Stromal Versican Accumulation and Proteolysis Regulate the Infiltration of CD8+ T Cells in Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Cancer Patient Specimens
2.2. Immunohistochemistry (IHC)
2.3. IHC Quantification
2.4. Estrogen Stimulation
2.5. qPCR
2.6. Gene Expression Profiling
2.7. Versican Proteolytic Phenotype Signatures and Gene Set Enrichment Analysis
2.8. Statistical Considerations
3. Results
3.1. Clinical Characteristics
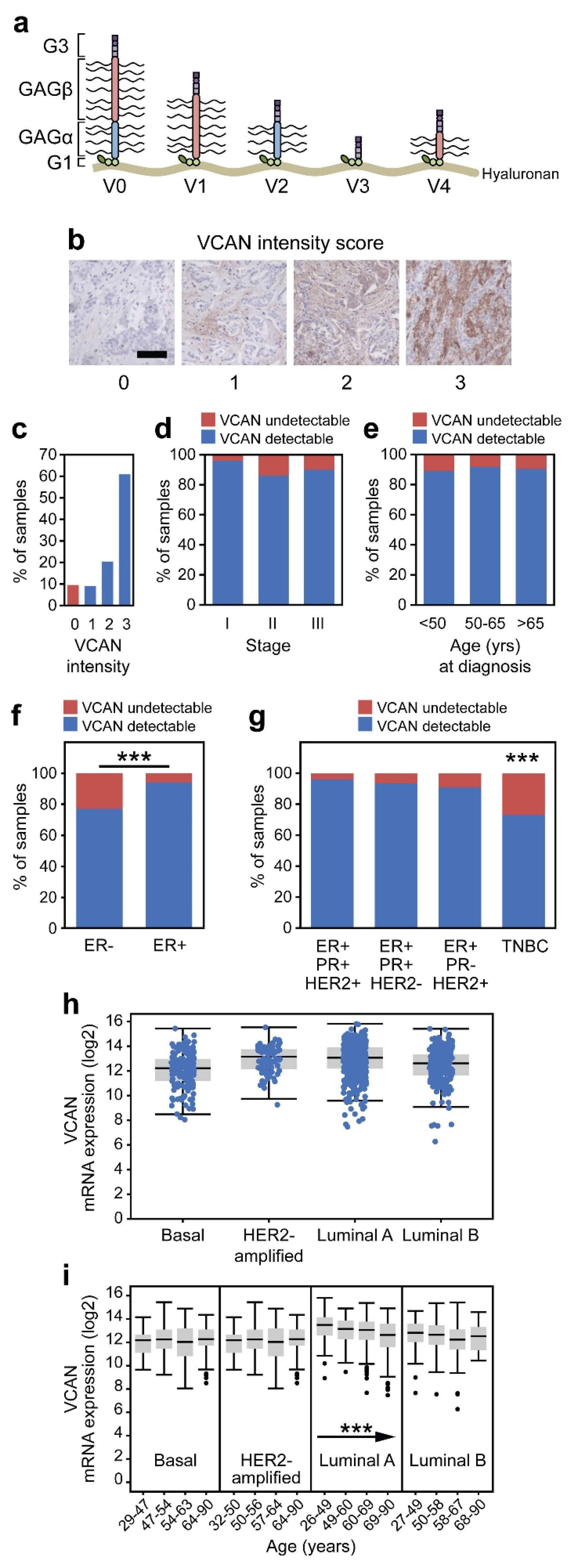
3.2. VCAN Accumulation Is a Common Feature of Breast Adenocarcinoma
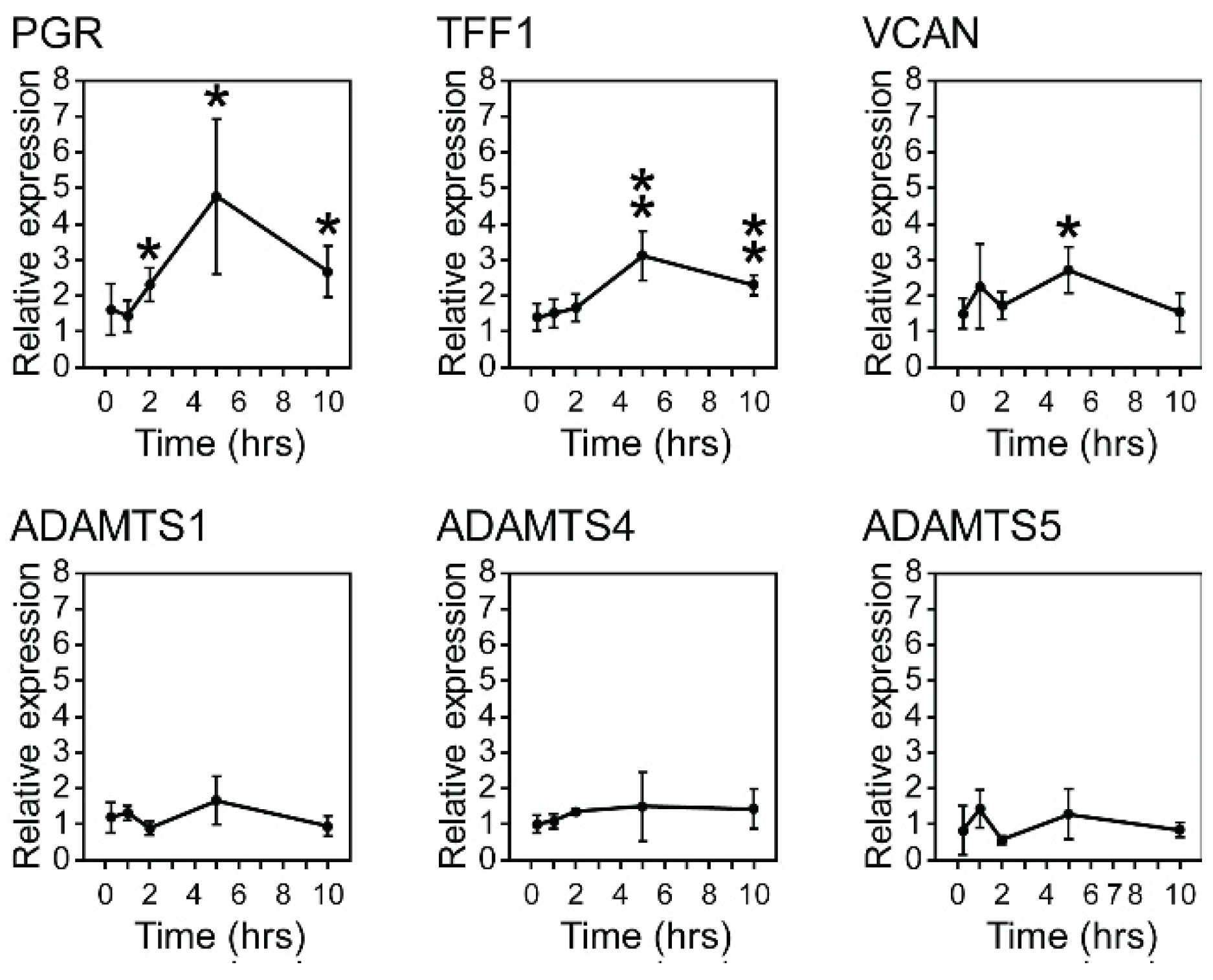
3.3. Estrogen Stimulation of VCAN Expression
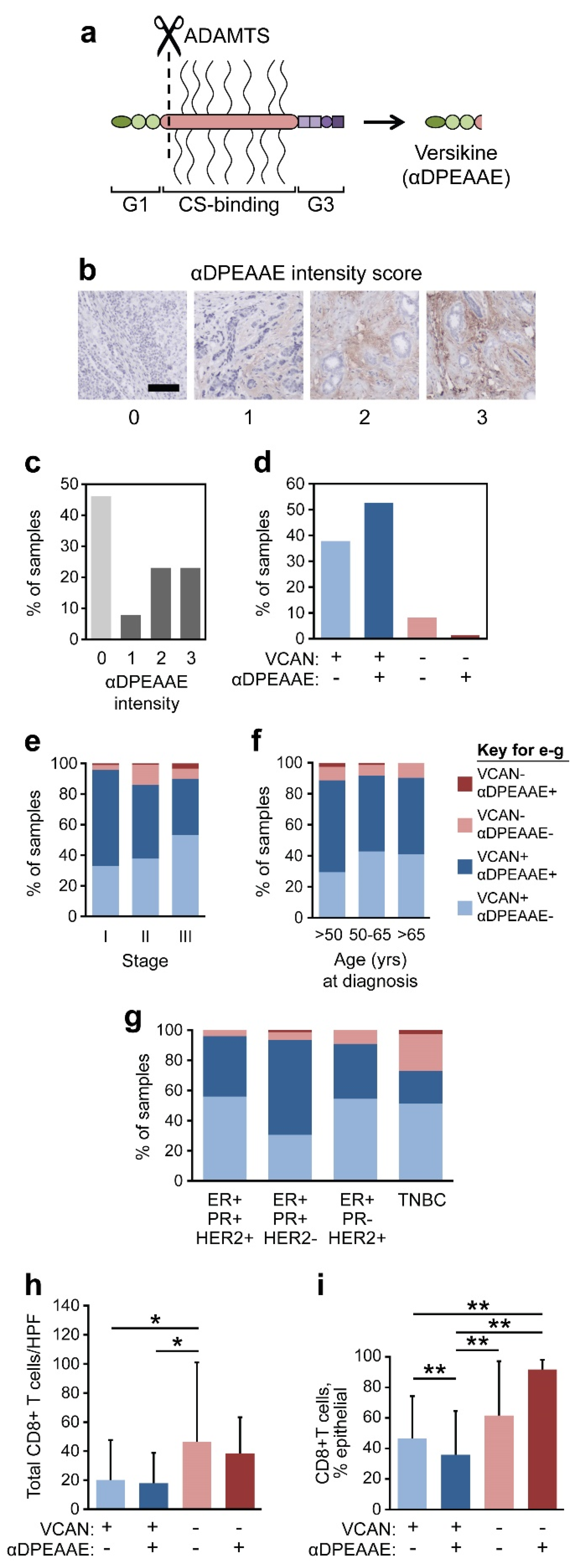
3.4. VCAN Accumulation Predicts CD8+ T Cell Exclusion in Breast Cancer
3.5. VCAN Predicts CD8+ T Cells Across Clinical Subtypes of Breast Cancer
3.6. VCAN Status Predicts Epithelial Infiltration of CD8+ T Cells
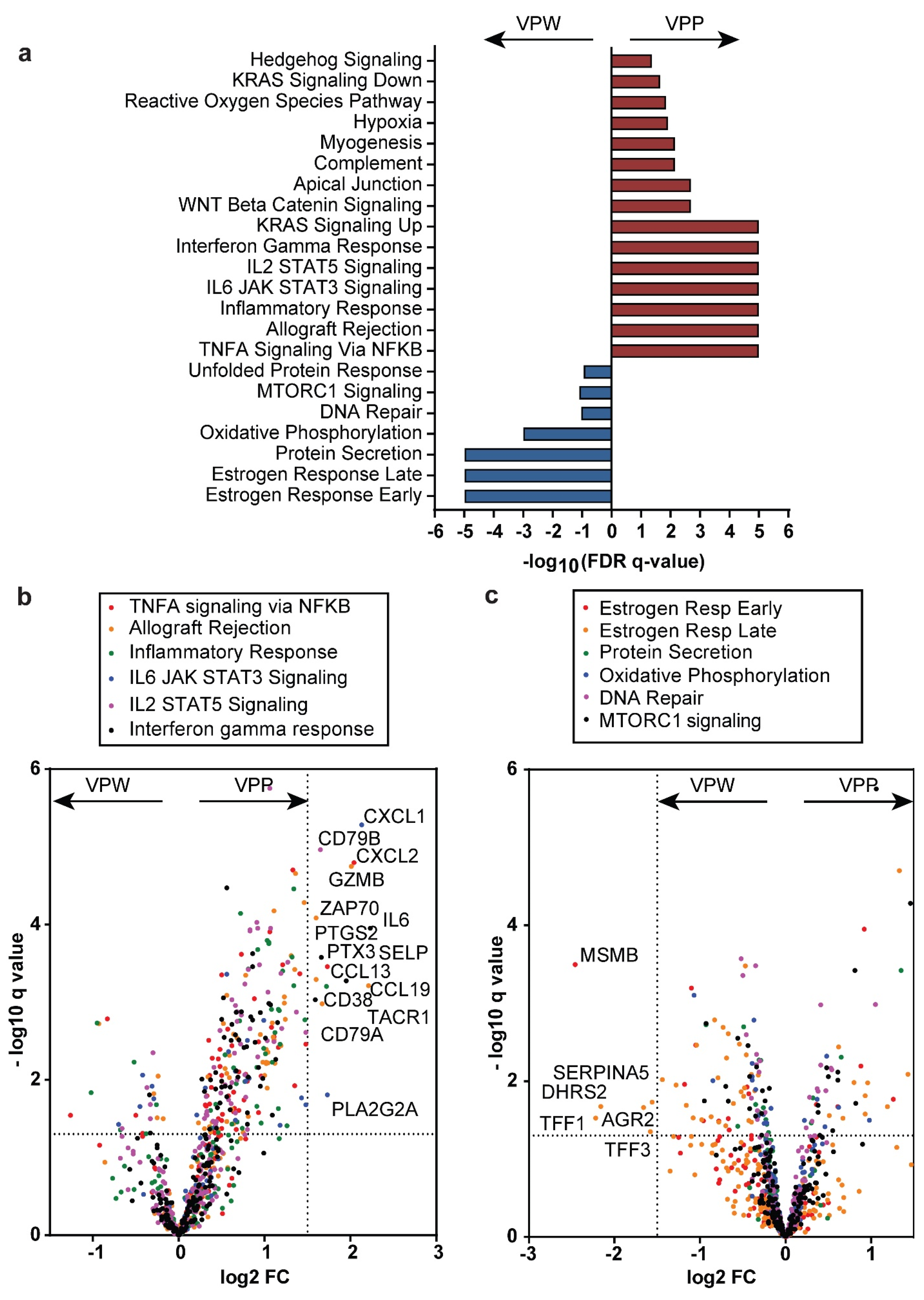
3.7. VCAN Proteolysis in Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Curigliano, G.; Salgado, R.F.; Romero Diaz, R.I.; Delaloge, S.; Rojas, C.; Kok, M.; Saura Manich, C.; Harbeck, N.; Mittendorf, E.A.; et al. LBA20 A Randomized, Double-Blind Trial of Nivolumab (NIVO) vs. Placebo (PBO) with Neoadjuvant Chemotherapy (NACT) Followed by Adjuvant Endocrine Therapy (ET) ± NIVO in Patients (Pts) with High-Risk, ER+ HER2− Primary Breast Cancer (BC). Ann. Oncol. 2023, 34, S1259–S1260. [Google Scholar] [CrossRef]
- Cardoso, F.; McArthur, H.L.; Schmid, P.; Cortés, J.; Harbeck, N.; Telli, M.L.; Cescon, D.W.; O’Shaughnessy, J.; Fasching, P.; Shao, Z.; et al. LBA21 KEYNOTE-756: Phase III Study of Neoadjuvant Pembrolizumab (Pembro) or Placebo (Pbo) + Chemotherapy (Chemo), Followed by Adjuvant Pembro or Pbo + Endocrine Therapy (ET) for Early-Stage High-Risk ER+/HER2− Breast Cancer. Ann. Oncol. 2023, 34, S1260–S1261. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Direkze, N.C.; Jeffery, R.; Hodivala-Dilke, K.; Hunt, T.; Playford, R.J.; Elia, G.; Poulsom, R.; Wright, N.A.; Alison, M.R. Bone Marrow-Derived Stromal Cells Express Lineage-Related Messenger RNA Species. Cancer Res. 2006, 66, 1265–1269. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in Cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Insua-Rodríguez, J.; Oskarsson, T. The Extracellular Matrix in Breast Cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vahdat, L.T.; Wong, S.; Chang, J.C.; Mittal, V. Microenvironmental Regulation of Epithelial-Mesenchymal Transitionsin Cancer. Cancer Res. 2012, 72, 4883–4889. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, Y.; Kim, H.J.; Zhang, L.; Ma, X. CCL5 as a Potential Immunotherapeutic Target in Triple-Negative Breast Cancer. Cell Mol. Immunol. 2013, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Santangelo, A.; Castioni, N.; Portararo, P.; Gulino, A.; Botti, L.; Parenza, M.; Cappetti, B.; Orlandi, R.; et al. Mesenchymal Transition of High-Grade Breast Carcinomas Depends on Extracellular Matrix Control of Myeloid Suppressor Cell Activity. Cell Rep. 2016, 17, 233–248. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal Cell Diversity Associated with Immune Evasion in Human Triple-negative Breast Cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Hope, C.; Foulcer, S.; Jagodinsky, J.; Chen, S.X.; Jensen, J.L.; Patel, S.; Leith, C.; Maroulakou, I.; Callander, N.; Miyamoto, S.; et al. Immunoregulatory Roles of Versican Proteolysis in the Myeloma Microenvironment. Blood 2016, 128, 680–685. [Google Scholar] [CrossRef]
- Hope, C.; Emmerich, P.B.; Papadas, A.; Pagenkopf, A.; Matkowskyj, K.A.; Van De Hey, D.R.; Payne, S.N.; Clipson, L.; Callander, N.S.; Hematti, P.; et al. Versican-Derived Matrikines Regulate Batf3–Dendritic Cell Differentiation and Promote T Cell Infiltration in Colorectal Cancer. J. Immunol. 2017, 199, 1933–1941. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Brooks, J.H.; Suwiwat, S.; Sakko, A.J.; Mayne, K.; Raymond, W.A.; Seshadri, R.; LeBaron, R.G.; Horsfall, D.J. Regulation of Stromal Versican Expression by Breast Cancer Cells and Importance to Relapse-Free Survival in Patients with Node-Negative Primary Breast Cancer. Clin. Cancer Res. 2002, 8, 1054–1060. [Google Scholar]
- Van Bockstal, M.; Lambein, K.; Van Gele, M.; De Vlieghere, E.; Limame, R.; Braems, G.; Van den Broecke, R.; Cocquyt, V.; Denys, H.; Bracke, M.; et al. Differential Regulation of Extracellular Matrix Protein Expression in Carcinoma-Associated Fibroblasts by TGF-Β1 Regulates Cancer Cell Spreading but Not Adhesion. Oncoscience 2014, 1, 634–648. [Google Scholar] [CrossRef]
- dos Reis, D.C.; Damasceno, K.A.; de Campos, C.B.; Veloso, E.S.; Pêgas, G.R.A.; Kraemer, L.R.; Rodrigues, M.A.; Mattos, M.S.; Gomes, D.A.; Campos, P.P.; et al. Versican and Tumor-Associated Macrophages Promotes Tumor Progression and Metastasis in Canine and Murine Models of Breast Carcinoma. Front. Oncol. 2019, 9, 577. [Google Scholar] [CrossRef]
- Suwiwat, S.; Ricciardelli, C.; Tammi, R.; Tammi, M.; Auvinen, P.; Kosma, V.M.; LeBaron, R.G.; Raymond, W.A.; Tilley, W.D.; Horsfall, D.J. Expression of Extracellular Matrix Components Versican, Chondroitin Sulfate, Tenascin, and Hyaluronan, and Their Association with Disease Outcome in Node-Negative Breast Cancer. Clin. Cancer Res. 2004, 10, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.S.; McGregor, S.M. Combined Use of SOX10 and GATA3 in Mammary Carcinoma. Pathol. Res. Pract. 2020, 216, 152801. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Schicketanz, K.H. Immunohistochemical Determination of Estrogen and Progesterone Receptor Content in Human Breast Cancer: Computer-Assisted Image Analysis (QIC Score) vs. Subjective Grading (IRS). Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Chan, C.K.; Johnson, P.Y.; Frevert, C.W.; Wight, T.N. The Biochemistry and Immunohistochemistry of Versican. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 143. [Google Scholar]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Gu, Z. Complex Heatmap Visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- R Core Team. R Core Team 2023 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2023. Available online: https://www.R-Project.org/ (accessed on 1 November 2023).
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in CBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Papadas, A.; Arauz, G.; Cicala, A.; Wiesner, J.; Asimakopoulos, F. Versican and Versican-Matrikines in Cancer Progression, Inflammation, and Immunity. J. Histochem. Cytochem. 2020, 68, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Foulcer, S.J.; Day, A.J.; Apte, S.S. Isolation and Purification of Versican and Analysis of Versican Proteolysis. In Glycosaminoglycans Chemistry and Biology; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1229, pp. 587–604. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Milne, K.; Derocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. CD103 and Intratumoral Immune Response in Breast Cancer. Clin. Cancer Res. 2016, 22, 6290–6297. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the Immune Contexture to the Immunoscore: The Role of Prognostic and Predictive Immune Markers in Cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef]
- Nandadasa, S.; Foulcer, S.; Apte, S.S. The Multiple, Complex Roles of Versican and Its Proteolytic Turnover by ADAMTS Proteases during Embryogenesis. Matrix Biol. 2014, 35, 34–41. [Google Scholar] [CrossRef]
- Papadas, A.; Deb, G.; Cicala, A.; Officer, A.; Hope, C.; Pagenkopf, A.; Flietner, E.; Morrow, Z.T.; Emmerich, P.; Wiesner, J.; et al. Stromal Remodeling Regulates Dendritic Cell Abundance and Activity in the Tumor Microenvironment. Cell Rep. 2022, 40, 111201. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Yee, A.J. Roles of Versican in Cancer Biology-Tumorigenesis, Progression and Metastasis. Histol. Histopathol. 2013, 28, 701–713. [Google Scholar]
- Du, W.W.; Fang, L.; Yang, X.; Sheng, W.; Yang, B.L.; Seth, A.; Zhang, Y.; Yang, B.B.; Yee, A.J. The Role of Versican in Modulating Breast Cancer Cell Self-Renewal. Mol. Cancer Res. 2013, 11, 443–455. [Google Scholar] [CrossRef]
- Kischel, P.; Waltregny, D.; Dumont, B.; Turtoi, A.; Greffe, Y.; Kirsch, S.; De Pauw, E.; Castronovo, V. Versican Overexpression in Human Breast Cancer Lesions: Known and New Isoforms for Stromal Tumor Targeting. Int. J. Cancer 2010, 126, 640–650. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kuwabara, H.; Yoneda, M.; Isogai, Z.; Tanigawa, N.; Shibayama, Y. Versican G1 and G3 Domains Are Upregulated and Latent Transforming Growth Factor-β Binding Protein-4 Is Downregulated in Breast Cancer Stroma. Breast Cancer 2012, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Labropoulou, V.T.; Ravazoula, P.; Likaki-Karatza, E.; Dobra, K.; Kalofonos, H.P.; Karamanos, N.K.; Theocharis, A.D. Versican but Not Decorin Accumulation Is Related to Malignancy in Mammographically Detected High Density and Malignant-Appearing Microcalcifications in Non-Palpable Breast Carcinomas. BMC Cancer 2011, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Gorter, A.; Zijlmans, H.J.; Van Gent, H.; Trimbos, J.B.; Fleuren, G.J.; Jordanova, E.S. Versican Expression Is Associated with Tumor-Infiltrating CD8-Positive T Cells and Infiltration Depth in Cervical Cancer. Mod. Pathol. 2010, 23, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Hirani, P.; McDermott, J.; Rajeeve, V.; Cutillas, P.R.; Jones, J.L.; Pennington, D.J.; Wight, T.N.; Santamaria, S.; Alonge, K.M.; Pearce, O.M.T. Versican Associates with Tumor Immune Phenotype and Limits T-Cell Trafficking via Chondroitin Sulfate. Cancer Res. Commun. 2024, 4, 970–985. [Google Scholar] [CrossRef]
- Pappas, A.G.; Magkouta, S.; Pateras, I.S.; Skianis, I.; Moschos, C.; Vazakidou, M.E.; Psarra, K.; Gorgoulis, V.G.; Kalomenidis, I. Versican Modulates Tumor-Associated Macrophage Properties to Stimulate Mesothelioma Growth. Oncoimmunology 2019, 8, e1537427. [Google Scholar] [CrossRef]
- Tang, M.; Diao, J.; Gu, H.; Khatri, I.; Zhao, J.; Cattral, M.S. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell Rep. 2015, 13, 2851–2864. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Choi, J.; Mani, N.; Sanmamed, M.F.; Datar, I.; Sowell, R.; Du, V.Y.; Kaftan, E.; Goldberg, S.; Dong, W.; et al. A Dormant TIL Phenotype Defines Non-Small Cell Lung Carcinomas Sensitive to Immune Checkpoint Blockers. Nat. Commun. 2018, 9, 3196. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Papadas, A.; Pagenkopf, A.; Flietner, E.; Morrow, Z.; Chaudhary, S.G.; Asimakopoulos, F. Tumor Matrix Remodeling and Novel Immunotherapies: The Promise of Matrix-Derived Immune Biomarkers. J. Immunother. Cancer 2018, 6, 65. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Glajcar, A.; Szpor, J.; Hodorowicz-Zaniewska, D.; Tyrak, K.E.; Okoń, K. The Composition of T Cell Infiltrates Varies in Primary Invasive Breast Cancer of Different Molecular Subtypes as Well as According to Tumor Size and Nodal Status. Virchows Arch. 2019, 475, 13–23. [Google Scholar] [CrossRef]
- Desmedt, C.; Salgado, R.; Fornili, M.; Pruneri, G.; Van Den Eynden, G.; Zoppoli, G.; Rothe, F.; Buisseret, L.; Garaud, S.; Willard-Gallo, K.; et al. Immune Infiltration in Invasive Lobular Breast Cancer. J. Natl. Cancer Inst. 2018, 110, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Chan, M.; Hirakawa, H.; Suzuki, A.; Tada, H.; Watanabe, G.; Nemoto, N.; Nakagawa, S.; et al. Tumor-Infiltrating CD8+ and FOXP3+ Lymphocytes in Triple-Negative Breast Cancer: Its Correlation with Pathological Complete Response to Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2014, 148, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Lachapelle, J.; Zlobec, I.; Bismar, T.A.; Terracciano, L.; Foulkes, W.D. Prognostic Significance of CD8+ T Lymphocytes in Breast Cancer Depends upon Both Oestrogen Receptor Status and Histological Grade. Histopathology 2011, 58, 1107–1116. [Google Scholar] [CrossRef]
- Liu, S.; Lachapelle, J.; Leung, S.; Gao, D.; Foulkes, W.D.; Nielsen, T.O. CD8+ lymphocyte Infiltration Is an Independent Favorable Prognostic Indicator in Basal-like Breast Cancer. Breast Cancer Res. 2012, 14, R48. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Clinical Significance of Tumor-Infiltrating Lymphocytes in Breast Cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef]
- Thompson, E.; Taube, J.M.; Elwood, H.; Sharma, R.; Meeker, A.; Warzecha, H.N.; Argani, P.; Cimino-Mathews, A.; Emens, L.A. The Immune Microenvironment of Breast Ductal Carcinoma in Situ. Mod. Pathol. 2016, 29, 249–258. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmerich, P.B.; Qyli, T.; Johnson, K.A.; Chaudhuri, S.; Clark, K.M.; Verhagen, N.B.; Depke, M.G.; Clipson, L.; Pasch, C.A.; Papadas, A.; et al. Stromal Versican Accumulation and Proteolysis Regulate the Infiltration of CD8+ T Cells in Breast Cancer. Cancers 2025, 17, 1435. https://doi.org/10.3390/cancers17091435
Emmerich PB, Qyli T, Johnson KA, Chaudhuri S, Clark KM, Verhagen NB, Depke MG, Clipson L, Pasch CA, Papadas A, et al. Stromal Versican Accumulation and Proteolysis Regulate the Infiltration of CD8+ T Cells in Breast Cancer. Cancers. 2025; 17(9):1435. https://doi.org/10.3390/cancers17091435
Chicago/Turabian StyleEmmerich, Philip B., Tonela Qyli, Katherine A. Johnson, Somak Chaudhuri, Kristen M. Clark, Nathaniel B. Verhagen, Mitchell G. Depke, Linda Clipson, Cheri A. Pasch, Athanasios Papadas, and et al. 2025. "Stromal Versican Accumulation and Proteolysis Regulate the Infiltration of CD8+ T Cells in Breast Cancer" Cancers 17, no. 9: 1435. https://doi.org/10.3390/cancers17091435
APA StyleEmmerich, P. B., Qyli, T., Johnson, K. A., Chaudhuri, S., Clark, K. M., Verhagen, N. B., Depke, M. G., Clipson, L., Pasch, C. A., Papadas, A., Burkard, M. E., Wisinski, K. B., McGregor, S. M., Asimakopoulos, F., & Deming, D. A. (2025). Stromal Versican Accumulation and Proteolysis Regulate the Infiltration of CD8+ T Cells in Breast Cancer. Cancers, 17(9), 1435. https://doi.org/10.3390/cancers17091435




