Mesoporous Silica-Supported Ionic Liquids as Catalysts for Styrene Carbonate Synthesis from CO2
Abstract
:1. Introduction
2. Results
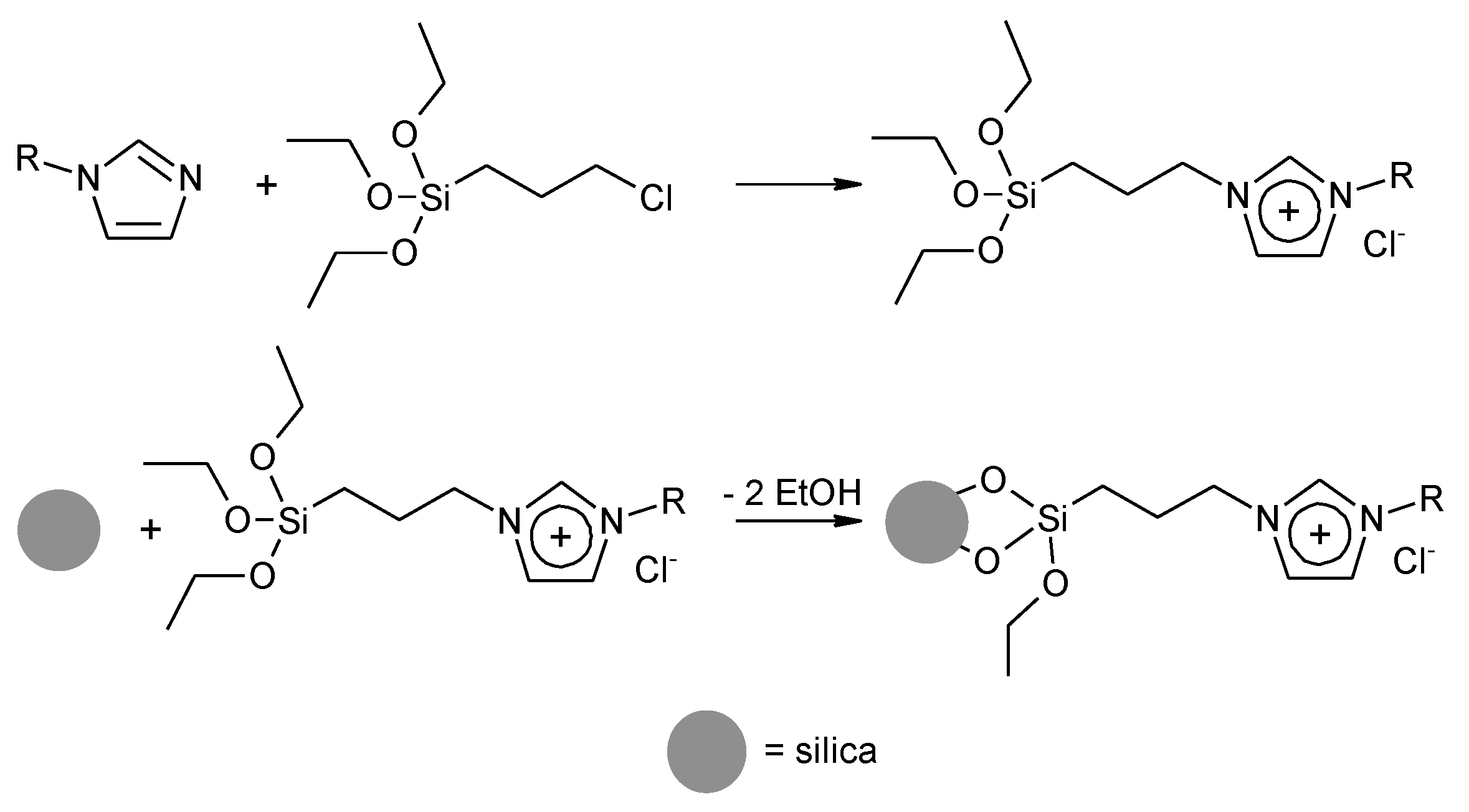
2.1. Synthesis of Immobilized Catalysts
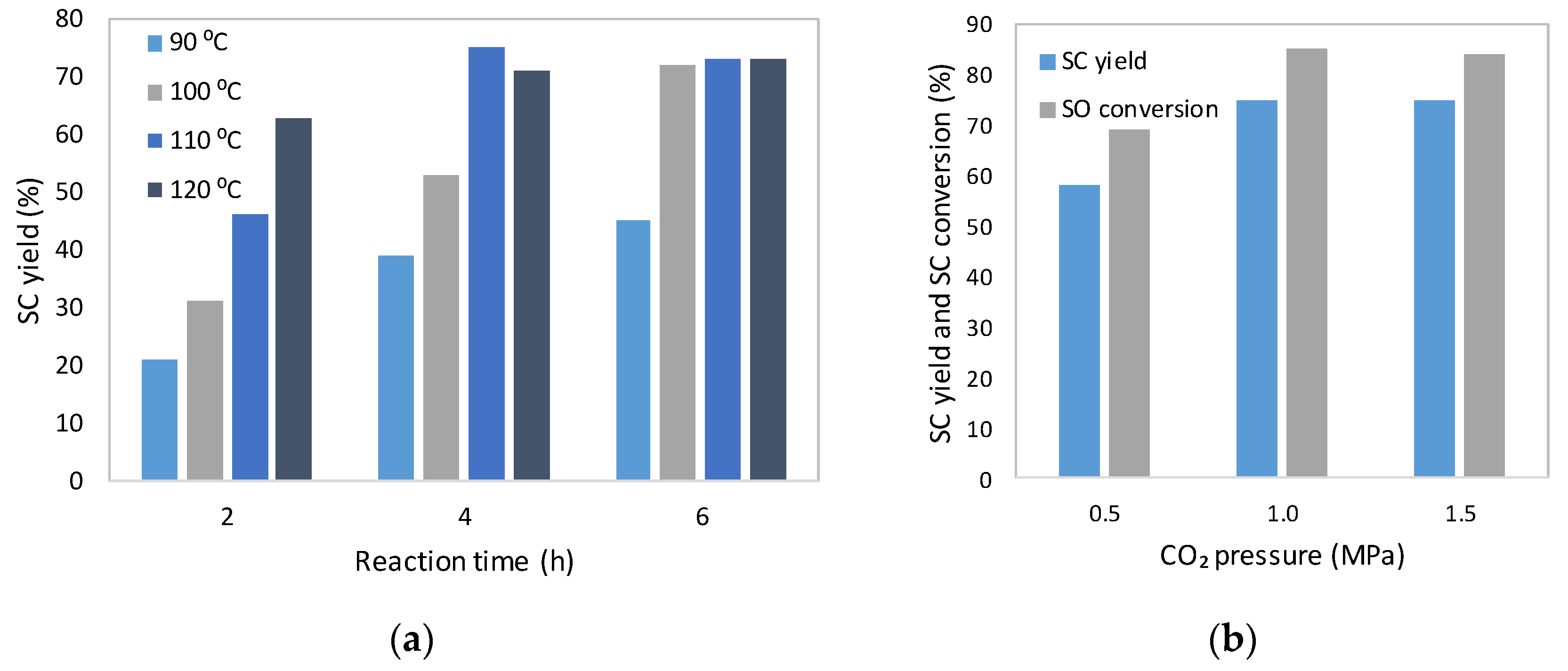
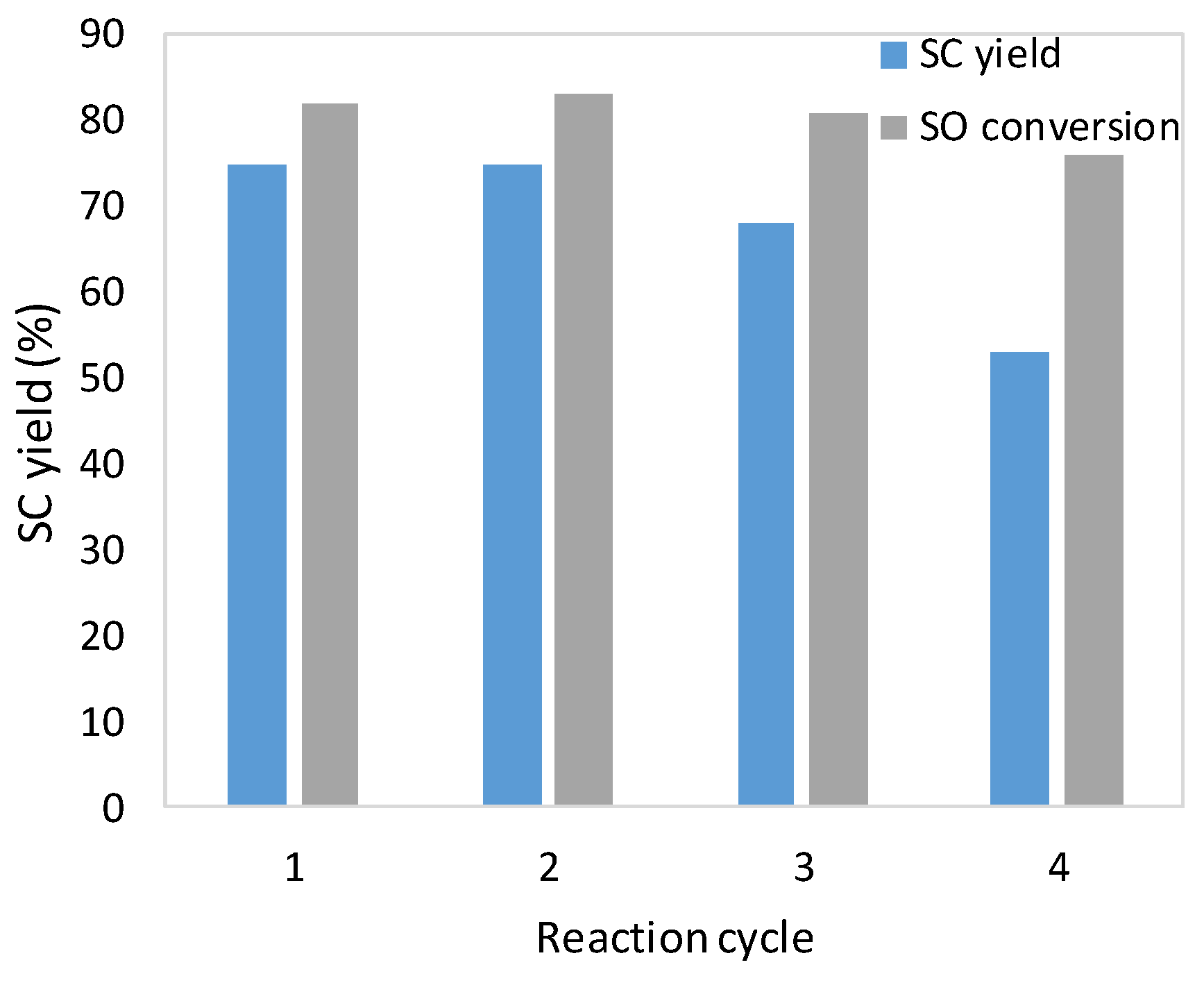
2.2. Catalytic Tests
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Synthetic Procedures
3.3.1. Synthesis of Silica Support
3.3.2. Synthesis of 1-alkyl-3-(triethosysilylpropyl) Imidazolium Chloride [atespim]Cl
3.3.3. Immobilization of 1-alkyl-3-(triethosysilylpropyl) Imidazolium Chloride on Silica
3.3.4. Reaction of Styrene Oxide with CO2
3.3.5. One-Pot Synthesis of Styrene Carbonate
3.3.6. Recycling of the Immobilized Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Wang, S.; Peng, J.; Yang, H.J.; Ban, B.; Wang, L.; Lei, B.; Guo, C.Y.; Hu, J.; Zhu, J.; Han, B. β-Cyclodextrin/Quaternary Ammonium Salt as an Efficient Catalyst System for Chemical Fixation of CO2. J. Nanosci. Nanotechnol. 2019, 19, 3263–3268. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, J.; Kubis, C.; Ludwig, R.; Werner, T. Mechanistic Study on the Addition of CO2 to Epoxides Catalyzed by Ammonium and Phosphonium Salts: A Combined Spectroscopic and Kinetic Approach. ACS Sustain. Chem. Eng. 2018, 6, 10778–10788. [Google Scholar] [CrossRef]
- Montoya, C.A.; Paninho, A.B.; Felix, P.M.; Zakrzewska, M.E.; Vital, J.; Najdanovic-Visak, V.; Nunes, A.V.M. Styrene carbonate synthesis from CO2 using tetrabutylammonium bromide as a non-supported heterogeneous catalyst phase. J. Supercrit. Fluids 2015, 100, 155–159. [Google Scholar] [CrossRef]
- Ema, T.; Fukuhara, K.; Sakai, T.; Ohbo, M.; Bai, F.Q.; Hasegawa, J. Quaternary ammonium hydroxide as a metal-free and halogen-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide. J. Catal. Sci. Technol. 2015, 5, 2314–2321. [Google Scholar] [CrossRef]
- Chen, J.J.; Xu, Y.C.; Gan, Z.L.; Peng, X.; Yi, X.Y. Zinc Complexes with Tridentate Pyridyl-Pyrrole Ligands and their Use as Catalysts in CO2 Fixation into Cyclic Carbonates. Eur. J. Inorg. Chem. 2019, 13, 1733–1739. [Google Scholar] [CrossRef]
- Takaishi, K.; Nath, B.D.; Yamada, Y.; Kosugi, H.; Ema, T. Unexpected Macrocyclic Multinuclear Zinc and Nickel Complexes that Function as Multitasking Catalysts for CO2 Fixations. Angew. Chem. Int. Ed. 2019, 58, 9984–9988. [Google Scholar] [CrossRef]
- Peng, J.; Yang, H.J.; Wang, S.; Ban, B.; Wei, Z.; Lei, B.; Guo, C.Y. Efficient solvent-free fixation of CO2 catalyzed by new recyclable bifunctional metal complexes. J. CO2 Util. 2018, 24, 1–9. [Google Scholar] [CrossRef]
- Li, P.; Cao, Z. Catalytic Preparation of Cyclic Carbonates from CO2 and Epoxides by Metal–Porphyrin and −Corrole Complexes: Insight into Effects of Cocatalyst and meso-Substitution. Organometallics 2018, 37, 406–414. [Google Scholar] [CrossRef]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coordin. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Li, B. A novel metal-organic framework as a heterogeneous catalysis for the solvent-free conversion of CO2 and epoxides into cyclic carbonate. Inorg. Chem. Commun. 2018, 88, 56–59. [Google Scholar] [CrossRef]
- Long, G.; Wu, D.; Pan, H.; Zhao, T.; Hu, X. Imidazolium hydrogen carbonate ionic liquids: Versatile organocatalysts for chemical conversion of CO2 into valuable chemicals. J. CO2 Util. 2020, 39, 101155. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Yin, L. Progress in the Heterogeneous Catalytic Cyclization of CO2 with Epoxides Using Immobilized Ionic Liquids. Catal. Lett. 2019, 149, 985–997. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Tamboli, A.H.; Kim, H. Ionic liquid as a catalyst for utilization of carbon dioxide to production of linear and cyclic carbonate. Fuel 2017, 200, 316–332. [Google Scholar] [CrossRef]
- Jasiak, K.; Siewniak, A.; Kopczyńska, K.; Chrobok, A.; Baj, S. Hydrogensulphate ionic liquids as an efficient catalyst for the synthesis of cyclic carbonates from carbon dioxide and epoxides. J. Chem. Technol. Biotechnol. 2016, 91, 2827–2833. [Google Scholar] [CrossRef]
- Xu, B.H.; Wang, J.Q.; Sun, J.; Huang, Y.; Zhang, J.P.; Zhang, X.P.; Zhang, S.J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Wang, L.; Que, S.; Ding, Z.; Vessally, E. Oxidative carboxylation of olefins with CO2: Environmentally benign access to five-membered cyclic carbonates. RSC Adv. 2020, 10, 9103–9115. [Google Scholar] [CrossRef] [Green Version]
- Zou, B.; Hu, C. Synthesis of Cyclic Carbonates from Alkenyl and Alkynyl Substrates. Chin. J. Chem. 2017, 35, 541–550. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Tost, R.M.; Millán, M.R. Mesoporous Materials: From Synthesis to Applications. Int. J. Mol. Sci. 2019, 20, 3213. [Google Scholar] [CrossRef] [Green Version]
- Kresge, C.T.; Roth, W.J. The discovery of mesoporous molecular sieves from the twenty year perspective. Chem. Soc. Rev. 2013, 42, 3663–3670. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fujita, S.; Zhao, F.; Hasegawa, M.; Arai, M. A direct synthesis of styrene carbonate from styrene with the Au/SiO2–ZnBr2/Bu4NBr catalyst system. J. Catal. 2005, 230, 398–405. [Google Scholar] [CrossRef]
- Dias, L.D.; Carrilho, R.M.B.; Henriques, C.A.; Calvete, M.J.F.; Masdeu-Bultó, A.M.; Claver, C.; Rossi, L.M.; Pereira, M.M. Hybrid Metalloporphyrin Magnetic Nanoparticles as Catalysts for Sequential Transformation of Alkenes and CO2 into Cyclic Carbonates. ChemCatChem 2018, 10, 2792–2803. [Google Scholar] [CrossRef]
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzębski, A. Supported hydrogensulfate ionic liquid catalysis in Baeyer–Villiger reaction. Appl. Catal. A Gen. 2009, 366, 22–28. [Google Scholar] [CrossRef]
- Szymańska, K.; Pietrowska, M.; Kocurek, J.; Maresz, K.; Koreniuk, A.; Mrowiec-Białoń, J.; Widłak, P.; Magner, E.; Jarzębski, A. Low back-pressure hierarchically structured multichannel microfluidic bioreactors for rapid protein digestion—Proof of concept. Chem. Eng. J. 2016, 287, 148–154. [Google Scholar] [CrossRef]
- Szymańska, K.; Odrozek, K.; Zniszczoł, A.; Pudło, W.; Jarzębski, A. A novel hierarchically structured siliceous packing to boost the performance of rotating bed enzymatic reactors. Chem. Eng. J. 2017, 315, 18–24. [Google Scholar] [CrossRef]
- Keller, J.U.; Staudt, R. Adsorption isotherms. In Gas Adsorption Equilibria. Experimental Methods and Adsorptive Isotherms; Springer: Boston, MA, USA, 2005; pp. 359–413. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications, 2nd ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Wu, S.S.; Zhang, X.W.; Dai, W.L.; Yin, S.F.; Li, W.S.; Ren, Y.Q.; Au, C.T. ZnBr2–Ph4PI as highly efficient catalyst for cyclic carbonates synthesis from terminal epoxides and carbon dioxide. Appl. Catal. A Gen. 2008, 341, 106–111. [Google Scholar] [CrossRef]
- Sun, J.; Fujita, S.; Bhanage, B.M.; Arai, M. One-pot synthesis of styrene carbonate from styrene in tetrabutylammonium bromide. Catal. Today 2004, 93–95, 383–388. [Google Scholar] [CrossRef]
- Kawanami, H.; Sasaki, A.; Matsui, K.; Ikushima, Y. A rapid and effective synthesis of propylene carbonate using a supercritical CO2–ionic liquid system. Chem. Commun. 2003, 7, 896–897. [Google Scholar] [CrossRef]
- Ted Oyama, S. Rates, Kinetics, and Mechanisms of Epoxidation: Homogeneous, Heterogeneous, and Biological Routes. In Mechanisms in Homogenous and Heterogeneous Epoxidation Catalysis, 1st ed.; Ted Oyama, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 16–21. [Google Scholar]
- Chen, F.; Dong, T.; Xu, T.; Li, X.; Hu, C. Direct synthesis of cyclic carbonates from olefins and CO2 catalyzed by a MoO2(acac)2-quaternary ammonium salt system. Green Chem. 2011, 13, 2518–2524. [Google Scholar] [CrossRef]
- Siewniak, A.; Jasiak-Jaroń, K.; Kotyrba, Ł.; Baj, S. Efficient Catalytic System Involving Molybdenyl Acetylacetonate and Immobilized Tributylammonium Chloride for the Direct Synthesis of Cyclic Carbonates from Carbon Dioxide and Olefins. Catal Lett. 2017, 147, 1567–1573. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, J.; Xiang, D.; Wang, L.; Sun, J.; Xiao, F.S. A Facile, Direct Synthesis of Styrene Carbonate from Styrene and CO2 Catalyzed by Au/Fe(OH)3–ZnBr2/Bu4NBr System. Catal. Lett. 2009, 129, 437–443. [Google Scholar] [CrossRef]
- Jasiak, K.; Krawczyk, T.; Pawlyta, M.; Jakóbik-Kolon, A.; Baj, S. One-Pot Synthesis of Styrene Carbonate from Styrene and CO2 Over the Nanogold-Ionic Liquid Catalyst. Catal. Lett. 2016, 146, 893–901. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, X.; Sun, J.; Xiao, F.S.; Sun, J. A novel route for synthesis of styrene carbonate using styrene and CO2 as substrates over basic resin R201 supported Au catalyst. Catal. Today 2009, 148, 383–388. [Google Scholar] [CrossRef]




| Entry | Material | BET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| 1 | @SiO2 | 305 | 15.0 | 1.2 |
| 2 | [mtespim]Cl/@SiO2 | 154 | 15.7 | 0.7 |
| 3 1 | MSU-F | 499 | 20.0 | 2.2 |
| 4 1 | MSU-H | 837 | 12.4 | 1.2 |
| 5 1 | MCM-41 | 930 | 2.9 | 0.8 |
| Entry | Catalyst | IL/SiO2 (mmol/g) 1 |
|---|---|---|
| 1 | [mtespim]Cl/@SiO2 | 1.02 |
| 2 | [mtespim]Cl/@SiO2 | 0.77 2 |
| 3 | [btespim]Cl/@SiO2 | 0.93 |
| 4 | [mtespim]Cl/MSU-F | 1.64 |
| 5 | [mtespim]Cl/MCM-4 | 2.20 |
| 6 | [mtespim]Cl/MSU-H | 1.53 |
| 7 | [otespim]Cl/MSU-H | 0.86 |
| Entry | Catalyst | Amount of Catalyst (mol%) | Amount of ZnBr2 (mol%) | SO Conversion (%) | SC Yield (%) |
|---|---|---|---|---|---|
| 1 | - | - | - | 0 | 0 |
| 2 | [mtespim]Cl | 0.50 | - | 27 | 7 |
| 3 | [mtespim]Cl/@SiO2 | 0.50 | - | 27 | 16 |
| 4 | - | - | 0.10 | 0 | 0 |
| 5 | [mtespim]Cl/@SiO2 | 0.50 | 0.10 | 83 | 72 |
| 6 | [mtespim]Cl/@SiO2 | 1.00 | 0.10 | 89 | 74 |
| 7 | [mtespim]Cl/@SiO2 | 0.25 | 0.10 | 58 | 24 |
| 8 | [mtespim]Cl/@SiO2 | 0.25 | 0.02 | 21 | 13 |
| Entry | Catalyst | SO Conversion (%) | SC Yield (%) |
|---|---|---|---|
| 1 | [mtespim]Cl/@SiO2 | 82 | 75 |
| 2 | [btespim]Cl/@SiO2 | 87 | 78 |
| 3 | [mtespim]Cl/MCM-41 | 66 | 48 |
| 4 | [mtespim]Cl/MSU-F | 81 | 66 |
| 5 | [mtespim]Cl/MSU-H | 76 | 52 |
| 6 | [mtespim]Cl/MSU-H | 78 | 61 |
| Entry | [mtespim]Cl/@SiO2 (mol%) | ST Conversion (%) | SO Yield (%) | BA Yield (%) | SC Yield (%) |
|---|---|---|---|---|---|
| 1 1 | 0.5 | 79 | 29 | 4 | 1 |
| 2 2 | 0.5 | 95 | 5 | 6 | 8 |
| 3 2 | 3.0 | 99 | 3 | 5 | 22 |
| 4 2 | 4.0 | 99 | 8 | 3 | 35 |
| 5 2 | 5.0 | 99 | 5 | 3 | 46 |
| 6 2 | 6.0 | 99 | 6 | 3 | 49 |
| 7 3 | 5.0 | 99 | 5 | 4 | 43 |
| 8 4 | 3.0 | 99 | 1 | 5 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siewniak, A.; Forajter, A.; Szymańska, K. Mesoporous Silica-Supported Ionic Liquids as Catalysts for Styrene Carbonate Synthesis from CO2. Catalysts 2020, 10, 1363. https://doi.org/10.3390/catal10111363
Siewniak A, Forajter A, Szymańska K. Mesoporous Silica-Supported Ionic Liquids as Catalysts for Styrene Carbonate Synthesis from CO2. Catalysts. 2020; 10(11):1363. https://doi.org/10.3390/catal10111363
Chicago/Turabian StyleSiewniak, Agnieszka, Adrianna Forajter, and Katarzyna Szymańska. 2020. "Mesoporous Silica-Supported Ionic Liquids as Catalysts for Styrene Carbonate Synthesis from CO2" Catalysts 10, no. 11: 1363. https://doi.org/10.3390/catal10111363





