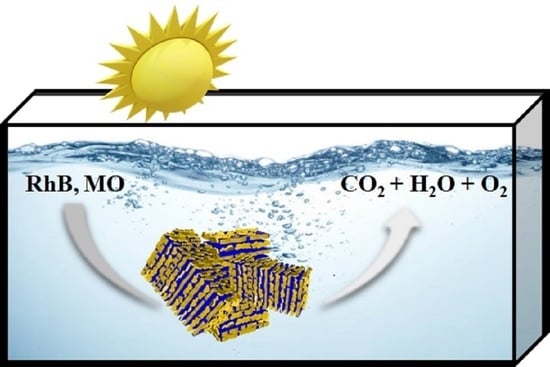
Rice Crust-Like ZnO/Ti3C2Tx MXene Hybrid Structures for Improved Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
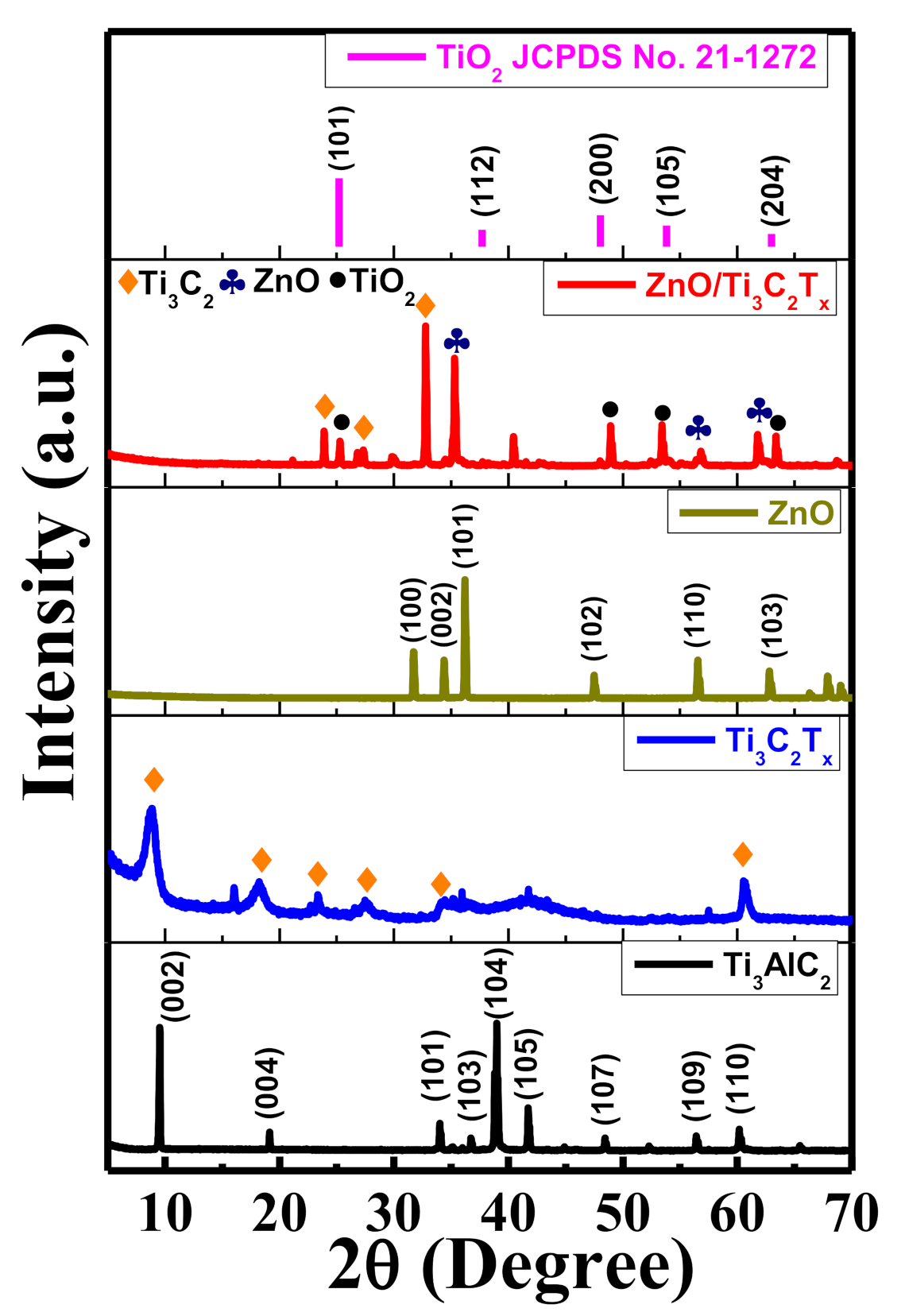
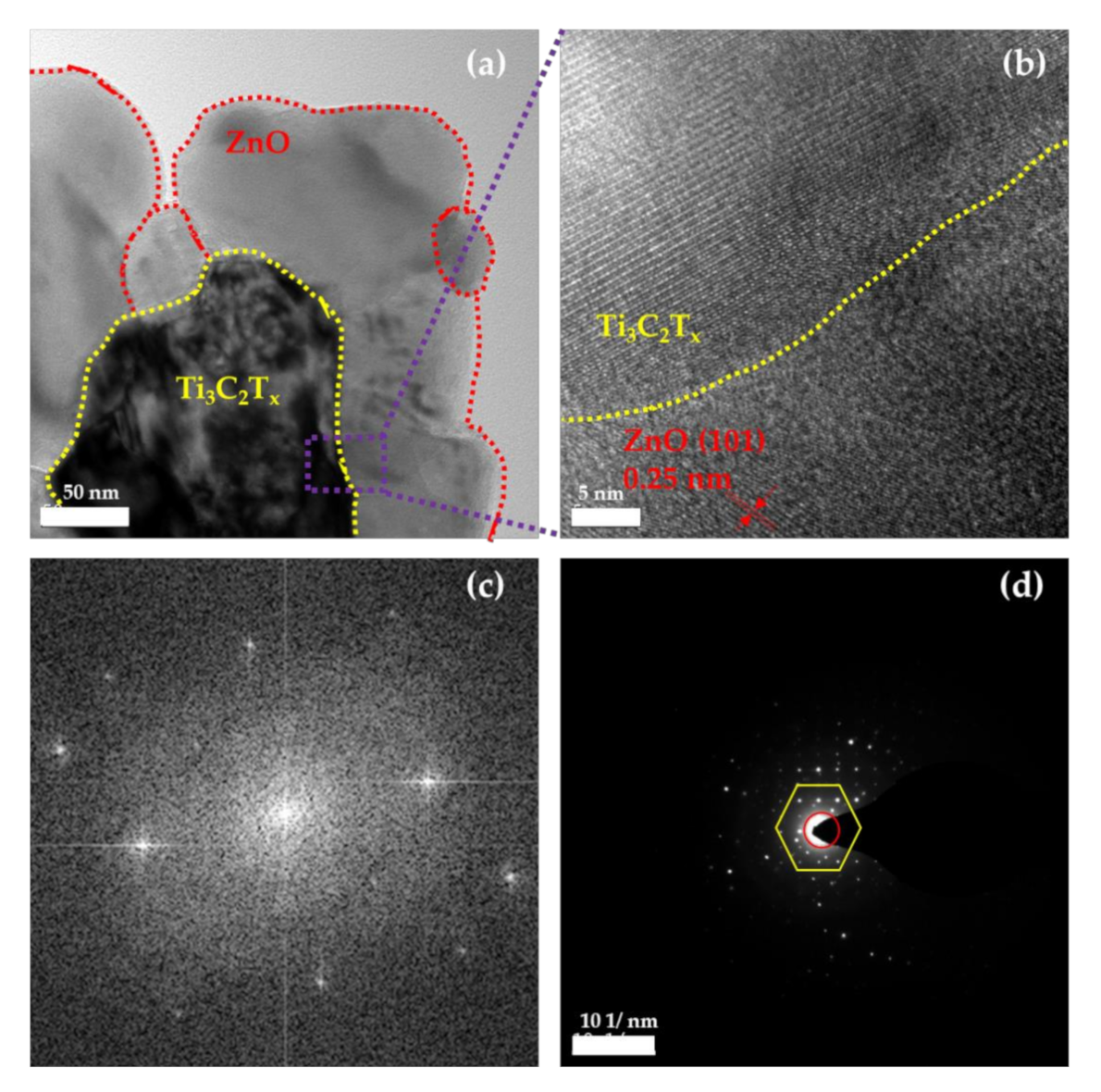
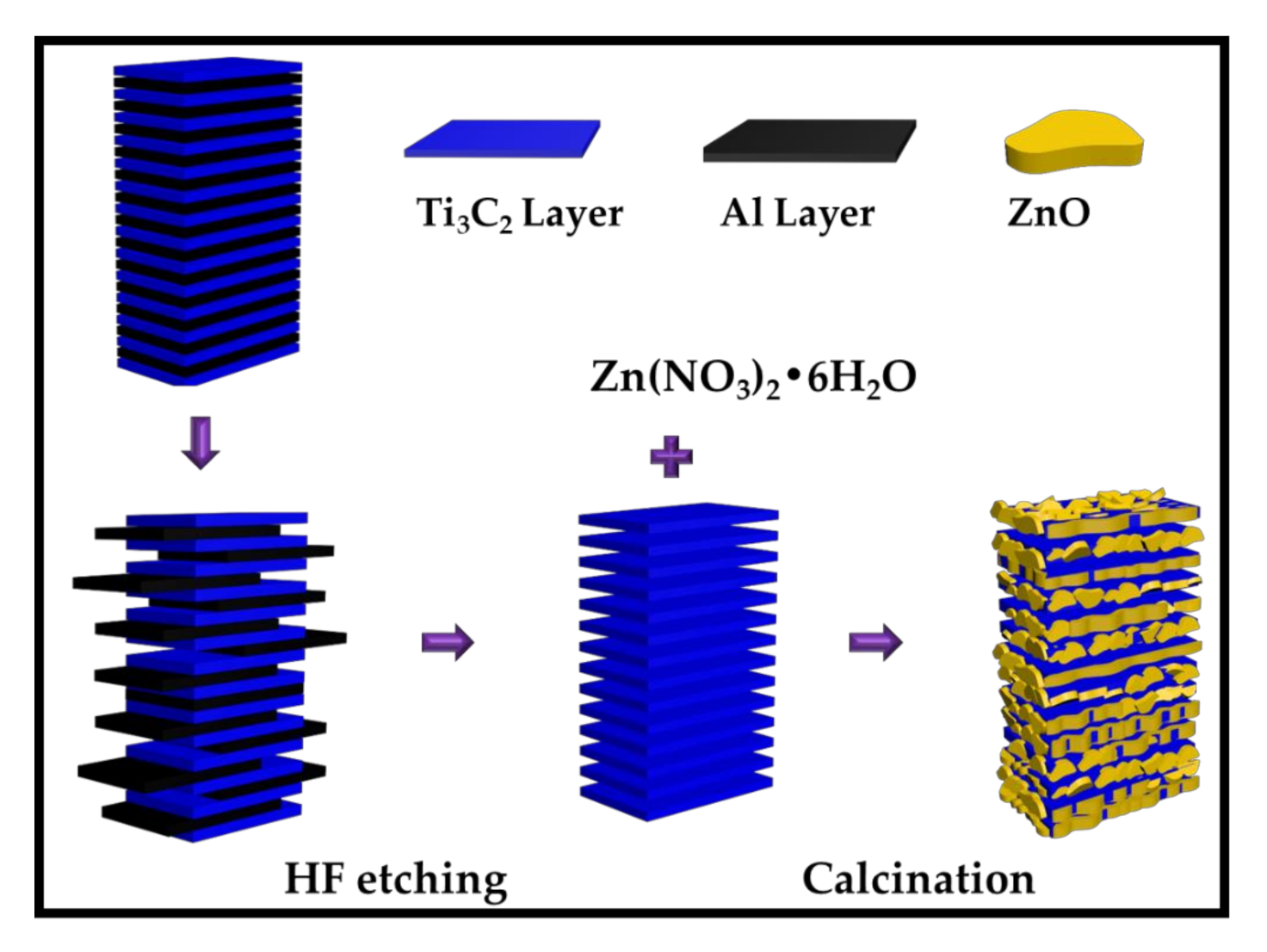
2.1. Crystal Structures and Morphologies
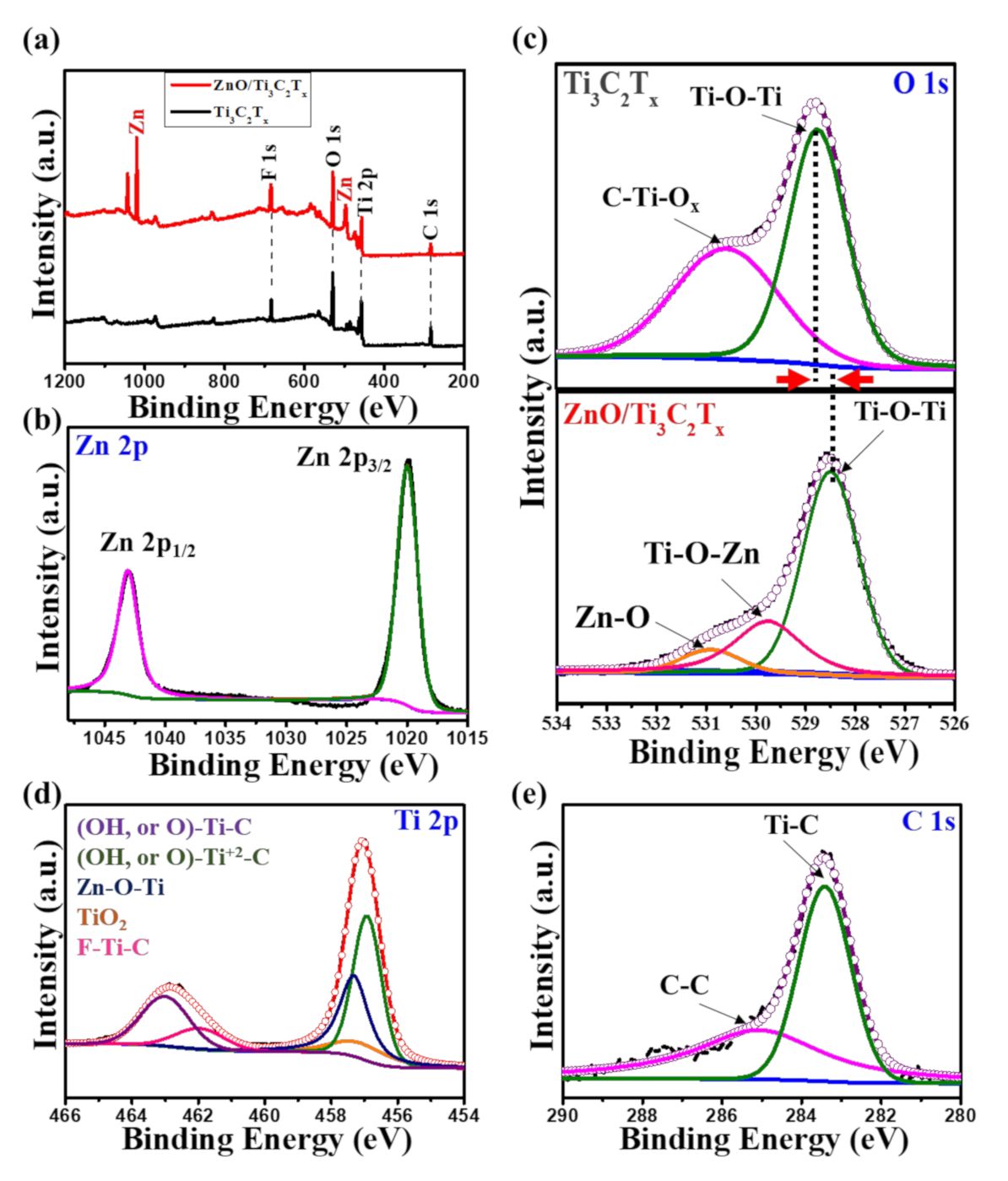
2.2. Bonding States
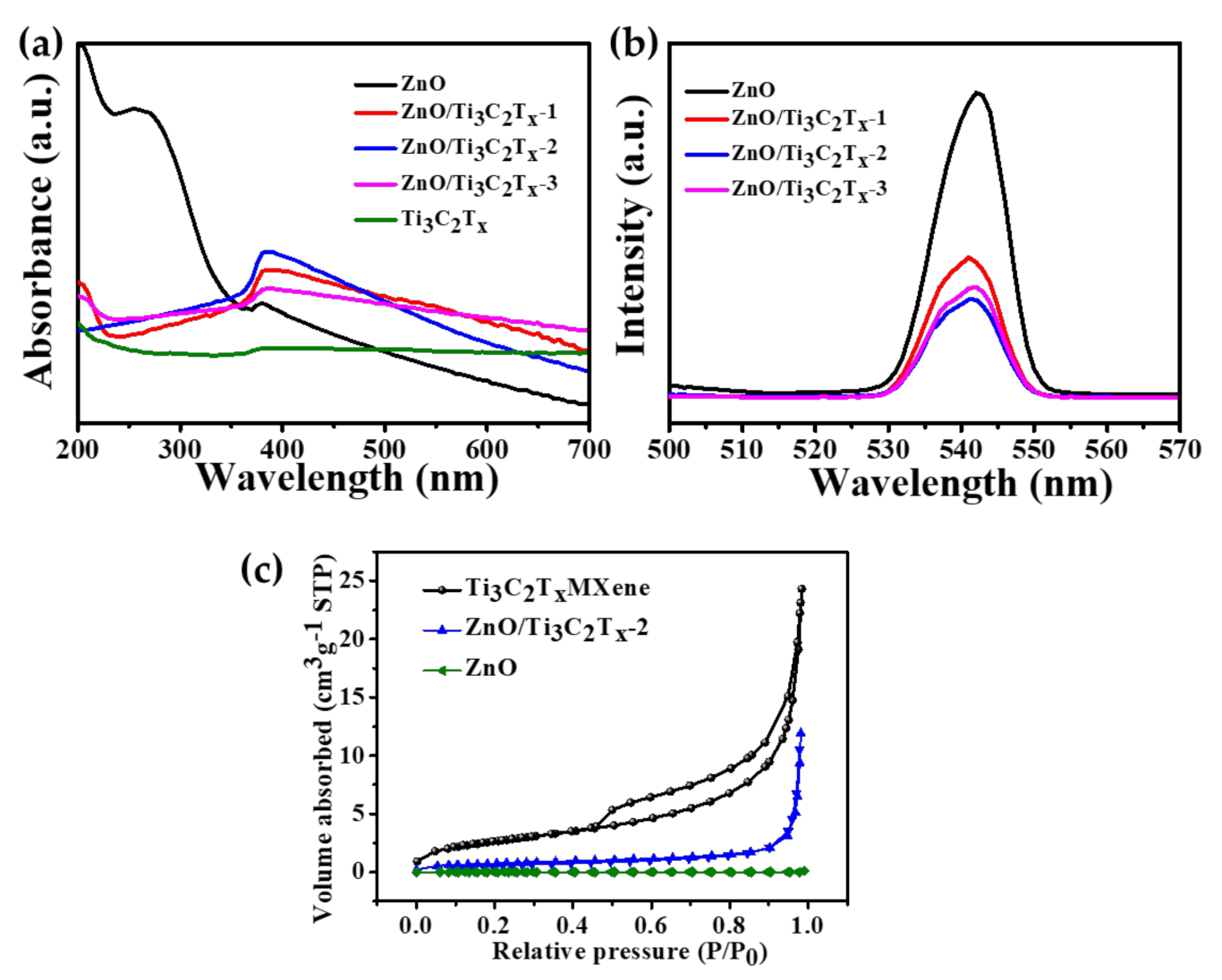
2.3. Optical Properties, Specific Surface Area, and Thermal Decomposition
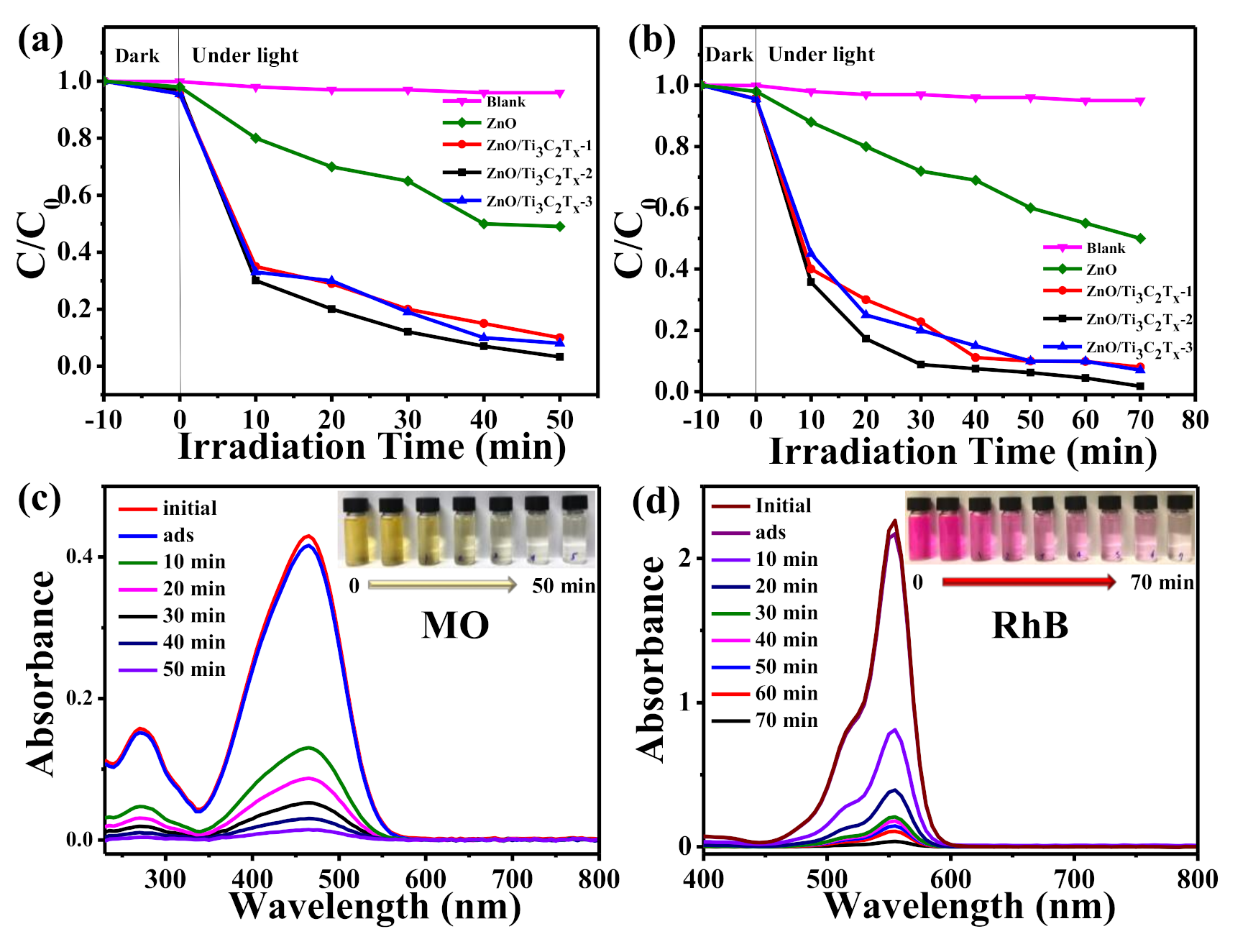
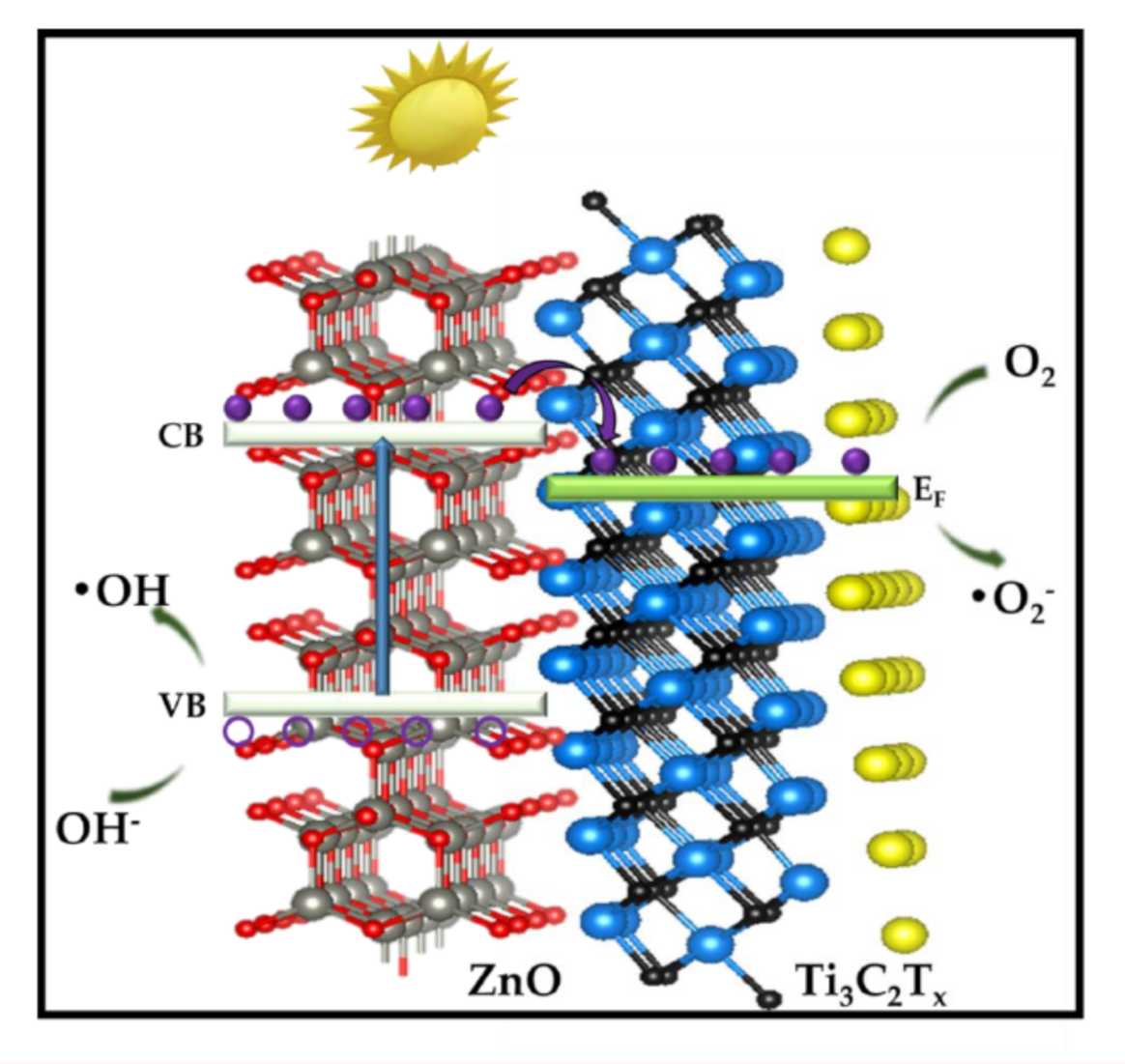
2.4. Photocatalytic Activities
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of ZnO/Ti3C2Tx Hybrid Structures
3.3. Characterizations
3.4. Photocatalytic Dye Degradation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater engineering. Management 1991, 7, 1–4. [Google Scholar]
- Rojas, S.; Horcajada, P. Metal–Organic frameworks for the removal of emerging organic contaminants in water. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carmen, Z.; Daniela, S. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Proceedings of the Organic Pollutants Ten Years after the Stockholm Convention-Environmental and Analytical Update, London, UK, 24 February 2012; Volume 10, p. 32373. [Google Scholar]
- Li, D.; Zheng, H.; Wang, Q.; Wang, X.; Jiang, W.; Zhang, Z.; Yang, Y. A novel double-cylindrical-shell photoreactor immobilized with monolayer TiO2-coated silica gel beads for photocatalytic degradation of Rhodamine B and Methyl Orange in aqueous solution. Sep. Purif. Technol. 2014, 123, 130–138. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Dashamiri, S.; Ghaedi, M.; Dashtian, K.; Rahimi, M.R.; Goudarzi, A.; Jannesar, R. Ultrasonic enhancement of the simultaneous removal of quaternary toxic organic dyes by CuO nanoparticles loaded on activated carbon: Central composite design, kinetic and isotherm study. Ultrason. Sonochem. 2016, 31, 546–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, U.; Sandoval, A.; Madrid, S.I.U.; Corro, G.; Sharma, V.; Mohanty, P. Mixed titanium, silicon, and aluminum oxide nanostructures as novel adsorbent for removal of rhodamine 6G and methylene blue as cationic dyes from aqueous solution. Chemosphere 2016, 163, 142–152. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar]
- Le, T.M.O.; Lam, T.H.; Pham, T.N.; Ngo, T.C.; Lai, N.D.; Do, D.B.; Nguyen, V.M. Enhancement of rhodamine B degradation by Ag nanoclusters-loaded g-C3N4 nanosheets. Polymers 2018, 10, 633. [Google Scholar] [CrossRef] [Green Version]
- AlHamedi, F.H.; Rauf, M.A.; Ashraf, S.S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Ombaka, L.; Curti, M.; Mcgettrick, J.D.; Davies, M.L.; Bahnemann, D.W. Nitrogen/carbon-coated zero valent copper as highly efficient co-catalysts for TiO2 applied in photocatalytic and photoelectrocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2020, 12, 30365–30380. [Google Scholar] [CrossRef]
- Sreedhar, A.; Jung, H.; Kwon, J.H.; Yi, J.; Sohn, Y.; Gwag, J.S. Novel composite ZnO/TiO2 thin film photoanodes for enhanced visible-light-driven photoelectrochemical water splitting activity. J. Electroanal. Chem. 2017, 804, 92–98. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Balayeva, N.O.; Granone, L.I.; Dillert, R.; Bahnemann, D.W.; Sillanpää, M. Anchoring lead-free halide Cs3Bi2I9 perovskite on UV100–TiO2 for enhanced photocatalytic performance. Sol. Energy Mater. Sol. Cells 2020, 204, 1–11. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2—Photocatalyzed acceptorless dehydrogenation of N—Heterocycles upon visible-light illumination. ACS Catal. 2020, 5542–5553. [Google Scholar] [CrossRef]
- Vo, V.; Van Kim, N.; Nga, N.T.V.; Trung, N.T.; Van Hanh, P.; Hoang, L.H.; Kim, S.-J. Preparation of gC3N4/Ta2O5 composites with enhanced visible-light photocatalytic activity. J. Electron. Mater. 2016, 45, 2334–2340. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.M.T.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Van Le, Q. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Ta, Q.T.H.; Cho, E.; Noh, J.-S. Highly supportive hydrogen peroxide as a hole scavenger to improve the visible light water splitting activity of flake-like Co-doped ZnO thin films. Sol. Energy 2019, 191, 151–160. [Google Scholar] [CrossRef]
- Sreedhar, A.; Sreekanth, T.V.M.; Kwon, J.H.; Yi, J.; Sohn, Y.; Gwag, J.S. Ag nanoparticles decorated ion-beam-assisted TiO2 thin films for tuning the water splitting activity from UV to visible light harvesting. Ceram. Int. 2017, 43, 12814–12821. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets. Appl. Surf. Sci. 2020, 502, 144189. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.-Q.; Weng, B.; Xu, Y.-J. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.-S.; Hu, C.; Nguyen, C.C.; Nguyen, D.L.T.; Nguyen Dinh, M.T.; Vo, D.-V.N.; Trinh, Q.T.; Shokouhimehr, M.; Hasani, A. Novel architecture titanium carbide (Ti3C2Tx) MXene cocatalysts toward photocatalytic hydrogen production: A mini-review. Nanomaterials 2020, 10, 602. [Google Scholar] [CrossRef] [Green Version]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-temperature reduction strategy synthesized Si/Ti3C2Tx MXene composite anodes for high-performance Li-Ion batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Û.G. 2D Metal Carbides and Nitrides (MXenes); Springer: Berlin/Heidelberg, Germany, 2019; ISBN 3030190269. [Google Scholar]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Low, I.M. An overview of parameters controlling the decomposition and degradation of Ti-based Mn+1AXn phases. Materials 2019, 12, 473. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Sun, Y.; Prenger, K.E.; Jiang, D.-E.; Naguib, M.; Pruski, M. Nature of Terminating Hydroxyl Groups and Intercalating Water in Ti3C2Tx MXenes: A Study by 1H Solid-State NMR and DFT Calculations. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Han, J.; Jin, Z.; Han, Y.; Wang, F.; Niu, Y.; Wu, Y.; Xu, Y. High-Performance Electrocatalytic Conversion of N2 to NH3 Using Oxygen-Vacancy-Rich TiO2 In Situ Grown on Ti3C2Tx MXene. Adv. Energy Mater. 2019, 9, 1803406. [Google Scholar] [CrossRef]
- Shuck, C.E.; Gogotsi, Y. Taking MXenes from the lab to commercial products. Chem. Eng. J. 2020, 401, 125786. [Google Scholar] [CrossRef]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-based photocatalysts. J. Mater. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.; Ma, T.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Su, Y.; Zhu, Y.; Zhang, Y.; Zhu, M. In-situ growing Bi/BiOCl microspheres on Ti3C2Tx nanosheets for upgrading visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 146339. [Google Scholar] [CrossRef]
- Sreedhar, A.; Kwon, J.H.; Yi, J.; Kim, J.S.; Gwag, J.S. Enhanced photoluminescence properties of Cu-doped ZnO thin films deposited by simultaneous RF and DC magnetron sputtering. Mater. Sci. Semicond. Process. 2016, 49, 8–14. [Google Scholar] [CrossRef]
- Bhaskar, R.; Li, J.; Xu, L. A comparative study of particle size dependency of IR and XRD methods for quartz analysis. Am. Ind. Hyg. Assoc. J. 1994, 55, 605–609. [Google Scholar] [CrossRef]
- Pan, Z.; Cao, F.; Hu, X.; Ji, X. A facile method for synthesizing CuS decorated Ti3C2Tx MXene with enhanced performance for asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 8984–8992. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Xu, J.; Niu, J.; Yang, D. Arrays of ZnO nanowires fabricated by a simple chemical solution route. Nanotechnology 2003, 14, 423. [Google Scholar] [CrossRef]
- Shuck, C.E.; Han, M.; Maleski, K.; Hantanasirisakul, K.; Kim, S.J.; Choi, J.; Reil, W.E.B.; Gogotsi, Y. Effect of Ti3AlC2 MAX phase on structure and properties of resultant Ti3C2Tx MXene. ACS Appl. Nano Mater. 2019, 2, 3368–3376. [Google Scholar] [CrossRef]
- Vuong, N.M.; Chinh, N.D.; Huy, B.T.; Lee, Y.-I. CuO-decorated ZnO hierarchical nanostructures as efficient and established sensing materials for H2S gas sensors. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Zhang, C.J.; Lu, P.; Dong, Y.; Wen, P.; Wu, Z.-S. Conducting and lithiophilic MXene/Graphene framework for high-capacity, dendrite-free lithium–metal anodes. ACS Nano 2019, 13, 14308–14318. [Google Scholar] [CrossRef]
- Schier, V.; Michel, H.-J.; Halbritter, J. ARXPS-analysis of sputtered TiC, SiC and Ti0.5Si0.5C layers. Fresenius. J. Anal. Chem. 1993, 346, 227–232. [Google Scholar] [CrossRef]
- Myhra, S.; Crossley, J.A.A.; Barsoum, M.W. Crystal-chemistry of the Ti3AlC2 and Ti4AlN3 layered carbide/nitride phases—Characterization by XPS. J. Phys. Chem. Solids 2001, 62, 811–817. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Halim, J.; Dyatkin, B.; Naguib, M.; Buranova, Y.S.; Barsoum, M.W.; Gogotsi, Y. Room-temperature carbide-derived carbon synthesis by electrochemical etching of max phases. Angew. Chem. 2014, 126, 4977–4980. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving high pseudocapacitance of 2D titanium carbide (MXene) by cation intercalation and surface modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Gilmanova, L.H.; Makhmutova, A.R.; Rakhimbekova, A.; Stoikov, I.I. Highly selective binding of methyl orange dye by cationic water-soluble pillar [5] arenes. Org. Biomol. Chem. 2016, 14, 4233–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, E.S.; Mihai, M.; Airinei, A. Thermochemically induced binding of methyl orange to polycations containing primary amine groups. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5898–5908. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Zhu, C.; Tang, Q.; Kang, P.; Cao, J. Preparation of magnetic α-Fe2O3/ZnFe2O4@Ti3C2Tx MXene with excellent photocatalytic performance. Ceram. Int. 2020, 46, 81–88. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Xiao, H.; Ren, E.; Song, Q.; Xiang, C.; Lai, X.; Lan, J.; Jiang, S. Bi2WO6/Nb2CTx MXene hybrid nanosheets with enhanced visible-light-driven photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2020, 505, 144595. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Li, C.; Wang, W.; Wang, L.; Feng, L.; Han, D. 2D visible-light-driven TiO2@Ti3C2/g-C3N4 ternary heterostructure for high photocatalytic activity. J. Mater. Sci. 2019, 54, 9385–9396. [Google Scholar] [CrossRef]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: Insights into electro-and photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Wang, G.; Zhang, X.; Dong, X. Oxygen vacancies modified TiO2/Ti3C2Tx derived from MXenes for enhanced photocatalytic degradation of organic pollutants: The crucial role of oxygen vacancy to schottky junction. Appl. Surf. Sci. 2020, 528, 146929. [Google Scholar] [CrossRef]


| Photocatalyst | Surface Area (m2g−1) | Pore Volume (cm3g−1) | Estimated Bandgap (eV) | Sheet Resistance (MΩsq−1) |
|---|---|---|---|---|
| ZnO | 0.01 | 1.6 × 10−4 | 3.3 | 23.94 |
| ZnO/Ti3C2Tx | 2.48 | 0.018 | 1.6 | 18.05 |
| Ti3C2Tx MXene | 9.19 | 0.037 | 0.95 |
| Report | Photocatalyst | Light Source | RhB Solution | Degradation Time |
|---|---|---|---|---|
| Zhang et al. [47] | Fe2O3/ZnFe2O4/Ti3C2 | visible light | 100 mL (20.8 μM) | 150 min |
| Cui et al. [48] | Bi2WO6/Nb2CTx | visible light | 100 mL (31.3 μM) | 100 min |
| This work | ZnO/Ti3C2Tx | visible light | 50 mL (20 μM) | 70 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, Q.T.H.; Tran, N.M.; Noh, J.-S. Rice Crust-Like ZnO/Ti3C2Tx MXene Hybrid Structures for Improved Photocatalytic Activity. Catalysts 2020, 10, 1140. https://doi.org/10.3390/catal10101140
Ta QTH, Tran NM, Noh J-S. Rice Crust-Like ZnO/Ti3C2Tx MXene Hybrid Structures for Improved Photocatalytic Activity. Catalysts. 2020; 10(10):1140. https://doi.org/10.3390/catal10101140
Chicago/Turabian StyleTa, Qui Thanh Hoai, Nghe My Tran, and Jin-Seo Noh. 2020. "Rice Crust-Like ZnO/Ti3C2Tx MXene Hybrid Structures for Improved Photocatalytic Activity" Catalysts 10, no. 10: 1140. https://doi.org/10.3390/catal10101140