, E.T.; Kim, E.J.; Tan, D.S.P.; Lee, J.Y.; Park, S.Y.; Fotopoulou, C.; Lim, M.C. Efficacy of immune-checkpoint inhibitors combined with cytotoxic chemotherapy in advanced or recurrent endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2024, 187, 85–91. [Google Scholar] [CrossRef]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Redman, M.W.; Dragnev, K.H.; Minichiello, K.; Villaruz, L.C.; Faller, B.; Al Baghdadi, T.; Hines, S.; Everhart, L.; Highleyman, L.; et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non–small-cell lung cancer previously treated with immunotherapy—Lung-MAP S1800A. J. Clin. Oncol. 2022, 40, 2295–2307. [Google Scholar] [CrossRef]
- Li, A.Q.; Fang, J.H. Anti-angiogenic therapy enhances cancer immunotherapy: Mechanism and clinical application. Interdiscip. Med. 2024, 2, e20230025. [Google Scholar] [CrossRef]
- Ma, X.; Ou, K.; Yang, W.; Cao, B.; Yang, L. Real world data and biomarker analysis of the efficacy and safety of PD-1 inhibitor combined with chemotherapy in first-line treatment of advanced gastric cancer. Am. Soc. Clin. Oncol. 2025, 43, 342. [Google Scholar] [CrossRef]
- Guan, J.; Sun, K.; Guerrero, C.A.; Zheng, J.; Xu, Y.; Mathur, S.; Teh, B.S.; Farach, A.; Zhang, J.; Butler, E.; et al. A phase 2 study of in situ oncolytic virus therapy and stereotactic body radiation therapy followed by pembrolizumab in metastatic non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Ning, Z.; Xu, L.; Shen, Y.; Zhu, X.; Yu, W.; Xie, J.; Meng, Z. The combination treatment of oncolytic adenovirus H101 with nivolumab for refractory advanced hepatocellular carcinoma: An open-label, single-arm, pilot study. ESMO Open 2024, 9, 102239. [Google Scholar] [CrossRef]
- Gainor, J.F.; Patel, M.R.; Weber, J.S.; Gutierrez, M.; Bauman, J.E.; Clarke, J.M.; Julian, R.; Scott, A.J.; Geiger, J.L.; Kirtane, K.; et al. T-cell responses to individualized neoantigen therapy mRNA-4157 (V940) alone or in combination with pembrolizumab in the phase 1 KEYNOTE-603 study. Cancer Discov. 2024, 14, 2209–2223. [Google Scholar] [CrossRef]
- Garcia-Reyes, K.; Gottlieb, R.A.; Menon, K.M.; Bishay, V.; Patel, R.; Patel, R.; Nowakowski, S.; Sung, M.W.; Marron, T.U.; Gansa, W.H.; et al. Radioembolization plus immune checkpoint inhibitor therapy compared with radioembolization plus tyrosine kinase inhibitor therapy for the treatment of hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2024, 35, 722–730.e1. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.; Pratumchai, I.; Marro, B.S.; Marquardt, K.L.; Zavareh, R.B.; Lairson, L.L.; Oldstone, M.B.A.; Varner, J.A.; Hegerova, L.; Cao, Q.; et al. JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma. Science 2024, 384, eade8520. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Marmarelis, M.E.; Foley, C.; Bauml, J.M.; Ye, D.; Ghinnagow, R.; Ngiow, S.F.; Klapholz, M.; Jun, S.; Zhang, Z.; et al. Combined JAK inhibition and PD-1 immunotherapy for non–small cell lung cancer patients. Science 2024, 384, eadf1329. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.G.; et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Chen, D.; Varanasi, S.K.; Hara, T.; Traina, K.; Sun, M.; McDonald, B.; Farsakoglu, Y.; Clanton, J.; Xu, S.; Garcia-Rivera, L.; et al. CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity 2023, 56, 2086–2104.e8. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Majem, M.; Forster, M.; Krebs, M.G.; Peguero, J.; Clay, T.; Felip, E.; Iams, W.; Roxburgh, E.; Doger de Spéville, B.; Bajaj, P.; et al. 11MO Final data from a phase II study (TACTI-002) of eftilagimod alpha (soluble LAG-3) and pembrolizumab in 2nd-line metastatic NSCLC pts resistant to PD-1/PD-L1 inhibitors. J. Thorac. Oncol. 2023, 18, S43–S44. [Google Scholar] [CrossRef]
- Mimura, K.; Kua, L.F.; Xiao, J.F.; Asuncion, B.R.; Nakayama, Y.; Syn, N.; Fazreen, Z.; Soong, R.; Kono, K.; Yong, W.P. Combined inhibition of PD-1/PD-L1, Lag-3, and Tim-3 axes augments antitumor immunity in gastric cancer–T cell coculture models. Gastric Cancer 2021, 24, 611–623. [Google Scholar] [CrossRef]
- Youssef, R.; Maniar, R.; Khan, J.; Mesa, H. Metabolic interplay in the tumor microenvironment: Implications for immune function and anticancer response. Curr. Issues Mol. Biol. 2023, 45, 9753–9767. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, K.E.; Dudzinski, S.O.; Rathmell, J.C. Dysfunctional T cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev. 2017, 35, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, S.; Gupta, P.; Corbali, H.I.; Nanez, S.; Riad, T.S.; Duong, L.K.; Barsoumian, H.B.; Masrorpour, F.; Jiang, H.; Welsh, J.W.; et al. The role of tumor metabolism in modulating T-Cell activity and in optimizing immunotherapy. Front. Immunol. 2023, 14, 1172931. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Lu, T.; Bi, G.; Li, M.; Liang, J.; Hu, Z.; Zheng, Y.; Yin, J.; Xi, J.; et al. HIF-1α switches the functionality of TGF-β signaling via changing the partners of smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 398. [Google Scholar] [CrossRef]
- Lim, S.A. Metabolic reprogramming of the tumor microenvironment to enhance immunotherapy. BMB Rep. 2024, 57, 388. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019, 8, LMT11. [Google Scholar] [CrossRef]
- Ying, L.; Cheng, M.; Lu, Y.; Tao, Q.; Chen, X.; Shen, B.; Xiong, F.; Hu, Z.; Wang, D.; Li, X. Glutamine metabolism scoring predicts prognosis and therapeutic resistance in hepatocellular carcinoma. Pathol. Oncol. Res. 2021, 27, 1610075. [Google Scholar] [CrossRef]
- Ratnayake, G.; Reinwald, S.; Edwards, J.; Wong, N.; Yu, D.; Ward, R.; Smith, R.; Haydon, A.; Au, P.M.; van Zelm, M.C.; et al. Blood T-cell profiling in metastatic melanoma patients as a marker for response to immune checkpoint inhibitors combined with radiotherapy. Radiother. Oncol. 2022, 173, 299–305. [Google Scholar] [CrossRef]
- Silk, A.W.; Kaufman, H.L.; Curti, B.; Mehnert, J.M.; Margolin, K.; McDermott, D.; Clark, J.; Newman, J.; Bommareddy, P.K.; Denzin, L.; et al. High-dose ipilimumab and high-dose interleukin-2 for patients with advanced melanoma. Front. Oncol. 2020, 9, 1483. [Google Scholar] [CrossRef]
- Ellingsen, E.B.; Bounova, G.; Kerzeli, I.; Anzar, I.; Simnica, D.; Aamdal, E.; Guren, T.; Clancy, T.; Mezheyeuski, A.; Inderberg, E.M.; et al. Characterization of the T cell receptor repertoire and melanoma tumor microenvironment upon combined treatment with ipilimumab and hTERT vaccination. J. Transl. Med. 2022, 20, 419. [Google Scholar] [CrossRef]
- Poran, A.; Scherer, J.; Bushway, M.E.; Besada, R.; Balogh, K.N.; Wanamaker, A.; Williams, R.G.; Prabhakara, J.; Ott, P.A.; Hu-Lieskovan, S.; et al. Combined TCR repertoire profiles and blood cell phenotypes predict melanoma patient response to personalized neoantigen therapy plus anti-PD-1. Cell Rep. Med. 2020, 1, 100141. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Gainor, J.F.; Callahan, M.K.; Falchook, G.S.; Pachynski, R.K.; LoRusso, P.; Kummar, S.; Gibney, G.T.; Burris, H.A.; Tykodi, S.S.; et al. First-in-human phase I/II ICONIC trial of the ICOS agonist vopratelimab alone and with nivolumab: ICOS-high CD4 T-cell populations and predictors of response. Clin. Cancer Res. 2022, 28, 3695–3708. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, E.; Besharat, Z.M.; Covre, A.; Maio, M.; Di Giacomo, A.M.; Ferretti, E. Immunotherapy in melanoma: Can we predict response to treatment with circulating biomarkers? Pharmacol. Ther. 2024, 256, 108613. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, X.; Wei, J.; Weng, Y.; Wang, J.; Wang, T.; Song, Q.; Min, P. Safety and efficacy of immune checkpoint inhibitor rechallenge in advanced non-small cell lung cancer: A retrospective study. Sci. Rep. 2024, 14, 2315. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising immunotherapy targets: TIM3, LAG3, and TIGIT joined the party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Carini, C. Insights and strategies of melanoma immunotherapy: Predictive biomarkers of response and resistance and strategies to improve response rates. Int. J. Mol. Sci. 2022, 24, 41. [Google Scholar] [CrossRef]
- Ding, H.; Wang, G.; Yu, Z.; Sun, H.; Wang, L. Role of interferon-gamma (IFN-γ) and IFN-γ receptor 1/2 (IFNγR1/2) in regulation of immunity, infection, and cancer development: IFN-γ-dependent or independent pathway. Biomed. Pharmacother. 2022, 155, 113683. [Google Scholar] [CrossRef]
- Ji, J.H.; Ha, S.Y.; Lee, D.; Sankar, K.; Koltsova, E.K.; Abou-Alfa, G.K.; Yang, J.D. Predictive biomarkers for immune-checkpoint inhibitor treatment response in patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2023, 24, 7640. [Google Scholar] [CrossRef]
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent advances of oncolytic virus in cancer therapy. Hum. Vaccines Immunother. 2020, 16, 2389–2402. [Google Scholar] [CrossRef]
- Tang, S.; Lyles, K.V.; Wang, Y.; Fan, D.; Luo, M. Enhancing the Efficacy of Breast Cancer Immunotherapy Using a Smac-Armed Oncolytic Virus. Cancers 2024, 16, 3248. [Google Scholar] [CrossRef]
- Sussman, T.; Ott, P. Adjuvant immunotherapy for melanoma patients: Progress and opportunities. ESMO Open 2024, 9, 102962. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.; McKean, M.; Long, G.; Sullivan, R.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Kyr, M.; Mudry, P.; Polaskova, K.; Dubska, L.Z.; Demlova, R.; Kubatova, J.; Hlavackova, E.; Pilatova, K.C.; Mazanek, P.; Vejmelkova, K.; et al. Personalized dendritic cell vaccine in multimodal individualized combination therapy improves survival in high-risk pediatric cancer patients. Int. J. Cancer 2024, 155, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Rubsamen, R. New Vaccine Therapy for Triple-Negative Breast Cancer. Curr. Breast Cancer Rep. 2024, 16, 288–301. [Google Scholar] [CrossRef]
- Meireson, A.; Tavernier, S.J.; Van Gassen, S.; Sundahl, N.; Demeyer, A.; Spaas, M.; Kruse, V.; Ferdinande, L.; Van Dorpe, J.; Hennart, B.; et al. Immune monitoring in melanoma and urothelial cancer patients treated with anti-PD-1 immunotherapy and SBRT discloses tumor specific immune signatures. Cancers 2021, 13, 2630. [Google Scholar] [CrossRef]
- Boutros, C.; Chaput-Gras, N.; Lanoy, E.; Larive, A.; Mateus, C.; Routier, E.; Sun, R.; Tao, Y.G.; Massard, C.; Bahleda, R.; et al. Dose escalation phase 1 study of radiotherapy in combination with anti-cytotoxic-T-lymphocyte-associated antigen 4 monoclonal antibody ipilimumab in patients with metastatic melanoma. J. Immunother. Cancer 2020, 8, e000627. [Google Scholar] [CrossRef]
- Ruff, S.M.; Pawlik, T.M. The role of immune checkpoint inhibitors and/or Yttrium-90 radioembolization in the management of hepatocellular carcinoma: Challenges of treatment sequence. Hepatoma Res. 2024, 10, 33. [Google Scholar] [CrossRef]
- Chen, R.; Monaco, G.; Stefanini, B.; Marseglia, M.; De Lorenzo, S.; Tovoli, F. Combination makes strength: A narrative review of transarterial radioembolization plus immune checkpoint inhibitors for hepatocellular carcinoma. Chin. Clin. Oncol. 2024, 13, 71. [Google Scholar] [CrossRef]
- Rafiq, Z.; Kang, M.; Barsoumian, H.B.; Manzar, G.S.; Hu, Y.; Leuschner, C.; Huang, A.; Masrorpour, F.; Lu, W.; Puebla-Osorio, N.; et al. Enhancing immunotherapy efficacy with synergistic low-dose radiation in metastatic melanoma: Current insights and prospects. J. Exp. Clin. Cancer Res. 2025, 44, 31. [Google Scholar] [CrossRef]
- Dong, S.; Ma, Z. Combination of JAK inhibitor and immune checkpoint inhibitor in clinical trials: A breakthrough. Front. Immunol. 2024, 15, 1459777. [Google Scholar] [CrossRef]
- Yang, J.; Kang, H.; Lyu, L.; Xiong, W.; Hu, Y. A target map of clinical combination therapies in oncology: An analysis of clinicaltrials.gov. Discov. Oncol. 2023, 14, 151. [Google Scholar] [CrossRef]
- Hamilton, M.P.; Sugio, T.; Noordenbos, T.; Shi, S.; Bulterys, P.L.; Liu, C.L.; Kang, X.; Olsen, M.N.; Good, Z.; Dahiya, S.; et al. Risk of Second Tumors and T-Cell Lymphoma after CAR T-Cell Therapy. N. Engl. J. Med. 2024, 390, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, X.; Wang, Y.; Lu, S. The Gut Microbiota Improves the Efficacy of Immune-checkpoint Inhibitor Immunotherapy against Tumors: From Association to Cause and Effect. Cancer Lett. 2024, 598, 217123. [Google Scholar] [CrossRef]
- Li, Q.; Chan, H.; Liu, W.X.; Liu, C.A.; Zhou, Y.; Huang, D.; Wang, X.; Li, X.; Xie, C.; Liu, W.Y.; et al. Carnobacterium maltaromaticum boosts intestinal vitamin D production to suppress colorectal cancer in female mice. Cancer Cell 2023, 41, 1450–1465.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kołat, D.; Yang, K.; Hu, J.K.; et al. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of immunotherapy combination strategies in cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Monti, E.; Vianello, C.; Leoni, I.; Galvani, G.; Lippolis, A.; D’Amico, F.; Roggiani, S.; Stefanelli, C.; Turroni, S.; Fornari, F. Gut Microbiome Modulation in Hepatocellular Carcinoma: Preventive Role in NAFLD/NASH Progression and Potential Applications in Immunotherapy-Based Strategies. Cells 2025, 14, 84. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Kim, D.; Sarvesh, S.; Valbak, O.; Backan, O.; Chen, D.; Morrow, C.; Benvensite, E.; Nabors, B.; Bekal, M.; Dono, A.; et al. MODL-06. THE GUT MICROBIOTA OF BRAIN TUMOR PATIENTS CAN IMPACT IMMUNOTHERAPY EFFICACY IN A PRECLINICAL MODEL OF GLIOMA. Neuro-Oncol. 2023, 25 (Suppl. S5), v299. [Google Scholar] [CrossRef]
- Li, R.; Hu, Y.; Hou, S. An exploration of oral-gut pathogens mediating immune escape of pancreatic cancer via miR-21/PTEN Axis. Front. Microbiol. 2022, 13, 928846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, Y.; Wu, T.; Ben, E.; Li, S.; Hu, L.; Xie, T. Role of gut microbiota in regulating immune checkpoint inhibitor therapy for glioblastoma. Front. Immunol. 2024, 15, 1401967. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, C.; Ren, K.; Xu, M.; Pan, C.; Ye, X.; Li, L. Modulation of gut microbiota by probiotics to improve the efficacy of immunotherapy in hepatocellular carcinoma. Front. Immunol. 2024, 15, 1504948. [Google Scholar] [CrossRef]
- Berland, L.; Gabr, Z.; Chang, M.; Ilié, M.; Hofman, V.; Rignol, G.; Ghiringhelli, F.; Mograbi, B.; Rashidian, M.; Hofman, P. Further knowledge and developments in resistance mechanisms to immune checkpoint inhibitors. Front. Immunol. 2024, 15, 1384121. [Google Scholar] [CrossRef]
- Wang, M.; Du, Q.; Jin, J.; Wei, Y.; Lu, Y.; Li, Q. LAG3 and its emerging role in cancer immunotherapy. Clin. Transl. Med. 2021, 11, e365. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Zhong, M.; Yarden, Y.; Fu, L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol. Cancer 2020, 19, 81. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Bu, F.; Zhang, H.; Fei, K.; Zhang, P. Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: A systematic review and meta-analysis. BMC Cancer 2020, 20, 707. [Google Scholar] [CrossRef]
- Gang, X.; Yan, J.; Li, X.; Shi, S.; Xu, L.; Liu, R.; Cai, L.; Li, H. Immune checkpoint inhibitors rechallenge in non-small cell lung cancer: Current evidence and future directions. Cancer Lett. 2024, 604, 217241. [Google Scholar] [CrossRef]
- Friedlaender, A.; Addeo, A.; Banna, G. New emerging targets in cancer immunotherapy: The role of TIM3. ESMO Open 2019, 4, e000497. [Google Scholar] [CrossRef]
- Chavanton, A.; Mialhe, F.; Abrey, J.; Baeza Garcia, A.; Garrido, C. LAG-3: Recent developments in combinational therapies in cancer. Cancer Sci. 2024, 115, 2494–2505. [Google Scholar] [CrossRef]
- Hasan, M.F.; Croom-Perez, T.J.; Oyer, J.L.; Dieffenthaller, T.A.; Robles-Carrillo, L.D.; Eloriaga, J.E.; Kumar, S.; Andersen, B.W.; Copik, A.J. TIGIT expression on activated NK cells correlates with greater anti-tumor activity but promotes functional decline upon lung cancer exposure: Implications for adoptive cell therapy and TIGIT-targeted therapies. Cancers 2023, 15, 2712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, J. Functions of immune checkpoint molecules beyond immune evasion. In Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy; Springer: Singapore, 2020; pp. 201–226. [Google Scholar]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Chen, X.Y.; Yan, Y.; Cheng, A.Y.; Lin, J.Y.; Jiang, Y.X.; Chen, H.Z.; Jin, J.M.; Luan, X. Targeting metabolism to enhance immunotherapy within tumor microenvironment. Acta Pharmacol. Sin. 2024, 45, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Stirling, E.R.; Song, Q.; Westwood, B.; Kooshki, M.; Forbes, M.E.; Holbrook, B.C.; Cook, K.L.; Alexander-Miller, M.A.; Miller, L.D.; et al. Circulating immune bioenergetic, metabolic, and genetic signatures predict melanoma patients’ response to anti–pd-1 immune checkpoint blockade. Clin. Cancer Res. 2022, 28, 1192–1202. [Google Scholar] [CrossRef]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and cancer: Metabolism, immune microenvironment, and therapeutic targets. Cell Commun. Signal. 2025, 23, 45. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, J.; Yin, M.; Liu, H.; Zhang, X.; Li, J.; Yan, B.; Guo, Y.; Zhou, J.; Tao, J.; et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol. Ther. 2021, 29, 2321–2334. [Google Scholar] [CrossRef]
- Best, S.A.; Gubser, P.M.; Sethumadhavan, S.; Kersbergen, A.; Negrón Abril, Y.L.; Goldford, J.; Sellers, K.; Abeysekera, W.; Garnham, A.L.; McDonald, J.A.; et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022, 34, 874–887.e6. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Thompson, J.W.; Yin, T.; Alexander, P.B.; Qin, D.; Mudgal, P.; Wu, H.; Liang, Y.; Tan, L.; et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 2022, 24, 230–241. [Google Scholar] [CrossRef]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Morozumi, K.; Kawasaki, Y.; Sato, T.; Maekawa, M.; Takasaki, S.; Shimada, S.; Sakai, T.; Yamasita, S.; Mano, N.; Ito, A. Glutamine metabolism and VEGF analysis to elucidate and overcome the mechanism of tyrosine kinase inhibitor resistance in renal cell carcinoma. Am. Soc. Clin. Oncol. 2024, 42, 464. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, Z.; Sun, X.; Wei, B.; Fu, Y.; Shi, D.; Zhu, Y. Identification of a glutamine metabolism reprogramming signature for predicting prognosis, immunotherapy efficacy, and drug candidates in bladder cancer. Front. Immunol. 2023, 14, 1111319. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.N.; Abbas, Z.; Lee, S.W. Efficacy and adverse events of immune checkpoint inhibitors: Evidence from non-small cell lung cancer and gastric cancer in Korea and Japan. Precis. Future Med. 2025, 9, 15–24. [Google Scholar] [CrossRef]
- Jung, H.; Kim, S.; Lee, C.S.; Byeon, S.H.; Kim, S.S.; Lee, S.W.; Kim, Y.J. Real-World Incidence of Incident Noninfectious Uveitis in Patients Treated With BRAF Inhibitors: A Nationwide Clinical Cohort Study. Am. J. Ophthalmol. 2024, 267, 142–152. [Google Scholar] [CrossRef]
- Chang, M.S.; Lee, S.W.; Kim, S.; Lee, C.S.; Byeon, S.H.; Kim, S.S.; Kim, Y.J. Incident noninfectious uveitis risk after immune checkpoint inhibitor treatment. Ophthalmology 2024, 131, 867–869. [Google Scholar] [CrossRef]
- Moon, H.; Kim, S.G.; Kim, S.K.; Kim, J.; Lee, S.R.; Moon, Y.W. A case report of re-challenge of immune checkpoint inhibitors after immune-related neurological adverse events: Review of literature. Medicine 2022, 101, e30236. [Google Scholar] [CrossRef]
- Satoh, T.K.; Neulinger, M.M.; Stadler, P.C.; Aoki, R.; French, L.E. Immune checkpoint inhibitor-induced epidermal necrolysis: A narrative review evaluating demographics, clinical features, and culprit medications. J. Dermatol. 2024, 51, 3–11. [Google Scholar] [CrossRef]

| Population and Design | Strategies Utilized | ORR | DCR | PFS (Months) | OS (Months) | Reference |
|---|---|---|---|---|---|---|
| Retrospective; n = 134 | Long-term ICI monotherapy (≥18 months) | 58.2% (overall); 33.3% (≥18 months) | 83.3% (≥18 months) | 10.6 | Not reached (↑ if ≥18 months ICI) | [15] |
| Retrospective; n = 145 | ICI + Chemotherapy or Anti-angiogenic | 29.3% | 85.4% | 6.77 | 18.60 | [16] |
| Meta-analysis; n = 2410 | ICI + ICI, ICI + chemo, various combos | RR 1.82 (↑) | RR 1.41 (↑) | HR 0.83 (↑) | HR 0.90 (↑) | [17] |
| Retrospective; n = 143 | ICI + TKIs, cross-line ICI | 11.2% | 72.7% | 4.6 | 11.8 | [18] |
| Real-world; n = 110 | ICIs (monotherapy or with novel/anti-angiogenic agents) | - | 75% | 5.5 | 20.3 | [19] |
| Real-world; n = 90 | ICI-based therapy | 36.7% | 78.9% | 4.9 | 13.9 | [20] |
| Real-world; n = 244 | First-line ICI monotherapy | - | - | 7.0; 11.3 (if >3 months immunotherapy) | 11.8; 15.4 (if >3 months immunotherapy) | [21] |
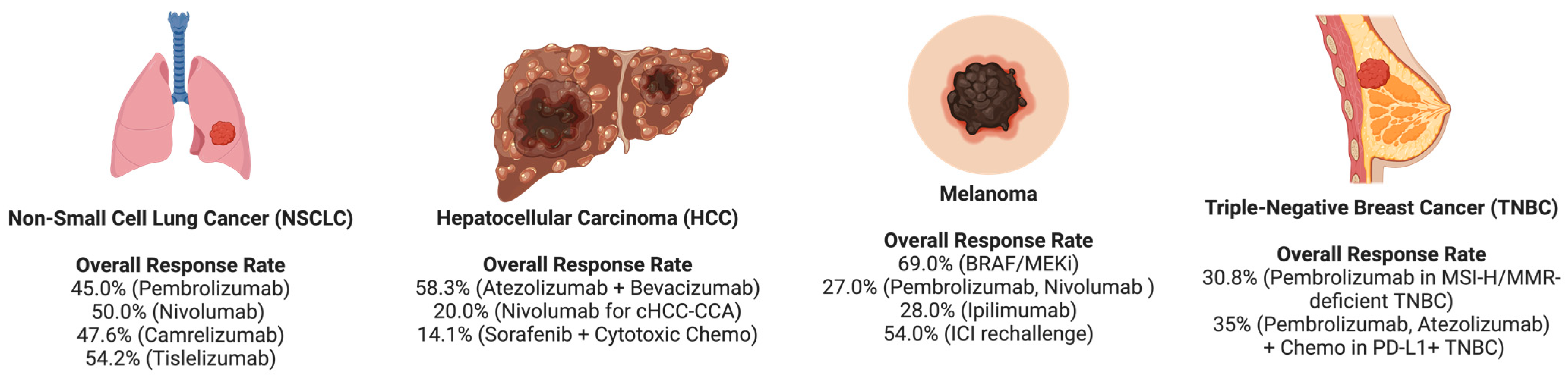
| Cancer Type | ORR | PFS | OS | DCR | References |
|---|---|---|---|---|---|
| NSCLC | Pembrolizumab: 45.0%, Nivolumab: 50% Camrelizumab: 47.6%, Tislelizumab: 54.2% | Median PFS for ICI-based therapies: 9.5 months. Pembrolizumab: 9.6 months, Nivolumab: 9.2 months, Camrelizumab: 10.4 months, Tislelizumab: 10.3 months | Median OS for ICI monotherapy: 10.9 months, chemotherapy: 10.7 months, ICI combination therapy: 20.3 months. In PD-L1 ≥ 1%, OS improved in ICI (22.4 months) vs. ICI + chemo (10.7 months) | ICI-treated elderly patients achieved 75% DCR | [19,27,28], |
| HCC | Atezo + Bev: 58.3%, Dur + Tre: 0%, Sorafenib and Cytotoxic Chemo: 14.1%, ICIs for cHCC-CCA: 20% | Atezo + Bev: 2.9 months, Lenvatinib (2L): 4.0 months, Sorafenib (2L): 2.3 months, TKI + ICI: 5.4 months, cHCC-CCA (ICIs): 3.5 months, Sorafenib and Cytotoxic Chemo: 3.8 months | Atezo + Bev: 8.0 months, Lenvatinib (2L): 8.0 months, Sorafenib (2L): 6.3 months, TKI + ICI: 12.6 months, cHCC-CCA (ICIs): 8.3 months, Sorafenib and Cytotoxic Chemo: 10.6 months | Atezo + Bev: 87.5%, Dur + Tre: 62.5% | [29,30,31,32] |
| Melanoma | BRAF/MEKi: 69%, Nivolumab: 27%, Nivolumab + Ipilimumab: 28% ICI rechallenge: 54% | BRAF/MEKi: 14.7 months, Anti-PD-1: 5.4 months, PD-1/CTLA-4: 5.8 months, ICI rechallenge: 21 months, Pembrolizumab (previously treated): 3.9 months, Pembrolizumab (naïve): 2.3 months | BRAF/MEKi: 34.6 months, Anti-PD-1: 37.0 months, Pembrolizumab (previously treated): 19.0 months, Pembrolizumab (naïve): 6.8 months, ICI rechallenge: Not reached (1-year OS: 78%, 2-year OS: 71%) | ICI rechallenge: 75% | [33,34,35,36] |
| TNBC | Pembrolizumab (CPS ≥ 10): Increased ORR, MSI-H/MMR-deficient: 30.8%, ICI + Chemo: OR 1.35 (95% CI) | Pembrolizumab (MSI-H/MMR-deficient): 3.5 months, ICI + Chemo (ITT): HR 0.80 (95% CI), ICI + Chemo (PD-L1+): HR 0.70 (95% CI), ICI + Chemo (no prior CT): HR 0.53 (95% CI) | ICI + Chemo (ITT): HR 0.89 (95% CI), ICI + Chemo (PD-L1+): HR 0.80 (95% CI), ICI + Chemo (no prior CT): HR 0.81 (95% CI), PD-1/PD-L1 + Chemo: No significant OS improvement | Not specified | [37,38,39,40] |
| Strategy | Key Findings | References |
|---|---|---|
| Combination Therapy | Anti-PD-1 + anti-CTLA-4 a dual checkpoint blockade improves outcomes. CAR-T cell therapy and mRNA vaccines enhances immune responses. Oncolytic viruses combined with chemotherapy or radiotherapy target metastatic tumors | [61,62,63,64,65] |
| ICI + Chemotherapy | Platinum-based chemo + ICI improved PFS and OS in endometrial cancer, with dMMR patients benefiting most | [66] |
| Anti-Angiogenesis + ICIs | Atezolizumab + bevacizumab in NSCLC improved OS. Ramucirumab + pembrolizumab enhanced OS in NSCLC and gastric cancer (GC). | [67,68,69] |
| Biomarker-guided Therapy | PD-L1, TMB, MSI as predictors; High LDH levels linked to poor OS; HER2-negative GC had ORR 61.9%, DCR 96.8%, PFS of 9 months and OS of 27 months. | [70] |
| Oncolytic Viruses | In situ ADV/HSV-tk + SBRT + pembrolizumab in mNSCLC had ORR of 33.3%, CBR of 70.4%, PFS of 7.4 months and an OS of 18.1 months. H101 + nivolumab in HCC: ORR 11.1%, DCR 38.9%, PFS 2.69 months, OS 15.04 months. | [71,72] |
| Cancer Vaccines | mRNA-4157 + pembrolizumab in melanoma extended recurrence-free survival of 18 months. α-lactalbumin vaccine in TNBC induced immune responses with no major adverse events. | [73] |
| Radiotherapy + ICIs | Radioembolization + ICI in HCC had ORR of 89.5%, DCR of 94.7% and LDRT enhanced ICI responses in preclinical models. | [74] |
| JAK Inhibitors + ICIs | JAK inhibitors improved ICI response rate (53%) and 2-year PFS in Hodgkin lymphoma and NSCLC; JAK1 inhibition post-pembrolizumab enhanced immune function. | [75,76] |
| Microbiome Modulation | Gut microbiota regulates ICI response via metabolite production. Fecal microbiota transplant enhances PD-1 therapy. Bile acid metabolism increases CXCR6+ NKT cells in HCC. | [77,78,79] |
| Alternative Checkpoints (LAG3, TIM3, TIGIT) | LAG3 inhibitors (relatlimab, eftilagimod alpha and pembrolizumab) improved ORR 8.3%, DCR 33% Dual inhibition of LAG3 + TIGIT enhances CD8+ T-cell response and reduces tumor growth. | [80,81] |
| Metabolic Reprogramming + ICIs | Targeting glucose/lactate metabolism enhances ICI efficacy. Metformin + PD-1 inhibitor (nivolumab or pembrolizumab) improved NSCLC response rate. | [82,83,84,85,86,87,88] |
| Glutamine Metabolism + ICIs | High glutamine metabolism linked to ICI resistance. Hight GMScore in HCC correlated with poor OS and high immune checkpoint expression. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mc Neil, V.; Lee, S.W. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers 2025, 17, 1408. https://doi.org/10.3390/cancers17091408
Mc Neil V, Lee SW. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers. 2025; 17(9):1408. https://doi.org/10.3390/cancers17091408
Chicago/Turabian StyleMc Neil, Valencia, and Seung Won Lee. 2025. "Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies" Cancers 17, no. 9: 1408. https://doi.org/10.3390/cancers17091408
APA StyleMc Neil, V., & Lee, S. W. (2025). Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers, 17(9), 1408. https://doi.org/10.3390/cancers17091408







