Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the MOF Mediated Catalysts
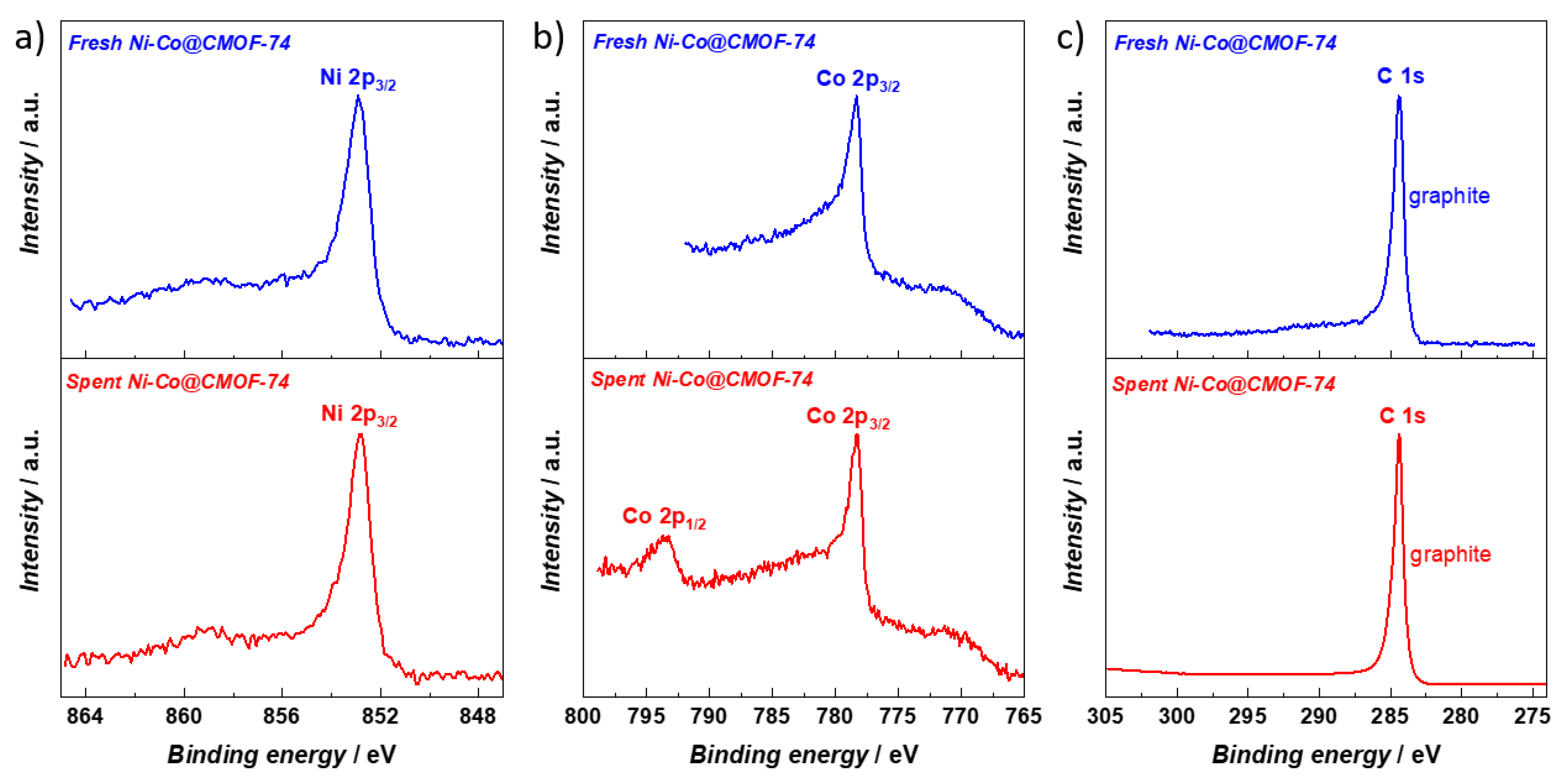
2.2. Catalytic DRM Tests
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.; Shah, N.; Maitland, G. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J.J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Dokania, A.; Ramirez, A.; Bavykina, A.V.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2018, 4, 167–176. [Google Scholar] [CrossRef]
- Tsang, S.; Claridge, J.B.; Green, M. Recent advances in the conversion of methane to synthesis gas. Catal. Today 1995, 23, 3–15. [Google Scholar] [CrossRef]
- Edwards, J.; Maitra, A. The chemistry of methane reforming with carbon dioxide and its current and potential applications. Fuel Process. Technol. 1995, 42, 269–289. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2005, 232, 268–275. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; AlBuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef]
- Ramirez, A.; Gascon, J. Support Was the Key to Success. Joule 2020. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2017, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.C.; Mandal, S. Potential Utilization of Metal–Organic Frameworks in Heterogeneous Catalysis: A Case Study of Hydrogen-Bond Donating and Single-Site Catalysis. Chem. Asian J. 2019, 14, 4087–4102. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zaworotko, M.J. Crystal Engineering of Hybrid Coordination Networks: From Form to Function. TrAC Trends Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Shen, K.; Chen, L.; Li, Y. Multimetal-MOF-derived transition metal alloy NPs embedded in an N-doped carbon matrix: Highly active catalysts for hydrogenation reactions. J. Mater. Chem. A 2016, 4, 10254–10262. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Yue, Y.; Wang, G.; Zu, Z.; Zhang, Y. Copper doped ceria porous nanostructures towards a highly efficient bifunctional catalyst for carbon monoxide and nitric oxide elimination. Chem. Sci. 2015, 6, 2495–2500. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-Q.; Zhao, J.; Wu, Y.-P.; Dong, W.-W.; Li, D.-S.; Li, J.-R.; Zhang, Q. Ultrafine Pt Nanoparticles and Amorphous Nickel Supported on 3D Mesoporous Carbon Derived from Cu-Metal–Organic Framework for Efficient Methanol Oxidation and Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2018, 10, 12740–12749. [Google Scholar] [CrossRef] [PubMed]
- Lippi, R.; Howard, S.; Escobar, H.B.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidalgo, B.; Zubizarreta, L.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Synthesis of carbon-supported nickel catalysts for the dry reforming of CH4. Fuel Process. Technol. 2010, 91, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, B.; Arenillas, A.; Menéndez, J.A. Synergetic effect of a mixture of activated carbon+Ni/Al2O3 used as catalysts for the CO2 reforming of CH4. Appl. Catal. A Gen. 2010, 390, 78–83. [Google Scholar] [CrossRef]
- Wang, L.J.; Deng, H.; Furukawa, H.; Gándara, F.; Cordova, K.E.; Peri, D.; Yaghi, O.M. Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals. Inorg. Chem. 2014, 53, 5881–5883. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Tovar, L.; Carne-Sanchez, A.; Carbonell, C.; Imaz, I.; Maspoch, D. Optimised room temperature, water-based synthesis of CPO-27-M metal-organic frameworks with high space-time yields. J. Mater. Chem. A 2015, 3, 20819–20826. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Olivos-Suarez, A.; Oar-Arteta, L.; Rozhko, E.; Osadchii, D.; Bavykina, A.V.; Kapteijn, F.; Gascon, J. Metal-Organic Framework Mediated Cobalt/Nitrogen-Doped Carbon Hybrids as Efficient and Chemoselective Catalysts for the Hydrogenation of Nitroarenes. ChemCatChem 2017, 9, 1854–1862. [Google Scholar] [CrossRef]
- Lin, H.-K.; Chiu, H.-C.; Tsai, H.-C.; Chien, S.-H.; Wang, C.-B. Synthesis, Characterization and Catalytic Oxidation of Carbon Monoxide over Cobalt Oxide. Catal. Lett. 2003, 88, 169–174. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Chen, C.-M. Amination of Polyethylene Glycol to Polyetheramine over the Supported Nickel Catalysts. Catal. Lett. 2001, 77, 147–154. [Google Scholar] [CrossRef]
- Rumble, J.R.; Bickham, D.M.; Powell, C.J. The NIST x-ray photoelectron spectroscopy database. Surf. Interface Anal. 1992, 19, 241–246. [Google Scholar] [CrossRef]
- Li, L.; Anjum, D.H.; Zhu, H.; Saih, Y.; Laveille, P.; D’Souza, L.; Basset, J.-M. Synergetic Effects Leading to Coke-Resistant NiCo Bimetallic Catalysts for Dry Reforming of Methane. ChemCatChem 2015, 7, 427–433. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Abu Tarboush, B.; Zeaiter, J.; Ahmad, M. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Chein, R.; Chen, Y.; Yu, C.; Chung, J. Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures. J. Nat. Gas Sci. Eng. 2015, 26, 617–629. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, D.; Wu, M.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Effect of catalytic site position: Nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming. Fuel 2013, 108, 430–438. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Li, Y.; Lin, Z.; Ma, X.; Zhang, Y.; Argyle, M.D.; Fan, M. Catalytic CH4 reforming with CO2 over activated carbon based catalysts. Appl. Catal. A Gen. 2014, 469, 387–397. [Google Scholar] [CrossRef]
- Zhang, G.; Su, A.; Du, Y.; Qu, J.; Xu, Y. Catalytic performance of activated carbon supported cobalt catalyst for CO2 reforming of CH4. J. Colloid Interface Sci. 2014, 433, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Materials | SBET (m2 g−1) | Metal wt % |
|---|---|---|
| Co-MOF-74 | 944 | 29 |
| Ni-MOF-74 | 1149 | 32 |
| Ni-Co-MOF-74 | 479 | 16/17 |
| Co@CMOF-74 | 265 | 68 |
| Ni@CMOF-74 | 230 | 69 |
| Ni-Co@CMOF-74 | 178 | 33/35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.S.; Ramirez, A.; Shterk, G.; Garzón-Tovar, L.; Gascon, J. Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane. Catalysts 2020, 10, 592. https://doi.org/10.3390/catal10050592
Khan IS, Ramirez A, Shterk G, Garzón-Tovar L, Gascon J. Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane. Catalysts. 2020; 10(5):592. https://doi.org/10.3390/catal10050592
Chicago/Turabian StyleKhan, Il Son, Adrian Ramirez, Genrikh Shterk, Luis Garzón-Tovar, and Jorge Gascon. 2020. "Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane" Catalysts 10, no. 5: 592. https://doi.org/10.3390/catal10050592
APA StyleKhan, I. S., Ramirez, A., Shterk, G., Garzón-Tovar, L., & Gascon, J. (2020). Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane. Catalysts, 10(5), 592. https://doi.org/10.3390/catal10050592





