Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
3. Results
3.1. Selected Articles
3.2. Cancer Incidence
3.3. Cancer Mortality
4. Discussion
Molecular Mechanisms Mediated the Relationship Between Physical Activity and Cancer
5. Limitations and Recommendations for Future Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSM | American College of Sports Medicine |
| BMI | Body Mass Index |
| CAT | catalase |
| CRP | C-reactive protein |
| GPAQ | Global Physical Activity Questionnaire |
| GTX | glutathione peroxidase |
| HR | Hazard Ratio |
| HRQoL | Health-related quality life |
| IGF-1 | insulin-like growth factor-1 |
| IGFBP-3 | insulin growth factor-binding protein-3 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IPAQ | International Physical Activity Questionnaire |
| LTPAQ | leisure-time physical activity questionnaire |
| MET | metabolic equivalent |
| MVPA | moderate-to-vigorous physical activity |
| OR | Odds Ratio |
| OS | oxidative stress |
| PA | physical activity |
| PAGAC | Physical Activity Guidelines Advisory Committee |
| PE | physical exercise |
| PI | physical inactivity |
| r | regression coefficient |
| RR | Relative Risk |
| SHBG | sex hormone-binding globulin |
| SOD | superoxidase |
| TNF-α | tumor necrosis factor-α |
| VILPA | vigorous intermittent lifestyle physical activity |
| WCRF/AICR | World Cancer Research Fund/American Institute for Cancer Research |
References
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity with Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.; Kwok, W.; Wallbank, G.; Tiedemann, A.; Michaleff, Z.A.; Ng, C.A.C.M.; Bauman, A. Evidence on physical activity and falls prevention for people aged 65+ years: Systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 144. [Google Scholar] [CrossRef]
- Jochem, C.; Leitzmann, M. Physical activity and sedentary behavior in relation to cancer survival: A narrative review. Cancers 2022, 14, 1720. [Google Scholar] [CrossRef]
- Racine, A.N.; Margaritis, I.; Duclos, M.; Carré, F.; Vuillemin, A.; Gautier, C. Costing the economic burden of prolonged sedentary behaviours in France. Eur. J. Public Health 2022, 32 (Suppl. S1), i3–i7. [Google Scholar] [CrossRef]
- Rezende, L.F.M.; Ferrari, G.; Bahia, L.R.; Rosa, R.D.S.; Quarti, M.; da Rosa, M.; de Souza, R.C.; Lee, D.H.; Giovannucci, E.; Eluf-Neto, J. Economic burden of colorectal and breast cancers attributable to lack of physical activity in Brazil. BMC Public Health 2021, 21, 1190. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Yang, L.; Courneya, K.S.; Friedenreich, C.M. The Physical Activity and Cancer Control (PACC) framework: Update on the evidence, guidelines, and future research priorities. Br. J. Cancer 2024, 131, 957–969. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar] [PubMed]
- Campbell, W.W.; Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. High-Intensity Interval Training for Cardiometabolic Disease Prevention. Med. Sci. Sports Exerc. 2019, 51, 1220–1226. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Kraus, W.E.; Powell, K.E.; Campbell, W.W.; Janz, K.F.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; 2018 Physical Activity Guidelines Advisory Committee. Association between Bout Duration of Physical Activity and Health: Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1213–1219. [Google Scholar] [CrossRef]
- van der Ploeg, H.P.; Hillsdon, M. Is sedentary behaviour just physical inactivity by another name? Int. J. Behav. Nutr. Phys. Act. 2017, 14, 142. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Y.; Chen, D.; Zhang, H.; Xue, E.; Shao, J.; Tang, L.; Zhao, B.; Lai, C.; Ye, Z. Sedentary behavior patterns and the risk of non-communicable diseases and all-cause mortality: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2023, 146, 104563. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Giallauria, F.; Testa, C.; Cuomo, G.; Di Lorenzo, A.; Venturini, E.; Lauretani, F.; Maggio, M.G.; Iannuzzo, G.; Vigorito, C. Exercise training in elderly cancer patients: A systematic review. Cancers 2023, 15, 1671. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Physical activity and cancer prevention: Updating the evidence. The role of oxidative stress in carcinogenesis. Curr. Cancer Ther. Rev. 2007, 3, 81–95. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Pedersen, B.K. The physiology of optimizing health with a focus on exercise as medicine. Annu. Rev. Physiol. 2019, 81, 607–627. [Google Scholar] [CrossRef]
- Kramer, A. An overview of the beneficial effects of exercise on health and performance. Adv. Exp. Med. Biol. 2020, 1228, 3–22. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Pan, X.F.; Chen, J.; Cao, A.; Zhang, Y.G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical activity in cancer prevention and survival: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Gauci, C.; Delicata, N. Prevention of cancer through lifestyle change and screening. J. Malta Coll. Pharm. Pract. 2011, 17, 21–24. Available online: https://www.um.edu.mt/library/oar//handle/123456789/14133 (accessed on 12 May 2024).
- WHO. Cancer. 2 February 2022. Available online: https://www.who.int/news-room/facts-in-pictures/detail/cancer (accessed on 17 June 2024).
- Misiąg, W.; Piszczyk, A.; Szymańska-Chabowska, A.; Chabowski, M. Physical activity and cancer care—A review. Cancers 2022, 14, 4154. [Google Scholar] [CrossRef]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report; WCRF: Washington, DC, USA, 2018; Available online: https://www.wcrf.org/wp-content/uploads/2024/11/Summary-of-Third-Expert-Report-2018.pdf (accessed on 26 September 2024).
- Katzmarzyk, P.T.; Powell, K.E.; Jakicic, J.M.; Troiano, R.P.; Piercy, K.; Tennant, B.; 2018 Physical Activity Guidelines Advisory Committee. Sedentary behavior and health: Update from the 2018 physical activity guidelines advisory committee. Med. Sci. Sports Exerc. 2019, 51, 1227–1241. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Friedenreich, C.M.; Stone, C.R.; Cheung, W.Y.; Hayes, S.C. Physical activity and mortality in cancer survivors: A systematic review and meta-analysis. JNCI Cancer Spectr. 2019, 4, pkz080. [Google Scholar] [CrossRef]
- An, H.; Liu, K.; Shirai, K.; Kawasaki, R.; Tamakoshi, A.; Iso, H. Physical Activity and Bladder Cancer Risk: Findings of the Japan Collaborative Cohort Study. Cancer Res. Treat. 2024, 56, 616–623. [Google Scholar] [CrossRef]
- Bigman, G.; Adebamowo, S.N.; Yawe, K.T.; Yilkudi, M.; Olaomi, O.; Badejo, O.; Famooto, A.; Ezeome, E.; Salu, I.K.; Miner, E.; et al. Leisure-time physical activity is associated with reduced risks of breast cancer and triple negative breast cancer in Nigerian women. Cancer Epidemiol. 2022, 79, 102195. [Google Scholar] [CrossRef]
- Fortner, R.T.; Brantley, K.D.; Tworoger, S.S.; Tamimi, R.M.; Rosner, B.; Holmes, M.D.; Willett, W.C.; Eliassen, A.H. Recreational physical activity and breast cancer risk by menopausal status and tumor hormone receptor status: Results from the Nurses’ Health Studies. Breast Cancer Res. Treat. 2024, 206, 77–90. [Google Scholar] [CrossRef]
- Liu, W.; An, J.; Jiao, C.; Zhi, L.; Guo, J.; Sun, L. The Association Between Physical Activity and Risk for Breast Cancer in US Female adults: A Cross-Sectional Study Based on NHANES 2011–2020. Eur. J. Surg. Oncol. 2024, 50, 108647. [Google Scholar] [CrossRef]
- An, S.; Park, S. Association of physical activity and sedentary behavior with the risk of colorectal cancer. J. Korean Med. Sci. 2022, 37, e158. [Google Scholar] [CrossRef]
- Hatime, Z.; El Kinany, K.; Huybrechts, I.; Murphy, N.; Gunter, M.J.; Khalis, M.; Meimouna, S.D.; Boudouaya, H.A.; Benslimane, A.; El Asri, A.; et al. Association of physical activity and sedentary behavior with colorectal cancer risk in moroccan adults: A large-scale, population-based case-control study. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 1859–1866. [Google Scholar] [CrossRef]
- Stein, M.J.; Baurecht, H.; Bohmann, P.; Fervers, B.; Fontvieille, E.; Freisling, H.; Friedenreich, C.M.; Konzok, J.; Peruchet-Noray, L.; Sedlmeier, A.M.; et al. Diurnal timing of physical activity and risk of colorectal cancer in the UK Biobank. BMC Med. 2024, 22, 399. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Sampson, J.N.; Michels, K.A.; Moore, S.C.; Loftfield, E.; McClain, K.; Cook, M.B.; Trabert, B.; Matthews, C.E. Physical activity from adolescence through midlife and associations with body mass index and endometrial cancer risk. JNCI Cancer Spectr. 2021, 5, pkab065. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, M.A.; Peres, S.V.; Assumpção, P.P.; Curado, M.P. Physical activity and gastric cancer risk: A case-control study in the Amazon region of Brazil. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2021, 30, 437–441. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Ma, Y.; Simon, T.G.; Meyerhardt, J.A.; Chan, A.T.; Giovannucci, E.L.; Zhang, X. Physical activity and risk of hepatocellular carcinoma among U.S. men and women. Cancer Prev. Res. 2020, 13, 707–714. [Google Scholar] [CrossRef]
- Han, W.; Han, K.; Hwang, S.G.; Ahn, S.H.; Kim, M.N. Association of physical activity, including amount and maintenance, with the risk of HCC among patients with type 2 diabetes. JHEP Rep. Innov. Hepatol. 2024, 6, 101166. [Google Scholar] [CrossRef]
- Chen, W.; Liu, A.; Jiang, Y.; Lin, Y.; Li, X.; Pan, C.; Wang, Y.; Yu, H.; Zhao, Y.; Li, J.; et al. Association between strenuous sports or other exercises and lung cancer risk: A mendelian randomization study. Transl. Lung Cancer Res. 2024, 13, 1210–1221. [Google Scholar] [CrossRef]
- Wang, T.; Jake-Schoffman, D.E.; Townsend, M.K.; Vinci, C.; Willett, W.C.; Tworoger, S.S. Early life physical activity and risk of ovarian cancer in adulthood. Int. J. Cancer 2021, 149, 2045–2051. [Google Scholar] [CrossRef]
- Sandhu, J.; De Rubeis, V.; Cotterchio, M.; Smith, B.T.; Griffith, L.E.; Brenner, D.R.; Borgida, A.; Gallinger, S.; Cleary, S.; Anderson, L.N. Trajectories of physical activity, from young adulthood to older adulthood, and pancreatic cancer risk; a population-based case-control study in Ontario, Canada. BMC Cancer 2020, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Jung, J.Y.; Oh, C.M.; Kim, M.H.; Ha, E.; Kim, Y.; Nam, D.J.; Ryoo, J.H. Daily vigorous intensity physical activity and its preventive effect on pancreatic cancer. Cancer Res. Treat. 2022, 54, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ihira, H.; Sawada, N.; Yamaji, T.; Goto, A.; Shimazu, T.; Inoue, M.; Iwasaki, M.; Tsugane, S.; Japan Public Health Centre-based Prospective Study Group. Physical activity and subsequent risk of kidney, bladder and upper urinary tract cancer in the Japanese population: The Japan Public Health Centre-based Prospective Study. Br. J. Cancer 2019, 120, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.H.; Al-Mallah, M.H.; Dardari, Z.; Brawner, C.A.; Lamerato, L.E.; Keteyian, S.J.; Ehrman, J.K.; Visvanathan, K.; Blaha, M.J. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: Results from the Henry Ford Exercise Testing (FIT) cohort. Cancer 2019, 125, 2594–2601. [Google Scholar] [CrossRef]
- Pang, Y.; Lv, J.; Kartsonaki, C.; Yu, C.; Guo, Y.; Du, H.; Bennett, D.; Bian, Z.; Chen, Y.; Yang, L.; et al. Association of physical activity with risk of hepatobiliary diseases in China: A prospective cohort study of 0.5 million people. Br. J. Sports Med. 2021, 55, 1024–1033. [Google Scholar] [CrossRef]
- Su, J.; Jiang, Y.; Fan, X.; Tao, R.; Wu, M.; Lu, Y.; Hua, Y.; Jin, J.; Guo, Y.; Lv, J.; et al. Association between physical activity and cancer risk among Chinese adults: A 10-year prospective study. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 150. [Google Scholar] [CrossRef]
- Bai, P.; Ning, X.; Gao, R.; Shao, X.; Zhou, S.; Li, J.; Lin, Y.; Liu, H.; Zhang, M.; Yu, P. Association between circadian physical activity patterns and cancer incidence through regulation of inflammation: A UK biobank study. Prev. Med. 2024, 179, 107831. [Google Scholar] [CrossRef]
- Franco-García, J.M.; Castillo-Paredes, A.; Rodríguez-Redondo, Y.; Carlos-Vivas, J.; García-Carrillo, R.M.; Denche-Zamorano, Á. Greater physical activity levels are associated with lower prevalence of tumors and risk of cancer in Spanish population: A cross-sectional study. Heliyon 2024, 10, e29191. [Google Scholar] [CrossRef]
- Stamatakis, E.; Ahmadi, M.N.; Friedenreich, C.M.; Blodgett, J.M.; Koster, A.; Holtermann, A.; Atkin, A.; Rangul, V.; Sherar, L.B.; Teixeira-Pinto, A.; et al. vigorous intermittent lifestyle physical activity and cancer incidence among nonexercising adults: The UK Biobank Accelerometry Study. JAMA Oncol. 2023, 9, 1255–1259. [Google Scholar] [CrossRef]
- Jung, A.Y.; Behrens, S.; Schmidt, M.; Thoene, K.; Obi, N.; Hüsing, A.; Benner, A.; Steindorf, K.; Chang-Claude, J. Pre- to postdiagnosis leisure-time physical activity and prognosis in postmenopausal breast cancer survivors. Breast Cancer Res. BCR 2019, 21, 117. [Google Scholar] [CrossRef]
- Cannioto, R.A.; Attwood, K.M.; Davis, E.W.; Mendicino, L.A.; Hutson, A.; Zirpoli, G.R.; Tang, L.; Nair, N.M.; Barlow, W.; Hershman, D.L.; et al. Adherence to cancer prevention lifestyle recommendations before, during, and 2 years after treatment for high-risk breast cancer. JAMA Netw. Open 2023, 6, e2311673. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Cook, L.S.; Wang, Q.; Kokts-Porietis, R.L.; McNeil, J.; Ryder-Burbidge, C.; Courneya, K.S. Prospective cohort study of pre- and postdiagnosis physical activity and endometrial cancer survival. J. Clin. Oncol. 2020, 38, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Gorzelitz, J.S.; Trentham Dietz, A.; Hampton, J.M.; Spencer, R.J.; Costanzo, E.; Koltyn, K.; Gangnon, R.E.; Newcomb, P.A.; Cadmus-Bertram, L.A. Mortality risk and physical activity across the lifespan in endometrial cancer survivors. Cancer Causes Control CCC 2022, 33, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, A.S.; Hagemann, A.R.; Morrison, L.; Lee, J.A.; Liao, L.M.; Brinton, L.A.; Park, Y.; Toriola, A.T. Pre-diagnosis body mass index, physical activity and ovarian cancer mortality. Gynecol. Oncol. 2019, 155, 105–111. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, D.; White, E.; Lee, D.H.; Blot, W.; Robien, K.; Sinha, R.; Park, Y.; Takata, Y.; Gao, Y.T.; et al. Prediagnosis leisure-time physical activity and lung cancer survival: A pooled analysis of 11 cohorts. JNCI Cancer Spectr. 2022, 6, pkac009. [Google Scholar] [CrossRef]
- Hansen, J.M.; Nagle, C.M.; Ibiebele, T.I.; Grant, P.T.; Obermair, A.; Friedlander, M.L.; DeFazio, A.; Webb, P.M.; Ovarian Cancer Prognosis and Lifestyle Study Group. A healthy lifestyle and survival among women with ovarian cancer. Int. J. Cancer 2020, 147, 3361–3369. [Google Scholar] [CrossRef]
- Wang, T.; Townsend, M.K.; Eliassen, A.H.; Terry, K.L.; Song, M.; Irwin, M.L.; Tworoger, S.S. Prediagnosis and postdiagnosis leisure time physical activity and survival following diagnosis with ovarian cancer. Int. J. Cancer 2021, 149, 1067–1075. [Google Scholar] [CrossRef]
- Cannioto, R.A.; Dighe, S.; Mahoney, M.C.; Moysich, K.B.; Sen, A.; Hulme, K.; McCann, S.E.; Ambrosone, C.B. Habitual recreational physical activity is associated with significantly improved survival in cancer patients: Evidence from the Roswell Park Data Bank and BioRepository. Cancer Causes Control CCC 2019, 30, 1–12. [Google Scholar] [CrossRef]
- Stamatakis, E.; Ahmadi, M.N.; Gill, J.M.R.; Thøgersen-Ntoumani, C.; Gibala, M.J.; Doherty, A.; Hamer, M. Association of wearable device-measured vigorous intermittent lifestyle physical activity with mortality. Nat. Med. 2022, 28, 2521–2529. [Google Scholar] [CrossRef]
- Watts, E.L.; Matthews, C.E.; Freeman, J.R.; Gorzelitz, J.S.; Hong, H.G.; Liao, L.M.; McClain, K.M.; Saint-Maurice, P.F.; Shiroma, E.J.; Moore, S.C. Association of leisure time physical activity types and risks of all-cause, cardiovascular, and cancer mortality among older adults. JAMA Netw. Open 2022, 5, e2228510. [Google Scholar] [CrossRef]
- Chang, Q.; Zhu, Y.; Liu, Z.; Cheng, J.; Liang, H.; Lin, F.; Li, D.; Peng, J.; Pan, P.; Zhang, Y. Replacement of sedentary behavior with various physical activities and the risk of all-cause and cause-specific mortality. BMC Med. 2024, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, G.; Petermann-Rocha, F.; Ferrari, G.; Lee, I.M.; Hamer, M.; Stamatakis, E.; Sarmiento, O.L.; Ibáñez, A.; Lopez-Jaramillo, P. Associations of the ‘weekend warrior’ physical activity pattern with all-cause, cardiovascular disease and cancer mortality: The Mexico City Prospective Study. Br. J. Sports Med. 2024, 58, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Ahmadi, M.N.; Elphick, T.L.; Huang, B.H.; Paudel, S.; Teixeira-Pinto, A.; Chen, L.J.; Cruz, B.D.P.; Lai, Y.J.; Holtermann, A.; et al. Occupational physical activity, all-cause, cardiovascular disease, and cancer mortality in 349,248 adults: Prospective and longitudinal analyses of the MJ Cohort. J. Sport Health Sci. 2024, 13, 579–589. [Google Scholar] [CrossRef]
- Chen, B.; Xie, Z.; Duan, X. Thyroid cancer incidence trend and association with obesity, physical activity in the United States. BMC Public Health 2022, 22, 1333. [Google Scholar] [CrossRef]
- Franco-García, J.M.; Carlos-Vivas, J.; Castillo-Paredes, A.; Mayordomo-Pinilla, N.; Rojo-Ramos, J.; Pérez-Gómez, J. Impacts of square stepping exercise on physical-cognitive function, biomarkers, body composition and mental health in healthy senior aged 60 and above: A systematic review. Healthcare 2024, 12, 2325. [Google Scholar] [CrossRef]
- Wilson, T.N.; Roquelaure, Y.; Evanoff, B.; Aublet-Cuvelier, A.; Porro, B. Physical activity in people diagnosed with cancer: A rapid review of recommendations and critical appraisal of international guidelines. Support. Care Cancer 2023, 31, 679. [Google Scholar] [CrossRef]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Cook, M.B.; Trabert, B.; Håkansson, N.; Larsson, S.C.; Wolk, A.; Gapstur, S.M.; Lynch, B.M.; et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J. Clin. Oncol. 2020, 38, 686–697. [Google Scholar] [CrossRef]
- Morishita, S.; Hamaue, Y.; Fukushima, T.; Tanaka, T.; Fu, J.B.; Nakano, J. Effect of exercise on mortality and recurrence in patients with cancer: A systematic review and meta-analysis. Integr. Cancer Ther. 2020, 19, 1534735420917462. [Google Scholar] [CrossRef]
- Takemura, N.; Chan, S.L.; Smith, R.; Cheung, D.S.T.; Lin, C.C. The effects of physical activity on overall survival among advanced cancer patients: A systematic review and meta-analysis. BMC Cancer 2021, 21, 242. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Ahn, J.; Lee, D.W.; Kim, H.R.; Kang, M.Y. Association of sedentary work with colon and rectal cancer: Systematic review and meta-analysis. Occup. Environ. Med. 2022, 79, 277–286. [Google Scholar] [CrossRef]
- Yuan, L.; Ni, J.; Lu, W.; Yan, Q.; Wan, X.; Li, Z. Association between domain-specific sedentary behaviour and endometrial cancer: A systematic review and meta-analysis. BMJ Open 2023, 13, e069042. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, H.; Sun, C.; Meng, M.; Qu, G.; Jiang, Y.; Wu, B.; Gao, J.; Feng, L.; Xie, P.; et al. Effect of physical activity on incidence and mortality in patients with gastric cancer: Evidence from real-world studies. Cancer Causes Control CCC 2023, 34, 1095–1111. [Google Scholar] [CrossRef]
- Diao, X.; Ling, Y.; Zeng, Y.; Wu, Y.; Guo, C.; Jin, Y.; Chen, X.; Feng, S.; Guo, J.; Ding, C.; et al. Physical activity and cancer risk: A dose-response analysis for the Global Burden of Disease Study 2019. Cancer Commun. 2023, 43, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Qie, R.; Han, M.; Huang, H.; Sun, P.; Xie, Y.; He, J.; Zhang, Y. Physical activity and risk of lung cancer: A systematic review and dose-response meta-analysis of cohort studies. J. Natl. Cancer Cent. 2023, 3, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, K.M.; Matthews, C.E.; Salerno, E.A.; Moore, S.C. Weight Training and Risk of 10 Common Types of Cancer. Med. Sci. Sports Exerc. 2019, 51, 1845–1851. [Google Scholar] [CrossRef]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O’Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts with All-Cause, Cancer, and Cardiovascular Mortality Endpoints. Am. J. Epidemiol. 2018, 187, 1102–1112. [Google Scholar] [CrossRef]
- Jee, H.; Park, E.; Hur, K.; Kang, M.; Kim, Y. High-Intensity Aerobic Exercise Suppresses Cancer Growth by Regulating Skeletal Muscle-Derived Oncogenes and Tumor Suppressors. Front. Mol. Biosci. 2022, 9, 818470. [Google Scholar] [CrossRef]
- Sheinboim, D.; Parikh, S.; Manich, P.; Markus, I.; Dahan, S.; Parikh, R.; Stubbs, E.; Cohen, G.; Zemser-Werner, V.; Bell, R.E.; et al. An exercise-induced metabolic shield in distant organs blocks cancer progression and metastatic dissemination. Cancer Res. 2022, 82, 4164–4178. [Google Scholar] [CrossRef]
- Ruaro, M.F.; Santana, J.O.; Gusmão, N.; Elías, D.; França Carvalho, B.N.; Farinazo, K.B.; Lencina, S.; Bonorino Corralo, V.D.; Clodoaldoantoniode, S.; Caperuto, É.C. Effects of strength training with and without blood flow restriction on quality of life in elderly women. J. Phys. Educ. Sport 2019, 19, 787–797. Available online: https://efsupit.ro/images/stories/iunie2019/Art%20112.pdf (accessed on 4 February 2025).
- Armenta-Guirado, B.I.; González-Rocha, A.; Mérida-Ortega, Á.; López-Carrillo, L.; Denova-Gutiérrez, E. Lifestyle quality indices and female breast cancer risk: A systematic review and meta-analysis. Adv. Nutr. 2023, 14, 685–709. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Y.; Bian, Z.; Zhou, S.; Xu, L.; Jiang, F.; Yuan, S.; Tan, X.; Chen, X.; Ding, Y.; et al. Sleep, physical activity, and sedentary behaviors in relation to overall cancer and site-specific cancer risk: A prospective cohort study. iScience 2024, 27, 109931. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Min, Y.; Xiang, Z.; Zeng, Y.; Wang, J.; Liu, L. Joint association of physical activity and dietary quality with survival among US cancer survivors: A population-based cohort study. Int. J. Surg. 2024, 110, 5585–5594. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H.; Wideman, L.; McKenzie, M.J.; Consitt, L.A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [CrossRef]
- Amirsasan, R.; Akbarzadeh, M.; Akbarzadeh, S. Exercise and colorectal cancer: Prevention and molecular mechanisms. Cancer Cell Int. 2022, 22, 247. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef]
- Du, F.; Wu, C. Review on the effect of exercise training on immune function. BioMed Res. Int. 2022, 2022, 9933387. [Google Scholar] [CrossRef]
- Assi, M.; Dufresne, S.; Rébillard, A. Exercise shapes redox signaling in cancer. Redox Biol. 2020, 35, 101439. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, L.; Yang, J.; Yin, X.; Zhang, T.; Wu, X.; Ma, X. Physical activity prevents tumor metastasis through modulation of immune function. Front. Pharmacol. 2022, 13, 1034129. [Google Scholar] [CrossRef] [PubMed]
- Spanoudaki, M.; Giaginis, C.; Karafyllaki, D.; Papadopoulos, K.; Solovos, E.; Antasouras, G.; Sfikas, G.; Papadopoulos, A.N.; Papadopoulou, S.K. Exercise as a promising agent against cancer: Evaluating its anti-cancer molecular mechanisms. Cancers 2023, 15, 5135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kenfield, S.A.; Yanagisawa, Y.; Newton, R.U. Why exercise has a crucial role in cancer prevention, risk reduction and improved outcomes. Br. Med. Bull. 2021, 139, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Jurdana, M. Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol. Oncol. 2021, 55, 7–17. [Google Scholar] [CrossRef]
- Arena, S.K.; Doherty, D.J.; Bellford, A.; Hayman, G. Effects of aerobic exercise on oxidative stress in patients diagnosed with cancer: A narrative review. Cureus 2019, 11, e5382. [Google Scholar] [CrossRef]
- Longobucco, Y.; Masini, A.; Marini, S.; Barone, G.; Fimognari, C.; Bragonzoni, L.; Dallolio, L.; Maffei, F. Exercise and oxidative stress biomarkers among adult with cancer: A systematic review. Oxidative Med. Cell. Longev. 2022, 2022, 2097318. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef]
- As’ad, M.R.F.; Liben, P.; Herawati, L. Mechanism of physical exercise on lowering levels of c-reactive protein (CRP) in overweight and obese. Folia Medica Indones. 2021, 57, 82–89. [Google Scholar] [CrossRef]
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; Di Luigi, L.; Caporossi, D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017, 18 (Suppl. S8), 802. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic health, mitochondrial fitness, physical activity, and cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tseng, L.M.; Lee, H.C. Role of mitochondrial alterations in human cancer progression and cancer immunity. J. Biomed. Sci. 2023, 30, 61. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical exercise: A novel tool to protect mitochondrial health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Turgeon, M.O.; Perry, N.J.S.; Poulogiannis, G. DNA Damage, repair, and cancer metabolism. Front. Oncol. 2018, 8, 15. [Google Scholar] [CrossRef]
- Wu, W.; Li, L.; Su, X.; Zhu, Z.; Lin, X.; Zhang, J.; Zhuang, Z.; Cai, H.; Huang, W. Nuclear factor-kappaB regulates the transcription of NADPH oxidase 1 in human alveolar epithelial cells. BMC Pulm. Med. 2021, 21, 98. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Körner, H.; Ge, C. Tumor necrosis factor: What is in a name? Cancers 2022, 14, 5270. [Google Scholar] [CrossRef]
- Sainz, R.M.; Lombo, F.; Mayo, J.C. Radical decisions in cancer: Redox control of cell growth and death. Cancers 2012, 4, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Sutkowy, P.; Wróblewska, J.; Wróblewski, M.; Nuszkiewicz, J.; Modrzejewska, M.; Woźniak, A. The impact of exercise on redox equilibrium in cardiovascular diseases. J. Clin. Med. 2022, 11, 4833. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Fujii, R.; Li, X.; Higashida, K.; Muraoka, I. Effects of exhaustive exercises, with different intensities, on oxidative stress markers in rat plasma and skeletal muscle. Sci. Sports 2018, 33, 169–175. [Google Scholar] [CrossRef]
- Chen, J.; Lai, T.F.; Lin, C.Y.; Hsueh, M.C.; Park, J.H.; Liao, Y. Associations between objectively measured overall and intensity-specific physical activity and phase angle in older adults. Sci. Rep. 2024, 14, 7309. [Google Scholar] [CrossRef]
- Oliveira Silvino, V.; Raffaela Barbosa Barros, K.; Machado Brito, F.; Matheus Dias Magalhães, F.; Augusto Ferreira Carioca, A.; César Carneiro Loureiro, A.; Salvador Veras-Silva, A.; Daniel Motta Drummond, M.; Antonio Pereira Dos Santos, M. Phase angle as an indicator of body composition and physical performance in handball players. BMC Sports Sci. Med. Rehabil. 2024, 16, 114. [Google Scholar] [CrossRef]
- Cunha, T.A.; Lopes, M.M.G.D.; de Araújo Brito, A.N.; Vermeulen-Serpa, K.M.; de Lima Vale, S.H.; Brandão-Neto, J.; Leite-Lais, L. Phase Angle and Bioelectrical Impedance Vector Analysis (BIVA) in Amyotrophic Lateral Sclerosis (ALS) Patients. Appl. Sci. 2024, 14, 1545. [Google Scholar] [CrossRef]
- da Silva, B.R.; Gonzalez, M.C.; Cereda, E.; Prado, C.M. Exploring the potential role of phase angle as a marker of oxidative stress: A narrative review. Nutrition 2022, 93, 111493. [Google Scholar] [CrossRef]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Rovida, E.; Tusa, I. Targeting MAPK in cancer 2.0. Int. J. Mol. Sci. 2022, 23, 5702. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, K.J.; Petersen, M.H.; Lien-Iversen, T.; Højlund, K.; Jensen, J. Effect of exercise training on fat loss-energetic perspectives and the role of improved adipose tissue function and body fat distribution. Front. Physiol. 2021, 12, 737709. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Kocot-Kępska, M.; Mitka, K.; Rybicka, M.; Janecki, M.; Przeklasa-Muszyńska, A. The role of physical activity in cancer patients: A narrative review. Palliat. Med. Pract. 2021, 15, 254–262. [Google Scholar]
- Neuzillet, C.; Anota, A.; Foucaut, A.M.; Védie, A.L.; Antoun, S.; Barnoud, D.; Bouleuc, C.; Chorin, F.; Cottet, V.; Fontaine, E.; et al. Nutrition and physical activity: French intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC, SFP-APA, SFNCM, AFSOS). BMJ Support. Palliat. Care 2021, 11, 381–395. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, M.; Cheng, A.S.; Zhou, Y.; So, W.K. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer 2014, 21, 262–274. [Google Scholar] [CrossRef]
- Mueller, A.K.; Vaeth, B.; Todd, A.C.; Dasaro, C.R.; Li, J.; Qiao, B.; Boffetta, P.; Prezant, D.J.; Hall, C.B.; Goldfarb, D.G.; et al. Importance of reference group selection in the evaluation of cancer incidence. Sci. Rep. 2025, 15, 270. [Google Scholar] [CrossRef]
- Stelmach, M. Physical activity assessment tools in monitoring physical activity: The Global Physical Activity Questionnaire (GPAQ), the International Physical Activity Questionnaire (IPAQ) or accelerometers—Choosing the best tools. Health Probl. Civiliz. 2018, 12, 57–63. [Google Scholar] [CrossRef]
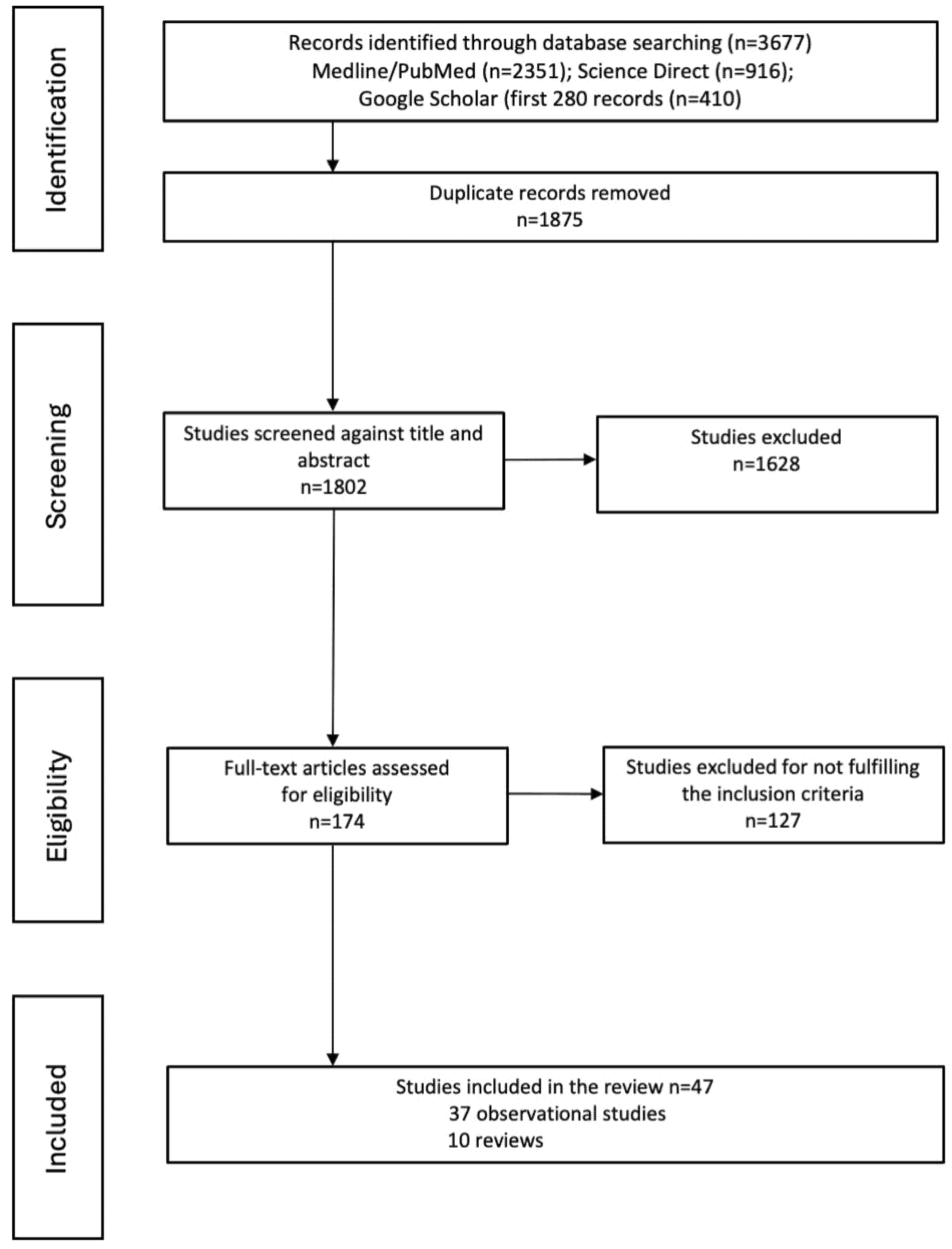
| Cancer Site | Magnitude Risk Reduction (%) | Level of Scientific Evidence | ||||
|---|---|---|---|---|---|---|
| Incidence | Survival | Incidence | Mortality | |||
| Prediagnosis PA | Postdiagnosis PA | WCRF/AICR [28] | PAGAC [29,30] | PAGAC | ||
| Colon/colorectal | 20 a, 24 b, 19 c | 23 b, 20 d | 30 b, 38 d | Convincing | Strong +• | Moderate |
| Breast premenopausal | 7 a | Probable # | ||||
| Breast postmenopausal | 13 a, 13 b | 18 b, 14 d | 31 b, 37 d | Probable | Strong + | Moderate |
| Endometrium | 27 a, 20 b, 20 c | Probable | Strong +• | |||
| Esophagus | 15 a, 21 b, 21 c | 23 d | 69 b | Limited-suggestive | Strong + | |
| Lung | 10 a, 24 b, 21–25 c | 22 b, 19 d | Limited-suggestive | Moderate • | ||
| Liver | 27 b | 22 d | Limited-suggestive | |||
| Kidney | 12 b | 50 b, 19 d | 43 d | Insufficient | ||
| Ovary | 8 c | Insufficient | Limited − | |||
| Prostate | contradicting results | ∗ b, 10 d | 33 b, 38 c, 30 d | Limited-suggestive | Limited − | Moderate |
| Pancreas | 11 c | Insufficient | Limited − | |||
| Bladder | 15 b | 23 d | Insufficient | Strong + | ||
| Stomach (gastric) | 17 b | 26 d | Insufficient | Strong + | ||
| All cancers combined | 10–20 c, 29 e | 18 d | 37 d | |||
| Author, Year, Country | Study Design/ Population, Number of Cases/Age/Period | Physical Activity Assessment Methods (Type of Activity, Detection) | Main Results (OR/HR, 95% CI) |
|---|---|---|---|
| bladder | |||
| An et al., 2024, Japan [32] | Prospective 50,374 individuals aged 40–79 years, 153 bladder cancer cases, follow-up 17.5 years | Japan Collaborative Study for Evaluation of Cancer questionnaires; determination of weekly duration of practicing sports or physical exercise, and sitting or reclining time over the past year or two, and occupational activity | Recreational sports participation of 5 h/wk vs. 1–2 h/wk; HR = 0.28 (0.09–0.89), p for trend 0.017, occupational PA (standing and walking), HR = 0.53 (0.32–0.85) vs. mostly sitting at the workplace. Protection stronger among men |
| breast cancer | |||
| Bigman et al., 2022, Nigeria [33] | Case-control; 508 breast cancer cases, 892 controls; mean age 45.5 and 40.1 years; 2014–2016 | Leisure-time PA (aerobic and resistance) based on questionnaire. Face-to-face interview, MET-h/wk calculated in the past year and divided by quartiles (Q1 < 3.75, Q2: 3.75–6.69, Q3: 6.70–14.74, Q4 ≥ 14.74) | OR = 0.51 (0.27–0.96) for Q4 vs. Q1; risk reduction varied by cancer subtypes and was more expressed in non-obese women |
| Fortner et al., 2024, USA [34] | Retrospective Nurses’ Health Studies, 187,278 women, 12,785 breast cancer cases, aged 30–55 and 25–42 years, 1986–2016 and 1989–2017 follow-up, respectively | Self-administered questionnaire, recreational PA (ten categories) reported every 4 years. Energy expenditure estimated by MET-h/wk for MVPA as annual average | ER+/PR+ breast cancer: ≥27 vs. <3 MET-h/wk: premenopausal women, OR = 0.83 (0.70–0.99), postmenopausal women OR = 0.86 (0.78–0.95) (total recreational activity). MVPA: premenopausal women OR = 0.88 (0.69–1.11), postmenopausal women OR = 0.71 (0.58–0.88). No association for ER-/PR—breast cancer |
| Liu et al., 2024, China [35] | Cross-sectional 233 breast cancer cases, 6395 controls from NHANES aged ≥ 20 years, 2011–2020 follow-up | Global Physical Activity Questionnaire (GPAQ), PA classification: vigorous work activity, moderate work activity, walking/cycling, vigorous leisure activity, moderate leisure activity during 7 last days (MET-min/wk), total activity level classification: low < 600, light 600–<1800, moderate 1800–<3000, high ≥ 3000 | Women light active, OR = 0.95 (0.68–1.34); moderate active, OR = 0.92 (0.57–1.49); high active, OR = 0.56 (0.37–0.86), p for trend 0.014. |
| colorectal | |||
| An and Park, 2022, Korea [36] | Cross-sectional; 33,403 participants, 193 colorectal cancer cases, aged ≥ 20 years; 2014–2019 | Self-administered GPAQ; recreational, occupational and transportation in three levels: sedentary behavior, moderate intensity, vigorous intensity, according to WHO recommended standard for activity, and sitting/reclining time evaluated. Sitting behavior dichotomized into <10 days and ≥10 days monthly | Individual with long sitting time (≥10 h/day vs. <10 h/day, OR = 1.64 (1.22–2.01)); No significant relation between colorectal cancer and the different domains of PA |
| Hatime et al., 2022, Morocco [37] | Case-control; 1516 case-control pairs; colorectal cancer; September 2009–February 2017 | Self-administered GPAQ; Occupational, household and leisure-time activity (last 7 days) (MET-min/wk) three levels: low intensity < 600, moderate 600–3000, vigorous ≥ 3000 | Vigorous PA vs. low intensity, OR = 0.77 (0.62–0.95) colon p for trend 0.05, OR = 0.65 (0.53–0.80) rectal p for trend 0.001, OR = 0.71 (0.61–0.82) colorectal p for trend 0.09 Sitting time ≥ 4 vs. <4 h/day: OR = 1.02 (0.87–1.20) colon OR = 1.17 (0.99–1.37) rectal OR = 1.09 (0.97–1.22) colorectal |
| Stein et al., 2024, Germany [38] | Prospective, 86,252 participants from UK Biobank aged 42–79 years, 529 colorectal cancer cases, 5.3-year follow-up | Accelerometer (Axivity AX3 wrist—worn triaxal), functional principal component analysis (fPCA) used to ascertain diurnal timing of PA patterns between 2013 and 2015 | Continuous day-long activity, HR = 0.94 (0.89–0.99) for higher vs. lower overall; early plus late-day activity vs. mid-day, HR = 0.89 (0.80–0.99), late-day activity vs. early-day, HR = 0.93 (0.85–1.02) mid-day plus night-time activity vs. early/late-day, HR = 1.02 (0.88–1.19) |
| endometrial | |||
| Saint-Maurice et al., 2021, USA [39] | Prospective cohort; 67,705 women, 1468 endometrial cancer cases, aged 50–71 years; 12.4-year follow-up period | Risk Factor Questionnaire identification of five long-term leisure-time PA patterns between adolescence and the cohort study entry at ages 15–18, 19–29, 30–35, and 10 years before cohort entry. Weekly duration of PA for each age period rarely or never, 0.5–<1 h, 1–3 h, 4–7 h, ≥7 h | High level PA (6–7 h/wk) over time, OR = 0.81 (0.67–0.98); low level PA (1–2 h/wk) over time, OR = 0.85 (0.69–1.04), increased activity level, OR = 0.74 (0.61–0.91); decreased activity level, OR = 0.98 (0.80–1.19) vs. <1 h/wk at each age period |
| gastric | |||
| Fagundes et al., 2021, Brazil [40] | Case-control; 147 gastric cancer cases, 150 controls; July 2017–April 2018 | Baecke Physical Activity Questionnaire; self-reported level of occupational, leisure-time, and transportation activities during three periods of 5, 10, and 15 years before the cancer diagnosis specified in three levels | PE performed 5 years before diagnosis: OR = 0.29 (0.12–0.75) for 1.75–2.00 and leisure and locomotion PE, OR = 1.66 (0.62–4.44) for 2.00–4.75 vs. 1.25–1.75. For 10 years before diagnosis, OR = 0.24 (0.09–0.69) for >3.25–4.50, for 15 years, OR = 0.22 (0.08–0.68) for >3.50–5.00 compared to 1.50–2.75 level |
| hepatocellular | |||
| Luo et al., 2020, USA [41] | Prospective cohort; two cohorts: the Nurses’ Health Study and Health Professionals Follow-up Study; 122,075 participants: 44,540 men, 77,535 women aged 40–75 years; 138 hepatocellular cancer cases; 23-year follow-up | Biennal questionnaire. Average time per week spent walking, jogging, running, swimming, bicycling, calisthenics and other aerobic exercise, squash/racquetball, tennis, weightlifting, chopping/digging, number of stairs climbed, yoga, stretching, and toning, estimated in MET-h/wk. Total activity coded into three-levels | Total PA, HR = 0.78 (0.51–1.18); moderate intensity activity: HR = 0.60 (0.38–0.94), p for trend 0.04 vigorous intensity, HR = 0.88 (0.56–1.37) highest vs. lowest tertile; brisk walking over 1 h/wk vs. non-brisk walking, HR = 0.56 (0.35–0.90) p for trend 0.006. |
| Han et al., 2024, South Korea [42] | Retrospective National Health Insurance Service cohort of 1439,152, 22,689 hepatocellular cancer cases in diabetic patients mean age 58.1 years, 5.2-year follow-up period | PA estimated in 2009 and 2011 using questionnaires. Dose of PA assessment in MET-min/wk: sedentary behavior < 500; moderate active 500–1500; active > 1500. Change in PA levels according to change of activity between 2009 and 2011: persistently sedentary; newly active, active, and persistently active | Moderate active, HR = 0.96 (0.93–0.99), active, HR = 0.95 (0.91–0.99) vs. sedentary group. Persistently active behavior vs. persistently sedentary group, HR = 0.91 (0.84–0.98), dose-dependent effects |
| lung | |||
| Chen et al., 2024, China [43] | Mendelian randomized 11,348 lung cancer cases, 15,861 controls | Self-report questionnaire and objective measure (accelerometer or wearable activity monitor) of moderate-to-vigorous PA duration (minimum of 30 min) of high-intensity activity. Moderate-intensity PA included brisk strolling, recreational sports, and moderate aerobic exercise. Mendelian randomization | Overall lung cancer, OR = 0.129 (0.021–0.779); lung adenocarcinoma and squamous cell lung cancer, OR = 0.045 (0.003–0.677). Strenuous sports effect, OR = 0.054 (0.010–0.302) |
| ovarian | |||
| Wang et al., 2021, USA [44] | Prospective cohort; 84,785 participants, two cohorts of Nurses’ Health Study 28,232 and 56,553, median age 69 and 42 years, respectively, 227 ovarian cancer cases; 15.1-year follow-up | Self-reported average weekly duration of transportation, moderate recreational PA (walking, cycling, hiking, yard work) and strenuous recreational activity (running, aerobics, lap swimming) at grades 7–8 (ages 12–13), grades 9–12 (ages 14–17), and ages 18–22. Total PA score weighted by intensity (MET-h/wk) | PA at ages 12–13, 14–17, and 18–22 years: HRs: 1.34 (0.87–2.05), 1.21 (0.77–1.89) and 1.08 (0.65–1.80), respectively, PA across all these periods, HR = 1.24 (0.80–1.92) for ≥78 vs. <24 MET-h/wk |
| pancreatic | |||
| Sandhu et al., 2020, Canada [45] | Case-control; 315 pancreatic cancer cases, 1254 controls aged 40–60 years; February 2011–January 2015 | Self-administered questionnaire applied to examine trajectories of moderate and vigorous recreational and occupational PA during participants’ 20s and 30s, mid-adulthood (40s and 50s), and 2 years ago. Estimated total weekly MET scores for combined moderate and vigorous activity | Life-course PA trajectories: low activity at all ages, OR = 1.11 (0.75–1.66), increasingly active, OR = 1.11 (0.56–2.21), high active in young adulthood and less in older adulthood, OR = 0.98 (0.62–1.53), and persistently high active, OR = 1.50 (0.86–2.62) |
| Park et al., 2022, Korea [46] | Retrospective cohort; 220,357 participants, 377 pancreatic cancer cases, mean age of 64.8 years; 4.38-year follow-up | Self-reported IPAQ short form assessed weekly frequency and durations of vigorous PA > 20 min (heavy lifting, digging, aerobic, fast bicycling) during the last 7 days; estimated total MET-hours. Four levels frequency of vigorous activity | HR = 0.47 (0.25–0.89), p for trend 0.014 for performing vigorous activity 6–7 days/wk vs. those declared no vigorous intensity PA |
| combined cancers | |||
| Ihira et al., 2019, Japan [47] | Prospective cohort; 76,795 individuals 36,670 men, 40,125 women, aged 45–74 years; cancer cases: 202 kidney, 373 bladder, and 83 upper urinary tract; 15.1-year follow-up | Self-administered PA questionnaire; Average time per day spent engaged in strenuous exercise, heavy physical work or walking and standing, and sitting time, estimated total METs/day score stratified in tertile. Leisure-time exercise, sports also stratified by weekly frequency | HRs for kidney, bladder, and upper urinary tract cancers: total activity 1.05 (0.74–1.49), 1.06 (0.81–1.39), 0.80 (0.49–1.35), leisure-time sports or PE: 0.87 (0.55–1.38), 0.95 (0.69–1.39), 0.81 (0.39–1.70), respectively, for the highest tertile vs. the lowest tertile |
| Marshal et al., 2019, USA [48] | Retrospective cohort; Henry Ford Exercise Project; 49,143 adults (mean age 54.0 years); 294 lung cancer and 188 colorectal cancer cases; followed ≥2 and ≥5 years, respectively; 46% women, 54% men; 7.7-year follow-up | Bruce protocol treadmill exercise stress test (pick METs) testing from 1991 through 2009 based on achieved speed. Calculated in MET by Quinton Controller and equations according to ACSM’s guidelines for exercise | Lung cancer: HR = 0.28 (0.17–0.46) (followed ≥ 2 years); HR = 0.27 (0.15–0.49) (followed ≥ 5 years) for the highest (≥12) vs. the lowest (<6) MET tertile, p for trend 0.01; colorectal cancer: HR = 0.32 (0.17–0.60) (followed ≥ 2 years) and HR = 0.30 (0.13–0.68) (followed ≥ 5 years) for ≥12 MET vs. <6 MET |
| Pang et al., 2021, China [49] | Prospective cohort; 460,937 participants, 22,012 cancer cases aged 30–79 years, (liver cancer, gallbladder cancer, biliary tract cancer); 10-year follow-up period | Self-administered questionnaire used in European Prospective Investigation into Cancer with additional modification that included occupational, commuting, household and leisure-time PA during the past 12 months; estimated in MET-h/wk | Liver cancer, HR = 0.81 (0.71–0.93); gallbladder cancer, HR = 0.51 (0.32–0.80); biliary tract cancer, HR = 0.53 (0.38–0.78), for the highest vs. the lowest quartile of total activity |
| Su et al., 2022, China [50] | Prospective study; 52,938, cancer-free individuals aged 30–79 years, 3674 cancer cases (lung, colorectal, liver, breast, esophageal, stomach); 10.1-year follow-up 2004–2008 | Self-reported information on occupational, recreational, and household activities collected by interview-administered questionnaire; estimated in quartiles of MET-h/day, sedentary leisure time quantified in h/day | Highest quartile vs. the lowest quartile, HRs: 0.89 (0.81–0.99) (total cancer); 0.75 (0.60–0.94) (lung cancer); 0.74 (0.55–1.00) (colorectal cancer). Lower risk magnitudes for females and never smokers |
| Bai et al., 2024, China [51] | Prospective 96,687 participants, 5995 several cancer-site cases; mean age 55.9 years, 7.1-year follow-up | Accelerometer measured PA over 7 days. Circadian patterns of activity delineated through PA trajectories for every 24 h acceleration data. Hourly mean acceleration, peaks (denoting intensity activity) and area under the curve (total PA volume), and the trajectory trend were measured | Vigorous activity pattern, HRs: 0.58 (0.04–0.86); bladder—0.58 (0.04–0.86); breast—0.73 (0.60–0.89); kidney—0.45 (0.26–0.78); lung—0.59 (0.41–0.84); myeloma—0.49 (0.27–0.88); oral and pharynx—0.51 (0.26–0.98), and 0.71 (0.54–0.93) for colorectal, in two distinct peaks of PA levels morning and afternoon |
| Franco-Garcia et al., 2024, Spain [52] | Cross-sectional 17,704 malignant cancer cases (men and women), median age 47 years October 2016, October 2017, follow-up | ENSE Adult Questionnaire PA levels (PAL): Inactive, Walkers, Actives, Very Actives, scores calculated on the basis of number of days/wk, duration and intensity of activity | Physically active group, OR = 0.62 (0.48–0.80); very active, OR = 0.32 (0.22–0.47), vs. sedentary group |
| Stamatakis et al., 2023, UK [53] | Prospective cohort; UK Biobank Accelerometry Subsample, 22,398 nonexercising adults (45.2% men, 54.8% women), 2356 total incident cancer cases (13 cancer sites) and 1084 individuals owing to PA-related cancer; mean age 62.0 years; 6.7-year follow-up | Daily vigorous intermittent lifestyle PA(VILPA) self-reported ≤ 1 min and ≤2 min duration bouts assessed using accelerometer | Median daily VILPA duration bouts (≤1 min) of 4.5 min/day. HR = 0.80 (0.65–0.92) for total cancer incidence and HR = 0.69 (0.55–0.86) for PA-related cancer. Minimal protection doses: 3.4 min/day for total cancer incidence, HR = 0.83 (0.73–0.93) and 3.7 min/day for PA-related cancer incidence, HR = 0.72 (0.59–0.88) |
| Author, Year, Country | Study Design/Population, Number of Cases/Age/Period | Physical Activity Assessment Methods (Type of Activity, Detection) | Main Results |
|---|---|---|---|
| breast cancer | |||
| Jung et al., 2019, Germany [54] | Prospective cohort; 2042 women from two regions with breast cancer; Vital status assessed in 2009 and 2015, 114 deaths from breast cancer; Age 50–74 years; 6-year follow-up | Telephone interviews based on questionnaire. PA index based on walking, commuting/transportation cycling, recreational activities, sports, and fitness from the age of 50 until diagnosis; leisure-time activities estimated in MET-h/wk: nonparticipant—0; low activity—>0–<7.5; sufficient—≥7.5 | HR = 0.54 (0.30–1.00) for increasingly active women. For decreasingly active from pre- to postdiagnosis, HR = 0.80 (0.45–1.42), sufficient activity in prediagnosis 0.90 (0.55–1.46) |
| Cannioto et al., 2023, USA [55] | Prospective cohort; 1340 women, 873 with hormone-receptor positive breast cancer, 222 deaths; mean age 50.89 years; study January 2005–December 2010, 7.7-year follow-up time updated through December 2018 | Interview-administered questionnaires meeting PA AICR and ACS guidelines, MVPA quantifications: inactive—no MVPA, insufficient—<7.5 MET-h/wk, meeting PA guidelines—≥7.5 MET-h/wk | For meeting PA guidelines, HR = 0.56 (0.41–0.76), p < 0.001 vs. no MVPA, insufficient activity, HR = 0.73 (0.52–1.03), p < 0.07 vs. no MVPA practice |
| endometrial | |||
| Friedenreich et al., 2020, Canada [56] | Prospective cohort, 425 women with endometrial cancer (2002–2006, observed to 2019), 18 deaths; age 30–80 years; 14.5-year follow-up, 60 deaths | Interview-administered LTPAQ. Frequency, duration, and intensity of occupational, household, and recreational PA from childhood until diagnosis estimated as average MET-h/wk/yr. Activity classification (MET): light (<3), moderate (<3–5.9), vigorous (≥6). Sedentary behavior in occupational activity (≤1.5) | Higher recreational activity > 14 vs. ≤8 MET-h/wk/yr, HR = 0.54 (0.30–0.96), p for trend 0.04. Recreational PA from pre- to postdiagnosis HR = 0.35 (0.18–0.69), |
| Gorzelitz et al., 2022, USA [57] | Population-based cancer registry, 745 endometrial cancer survivors, mean age 40–79 years; 1991–1994 | Self-reported frequency of MVPA, interview (number of session/wk) at ages 12, 20, and 5 years pre-interview. Specification of PA: vigorous (running, lap swimming, basketball, gymnastics), moderate (volleyball, softball, brisk walking, leisurely biking) | HR = 0.61 (0.41–0.92) for women engaged in one MVPA session per week 5 years before diagnosis vs. nonparticipants. For one session of activity engaged at ages 12 and 20 years, HR = 0.95 (0.86–1.06) and HR = 0.87 (0.65–1.16), respectively |
| ovarian | |||
| Zamorano et al., 2019, USA [58] | Retrospective cohort; Women enrollment into NIH-AARP Diet and Health Study; 566,398 individuals: 339,666 men and 226,732 women, 489 of 741 cases of epithelial ovarian cancer included in analysis; mean age 62.7 years; One-year follow-up | Self-administered questionnaire; questions on intensity and frequence of light and vigorous PA during the past 10 years, estimated in times per week or month. Vigorous activities ≥ 20 min duration and increase in heart rate or heavy sweating. | Frequency ≥ 5 times/wk, HR = 1.03 (0.76–1.39), p for trend 0.74 PA in past 10 years: light intensity for ≥7 h/wk, HR = 0.84 (0.48–1.47), p for trend 0.50; vigorous intensity, HR = 0.95 (0.65–1.39) HR = 0.60 (0.41–0.0.87) 4–7 h/wk, p for trend 0.06, vs. never/rarely practice |
| lung | |||
| Yang et al., 2022, USA [59] | Record linkage 11 cohorts (7 US, 2 European, 2 Asian); 1588,378 participants, 20,494 lung cancer cases, 13,596 deaths due to lung cancer; One-year follow-up | Self-administered LTPA valid cohort questionnaire; quantification of regular engagement in exercise and sport activities in MET-h/wk based on PA guidelines: none MET (nonparticipants referent), >0–<8.3 (low active), 8.3–16.0 (moderate active), >16.0 (highly active) | Lung cancer specific energy expenditure: 0–<8.3 MET-h/wk HR = 1.00 (0.96–1.05); ≥8.3 MET-h/wk HR = 0.99 (0.95–1.04); localized lung cancer, HR = 0.84 (0.68–1.04) and HR = 0.80 (0.65–0.99), respectively |
| ovarian | |||
| Hansen et al., 2020, Australia [60] | Prospective cohort; 18 major Australian treatment centers, 958 women with invasive epithelial ovarian cancer; age 18–79 years; January 2012–May 2015 | Active Australia Survey, three specific levels (MET-h/wk): least active (0–≤10.5), second tertile (>10.5–≤29.3), most active (>29.3) | HR, second tertile 0.98 (0.74–1.30), third tertile 0.93 (0.79–1.39) vs. first tertile, p for trend 0.6 |
| Wang et al., 2021, USA [61] | Prospective cohort; Nurses’ Health Study, two cohorts of Afro-American women from 14 states; 1431 ovarian cancer cases, 901 deaths from ovarian cancer; aged 25–42 years. Assessment every 2–4 years since 1986 in NHS I and 1989 in NHS II, with a median assessment of 4.6 years | Self-administered questionnaire on PA and sedentary behavior. Past-week recalls over 7 days. Recreational PA (average duration of eight common types of activity); estimated total weekly MET-hours. | Total PA (MET-h/wk) 1–8 years before diagnosis 1.5–<7.5 vs. <1.5 HR = 0.91 (0.68–1.22) ≥7.5 vs. <1.5, HR = 0.96 (0.72–1.27). Activity changes 1–8 years before diagnosis vs. 1–4 years after diagnosis: increased from <7.5 to ≥7.5, HR = 0.88 (0.58–1.35); decreased from ≥7.5 to <7.5, HR = 1.49 (1.07–2.08) |
| pancreatic | |||
| Marshall et al., 2019, USA [48] | Retrospective cohort; Henry Ford Exercise Project; 49,143 adults, (46% women, 54% men), Lung cancer 282 deaths, colorectal cancer 89 deaths; mean age 54.0 years; 7.7-year follow-up | Bruce protocol treadmill exercise stress test (pick METs) based on achieved speed. Calculated by Quinton Controller and equations according to ACSM’s guidelines for exercise | Lung cancer: HR = 0.56 (0.32–1.00); colorectal cancer: HR = 0.11 (0.03–0.37) for the highest vs. the lowest tertile (≥12 vs. <6 MET). p for trends: 0.01 and <0.01, respectively |
| Cannioto et al., 2019, USA [62] | Prospective cohort; 5807 participants (55% women and 45% men) with 19 cancer types, from Roswell Park Comprehensive Cancer Center, 1956 deaths; mean age 60.63 years; 52.7-month follow-up | Self-administered Data Bank and BioRepository questionnaire; Questions on activity mode, frequency, intensity, and duration in the decade prior to study enrollment; MVPA assessed | HR for any regular/weekly MVPA: 0.68 (0.67–0.75) vs. no regular activity, HRs: 0.81 (0.69–0.95), 0.68 (0.60–0.0.78) and 0.85 (0.74–0.98) for engaging frequency: 1–2 days, 3–4 days, and 5–7 days, respectively |
| Stamatakis et al., 2022, UK [63] | Prospective cohort; UK Biobank Accelerometry Subsample; 22,699 nonexercising adults (56.2% women), 511 cancer death (13 cancer sites); mean age 61.8 years: 6.9-year follow-up | Daily vigorous intermittent lifestyle PA (VILPA), self-reported ≤ 1 min and ≤2 min duration bouts assessed using accelerometer | Three doses up to 1 min bout VILPA, HR = 0.60 (0.46–0.78), three doses up to 2 min, HR = 0.62 (0.48–0.80). VILPA duration: 4.4 min/day (up to 1 min bout): HR = 0.70 (0.59–0.84), 4.4 min/day (up to 2 bouts): HR = 0.70 (0.60–0.83) |
| Watts et al., 2022, USA [64] | Prospective cohort; National Institutes of Health—AARP Diet and Health Study Cohort: 272550 participants (58% men), 32,366 cancer deaths; mean age 70.5-year, 12.4-year follow-up | AARP Diet and Health Study questionnaire estimated weekly, duration and frequences of aerobics, exercise (e.g., running, cycling, swimming), racquet sports, golf, and walking for exercise. Activity estimated in MET-h/wk. Participation categorization in each activity type: nonpartcipant (control); 0.1–<7.5 (moderate active); 7.5–<15 (active); 15–<22.5 (highly active), ≥22.5 (very highly active) | HRs for total activity combination of the 7 activities: moderate active: 0.95 (0.94–0.97), active 0.87 (0.85–0.89), dose-response, compared with the first level. Racquet sports, running, aerobic exercise participation: HRs: 1.01 (0.85–1.21), 0.81 (0.69–0.95), and 0.91 (0.86–0.97), respectively |
| Chang et al., 2024, UK [65] | Prospective 490,659 participants from UK Biobank and 33,534 from NHANES datasets, 36,109 and 3057 deaths due to cancer, aged 37–73 years, 13.5- and 6.7-year follow-ups, respectively | Sedentary behavior determined by interview or self-assessment: time spent sitting or reclining per day (hour/day). PA assessment: walking for pleasure, light activity, strenuous sports and other activities (UK Biobank), recreational, household chores, yard work, walking, and bicycling daily duration | Subjects meeting the daily PA guidelines: sitting time 5–8 h/day vs. <5 h/day, HR = 1.034 (1.002–1.066) UK Biobank, HR = 1.072 (0.904–1.271) NHANES; >8 h/day, HR = 1.106 (1.047–1.167) UK Biobank, HR = 1.216 (0.977–1.513) NHANES. Replacing sedentary behavior with a 30 min/day PA, HR = 0.949 (0.943–0.955) UK Biobank, HR = 0.944 (0.933–0.957) NHANES, strenuous sports (60 min/day), HR = 0.923 (0.888–1.017) walking for pleasure (60 min/day), HR = 0.968 (0.936–1.000) |
| O’Donovan, 2024, Colombia [66] | Prospective Mexico City, 10023 subjects, mean age 53.3 years, 3409 deaths due to cancer, 17.6-year follow-up | Leisure-time PA (exercise and sports) frequency per week and duration using questionnaire. Categorization: no sport or exercise, “weekend” warrior (exercise and playing sports 1–2 times/wk), regularly active ≥ 3 times/wk | The weekend warrior group, HR = 0.82 (0.71–0.95), regularly active, HR = 0.94 (0.86–1.04) vs. non-sports exercise practice |
| Stamatakis et al., 2024, China [67] | Prospective longitudinal 349,248 participants aged ≥ 18 years, 4631 cancer deaths (men), and 3689 (women), 16.2- and 16.4-year follow-ups, respectively | Self-administered questionnaire, leisure-time PA in MET-h/wk: inactive (<1), low (1.00–7.49), moderate (7.5–14.99), high (≥15) (based on current PA guidelines). Occupational PA: light (mostly sedentary), moderately heavy/heavy (mostly standing or walking/loading or moving, heavy lifting) | Baseline occupational PA Moderate activity HR = 1.18 (0.97–1.43), moderately heavy/heavy HR = 1.11 (0.86–1.42) vs. light. Activity changes: decreased, HR = 1.20 (0.99–1.46); increased, HR = 1.07 (0.85–1.33) (in women) |
| Potential Mechanisms | Effect of Physical Activity and Cancer Site |
|---|---|
| Reduced body fat and prevented weight gain | Activity reduces body fat, followed by decreasing levels of adipocytes, pro-inflammatory markers, estrogens, and exposure to bioavailable sex hormones (colon, postmenopausal breast, endometrium, ovaries). |
| Metabolic effects | Decreases C-peptide, insulin, IGF-1, fasting glucose levels, and fatty acids synthesis; increases glucose transport into muscle, muscle mass, IGFBP-3 level, insulin sensitivity; stimulates mitochondrial biogenesis, enhances cell resistance to environmental stressors (colon, postmenopausal breast, endometrium, prostate, ovaries, lung). |
| Hormonal effects | Regulates insulin resistance, reduces the level of bioavailable sex hormones, i.e., estrogens and androgens, decreases the level of free testosterone, and increases the formation of SHBG (breast, endometrium, ovaries, prostate). |
| Anti-inflammatory effects | Exercise decreases chronic inflammation by lowering levels of pro-inflammatory adipokines secreted by adipose tissue. Thus, it decreases levels of leptin and increases levels of adiponectin. Moreover, exercise decreases pro-inflammatory cytokines (IL-6, TNF-α, IL-1β), which can decrease CRP and serum amyloid levels (most cancers). |
| Innate immune system | Physical activity may trigger apoptosis, generate the responses of NK cells and lymphocytes, and thus enhance their activity in the immune system. It also participates in controlling cancerous and microbial cells, limiting their spread (most cancers). |
| Antioxidant refence, Prostaglandins | May control redox homeostasis by enhancing total endogenous antioxidant capacity, reduces oxidative stress by up-regulating levels of enzymatic antioxidants, e.g., CAT, GPX, SOD, and increases non-enzymatic antioxidants synthesis, e.g., glutathione, tocopherols, and stimulation of Vitamin D release from adipose tissue. Exercise may decrease DNA damage and enhance its repair. Exercise also inhibits the synthesis of prostaglandin E2 (promotor of colon cancer) and stimulates the synthesis of prostaglandin F2α, which is opposite to the effect of prostaglandin E2 (colon and most cancers). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, J.; Aboul-Enein, B.H.; Gołębiewska, M.E.; Duchnik, E.; Czerniak, U.; Marchlewicz, M. Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms. Cancers 2025, 17, 1410. https://doi.org/10.3390/cancers17091410
Kruk J, Aboul-Enein BH, Gołębiewska ME, Duchnik E, Czerniak U, Marchlewicz M. Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms. Cancers. 2025; 17(9):1410. https://doi.org/10.3390/cancers17091410
Chicago/Turabian StyleKruk, Joanna, Basil Hassan Aboul-Enein, Marta Ewelina Gołębiewska, Ewa Duchnik, Urszula Czerniak, and Mariola Marchlewicz. 2025. "Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms" Cancers 17, no. 9: 1410. https://doi.org/10.3390/cancers17091410
APA StyleKruk, J., Aboul-Enein, B. H., Gołębiewska, M. E., Duchnik, E., Czerniak, U., & Marchlewicz, M. (2025). Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms. Cancers, 17(9), 1410. https://doi.org/10.3390/cancers17091410






