Annealing Temperature-Dependent Effects of Fe-Loading on the Visible Light-Driven Photocatalytic Activity of Rutile TiO2 Nanoparticles and Their Applicability for Air Purification
Abstract
1. Introduction
2. Results and Discussion
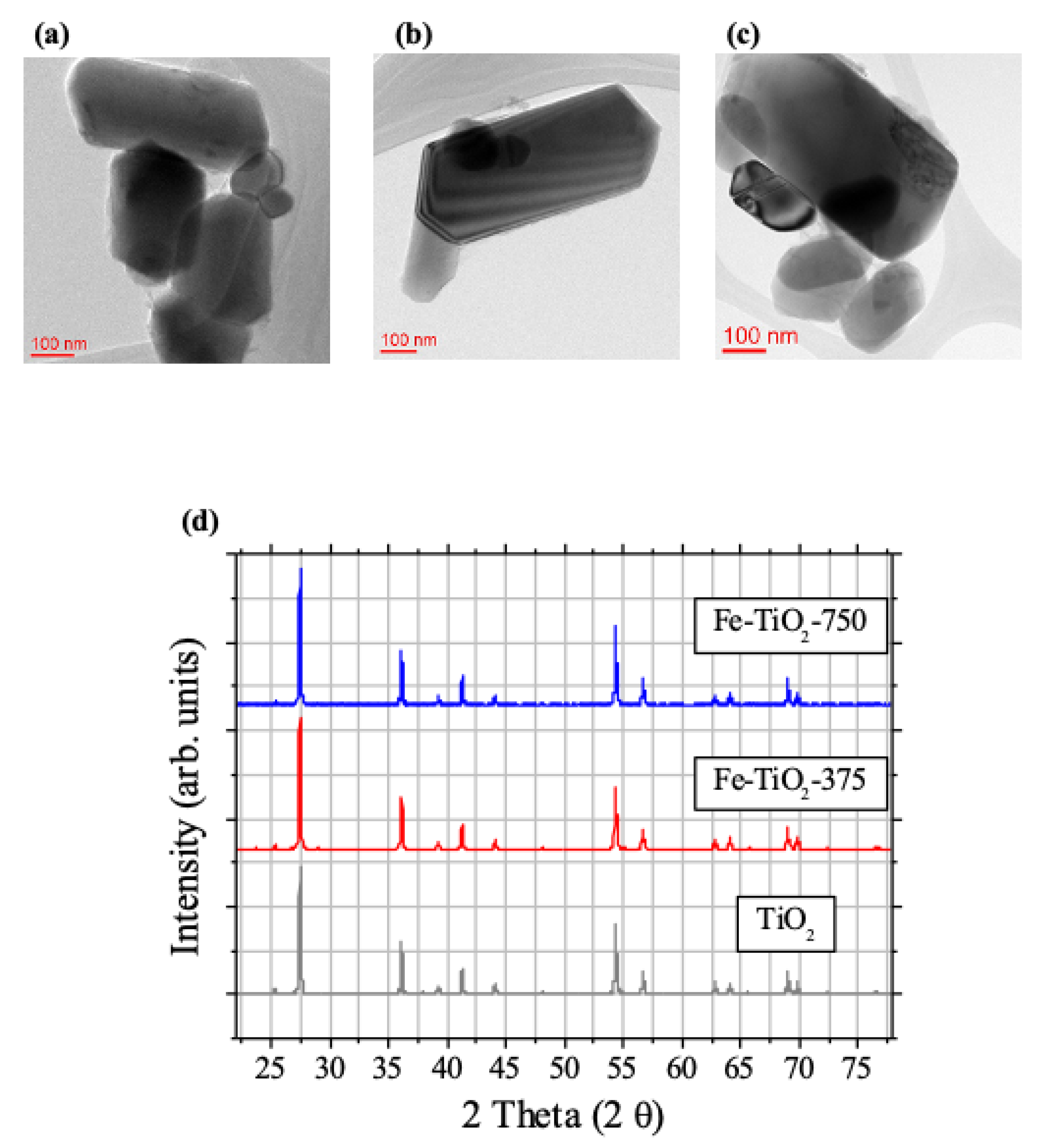
2.1. Characterization
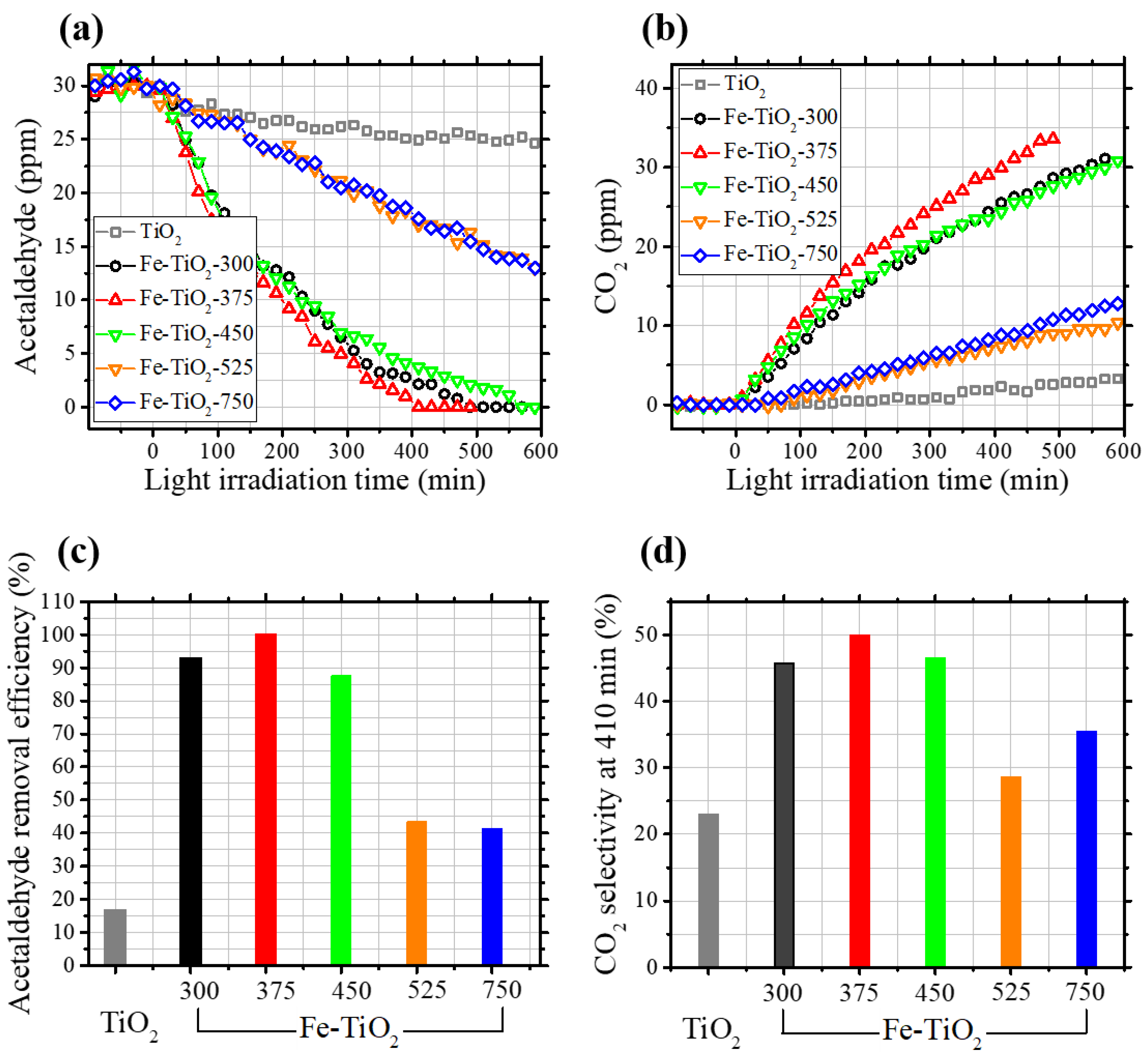
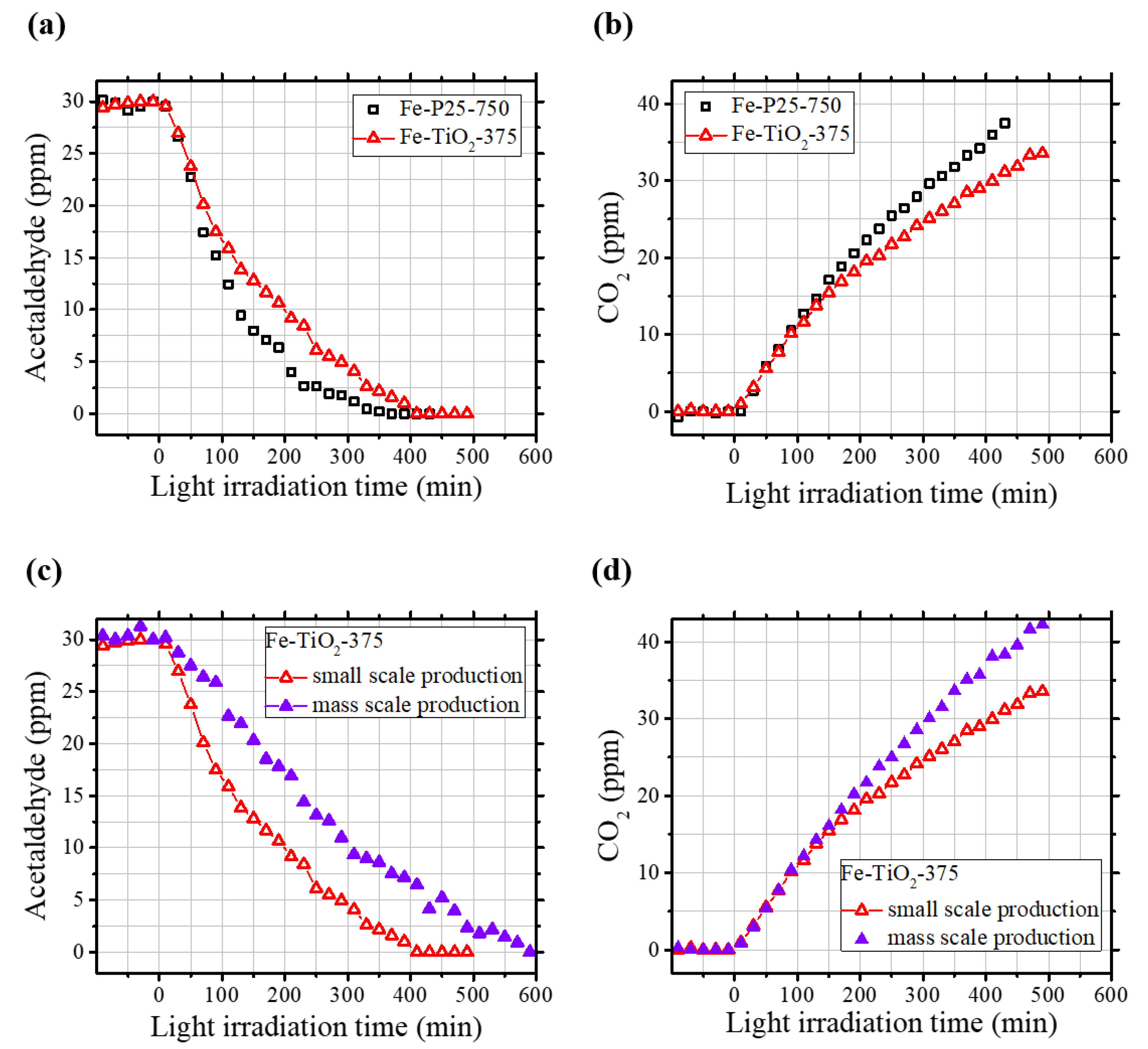
2.2. Photocatalytic Removal of Acetaldehyde
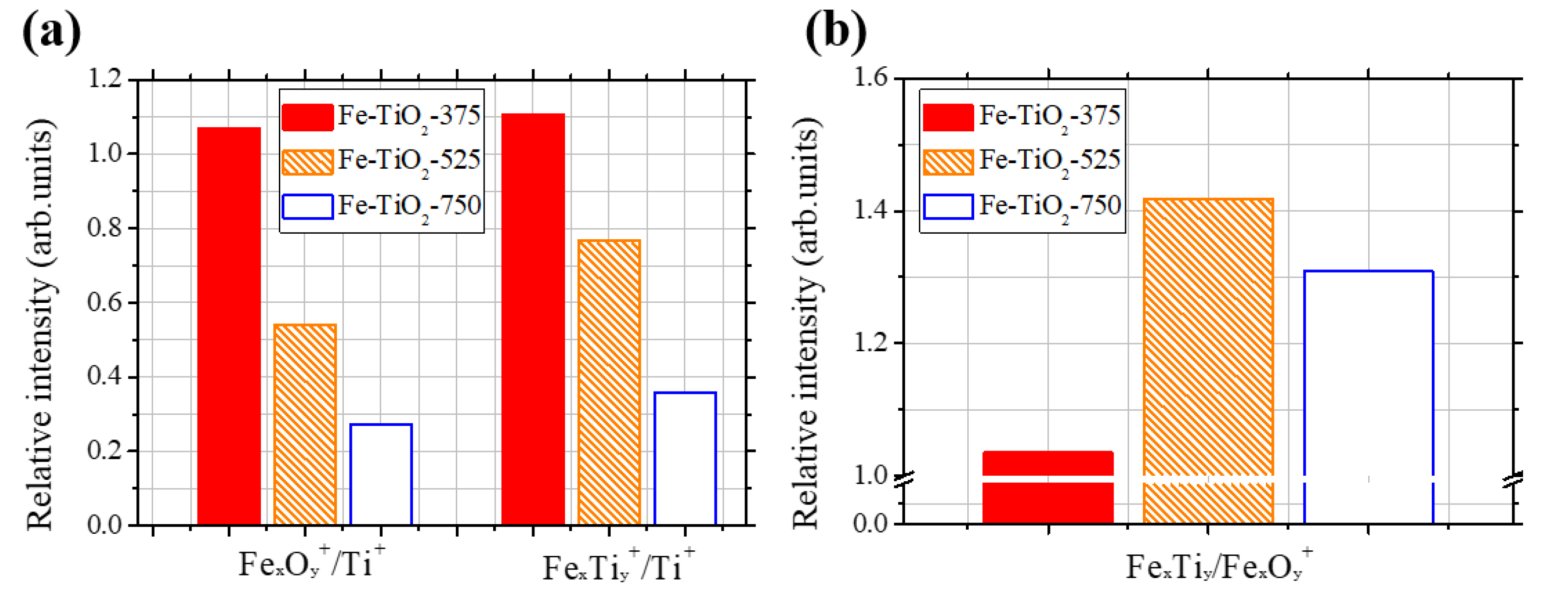
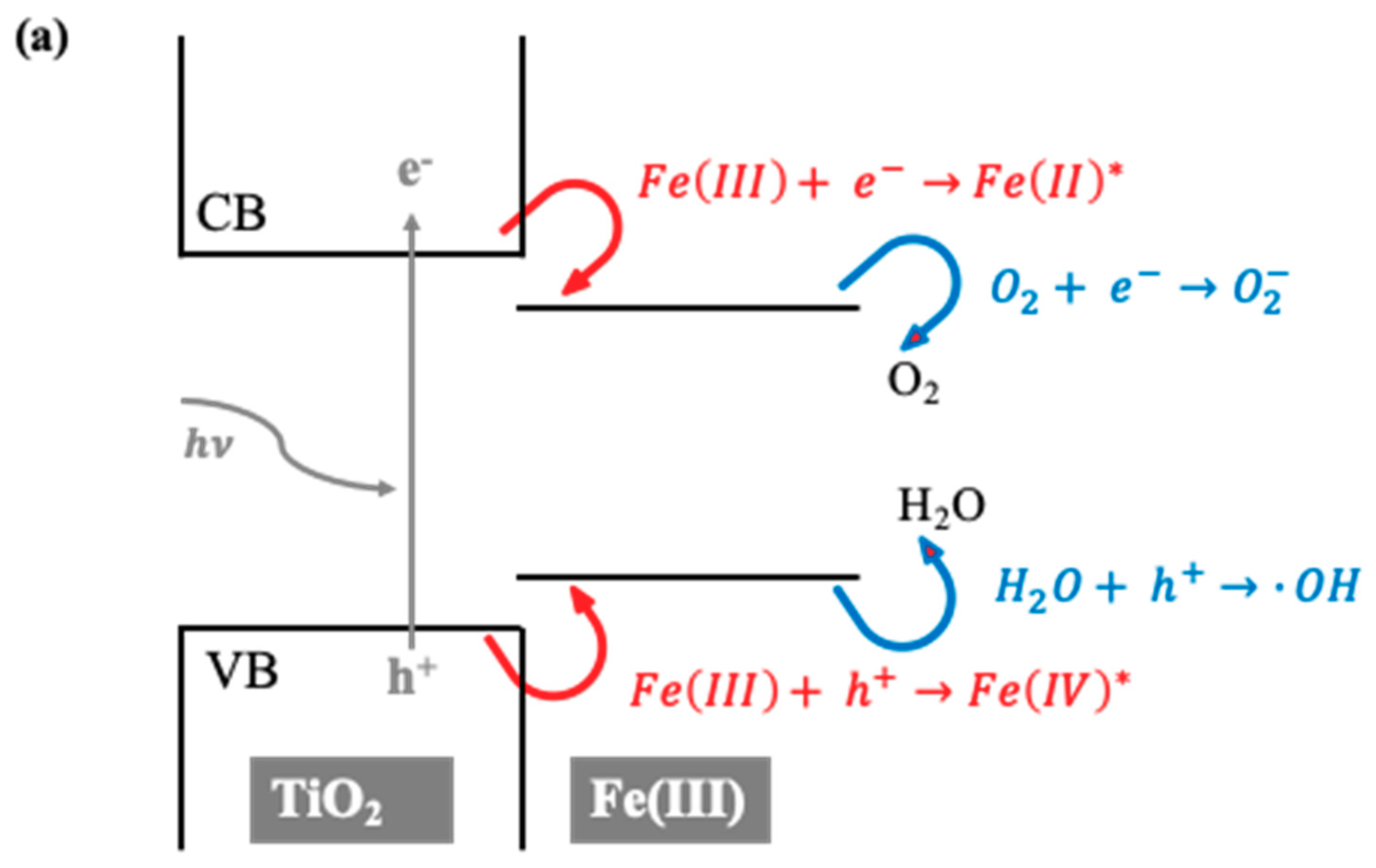
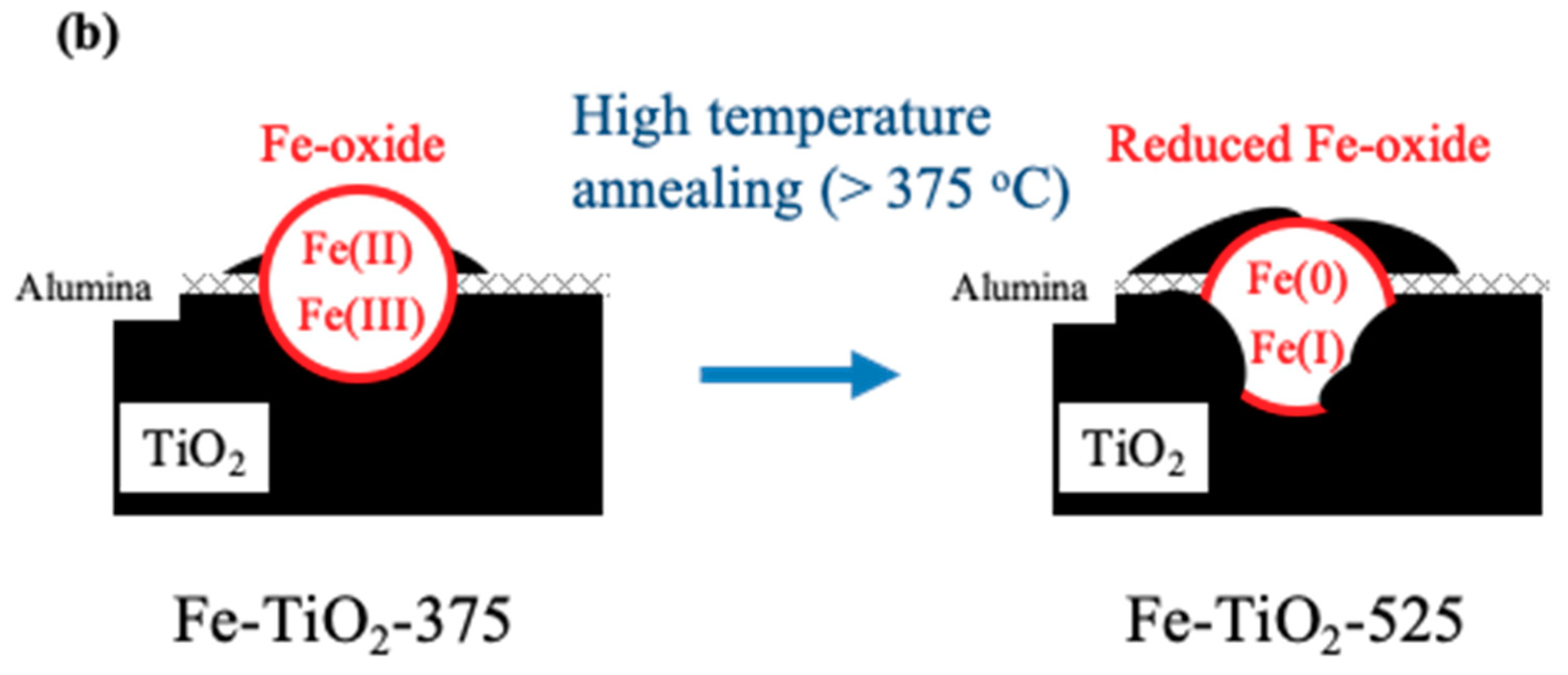
2.3. Further Analyses (UV Diffuse Reflectance Spectrophotometry (UV-DRS), Photoluminescence (PL), XPS, and TOF-SIMS)
2.4. Large-Scale Production of Catalysts and Their Potential Applications
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.1.1. Small-Scale Production
3.1.2. Large-Scale Production
3.2. Characterization of Photocatalysts
3.3. Photocatalytic Decomposition of Acetaldehyde
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Brunekreef, B.; Knöppe, H.; Lindvall, T.; Maroni, M.; Mølhave, L.; Skov, P. Effects of Indoor air pollution on human health. Indoor Air 1992, 2, 2–25. [Google Scholar] [CrossRef]
- Feron Victor, J.; Cassee Flemming, R.; Groten John, P.; van Vliet Petronella, W.; van Zorge Job, A. International issues on human health effects of exposure to chemical mixtures. Environ. Health Perspect. 2002, 110, 893–899. [Google Scholar] [CrossRef]
- Lovreglio, P.; Carrus, A.; Iavicoli, S.; Drago, I.; Persechino, B.; Soleo, L. Indoor formaldehyde and acetaldehyde levels in the province of Bari, South Italy, and estimated health risk. J. Environ. Monit. 2009, 11, 955–961. [Google Scholar] [CrossRef]
- Cavalcante, R.M.; Seyffert, B.H.; D’Oca, M.G.M.; Nascimento, R.F.; Campelo, C.S.; Pinto, I.S.; Anjos, F.B.; Costa, A.H.R. Exposure Assessment for Formaldehyde and Acetaldehyde in the Workplace. Indoor Built Environ. 2005, 14, 165–172. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gondal, M.A.; Drmosh, Q.A.; Yamani, Z.H.; Al-yamani, A. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 407–412. [Google Scholar] [CrossRef]
- Chang, C.-A.; Ray, B.; Paul, D.K.; Demydov, D.; Klabunde, K.J. Photocatalytic reaction of acetaldehyde over SrTiO3 nanoparticles. J. Mol. Catal. A Chem. 2008, 281, 99–106. [Google Scholar] [CrossRef]
- Arai, T.; Horiguchi, M.; Yanagida, M.; Gunji, T.; Sugihara, H.; Sayama, K. Complete oxidation of acetaldehyde and toluene over a Pd/WO3 photocatalyst under fluorescent- or visible-light irradiation. Chem. Commun. 2008, 5565–5567. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient complete oxidation of acetaldehyde into CO2 over CuBi2O4/WO3 composite photocatalyst under visible and UV light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]
- Wu, H.B.; Hng, H.H.; Lou, X.W. Direct Synthesis of Anatase TiO2 Nanowires with Enhanced Photocatalytic Activity. Adv. Mater. 2012, 24, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, H.; Cheng, B.; Zhao, X.; Zhang, Q. Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J. Photochem. Photobiol. A 2006, 182, 121–127. [Google Scholar] [CrossRef]
- Lv, K.; Yu, J.; Cui, L.; Chen, S.; Li, M. Preparation of thermally stable anatase TiO2 photocatalyst from TiOF2 precursor and its photocatalytic activity. J. Alloy. Compd. 2011, 509, 4557–4562. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N−F−Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- In, S.; Orlov, A.; Berg, R.; García, F.; Pedrosa-Jimenez, S.; Tikhov, M.S.; Wright, D.S.; Lambert, R.M. Effective Visible Light-Activated B-Doped and B,N-Codoped TiO2 Photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Liu, S.; Low, M.; Zhang, S.-Y.; Liu, Z.; Mlayah, A.; Han, M.-Y. Janus Au-TiO2 Photocatalysts with Strong Localization of Plasmonic Near-Fields for Efficient Visible-Light Hydrogen Generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Hasegawa, K.; Kagaya, S. Photocatalytic degradation of phenol by visible light-responsive iron-doped TiO2 and spontaneous sedimentation of the TiO2 particles. Chemosphere 2006, 65, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xing, M.; Zhang, J.; Tian, B. Visible light activated sulfur and iron co-doped TiO2 photocatalyst for the photocatalytic degradation of phenol. Catal. Today 2013, 201, 159–166. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Neubert, S.; Pulisova, P.; Wiktor, C.; Weide, P.; Mei, B.; Guschin, D.A.; Fischer, R.A.; Muhler, M.; Beranek, R. Enhanced photocatalytic degradation rates at rutile TiO2 photocatalysts modified with redox co-catalysts. Catal. Today 2014, 230, 97–103. [Google Scholar] [CrossRef]
- Ohno, T.; Murakami, N.; Tsubota, T.; Nishimura, H. Development of metal cation compound-loaded S-doped TiO2 photocatalysts having a rutile phase under visible light. Appl. Catal. A 2008, 349, 70–75. [Google Scholar] [CrossRef]
- Saqlain, S.; Cha, B.J.; Kim, S.Y.; Ahn, T.K.; Park, C.; Oh, J.-M.; Jeong, E.C.; Seo, H.O.; Kim, Y.D. Visible light-responsive Fe-loaded TiO2 photocatalysts for total oxidation of acetaldehyde: Fundamental studies towards large-scale production and applications. Appl. Surf. Sci. 2020, 505, 144160. [Google Scholar] [CrossRef]
- Arai, T.; Horiguchi, M.; Yanagida, M.; Gunji, T.; Sugihara, H.; Sayama, K. Reaction mechanism and activity of WO3-catalyzed photodegradation of organic substances promoted by a CuO cocatalyst. J. Phys. Chem. C 2009, 113, 6602–6609. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Promotion effect of CuO co-catalyst on WO3-catalyzed photodegradation of organic substances. Catal. Commun. 2008, 9, 1254–1258. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Nobbs, J.H. Kubelka—Munk Theory and the Prediction of Reflectance. Rev. Prog. Color. Relat. Top. 1985, 15, 66–75. [Google Scholar] [CrossRef]
- Ishibai, Y.; Sato, J.; Nishikawa, T.; Miyagishi, S. Synthesis of visible-light active TiO2 photocatalyst with Pt-modification: Role of TiO2 substrate for high photocatalytic activity. Appl. Catal. B 2008, 79, 117–121. [Google Scholar] [CrossRef]
- Gonbeau, D.; Guimon, C.; Pfister-Guillouzo, G.; Levasseur, A.; Meunier, G.; Dormoy, R. XPS study of thin films of titanium oxysulfides. Surf. Sci. 1991, 254, 81–89. [Google Scholar] [CrossRef]
- Biener, J.; Bäumer, M.; Wang, J.; Madix, R.J. Electronic structure and growth of vanadium on TiO2(110). Surf. Sci. 2000, 450, 12–26. [Google Scholar] [CrossRef]
- Mayer, J.T.; Diebold, U.; Madey, T.E.; Garfunkel, E. Titanium and reduced titania overlayers on titanium dioxide(110). J. Electron Spectrosc. Relat. Phenom. 1995, 73, 1–11. [Google Scholar] [CrossRef]
- Madhusudhan Rao, P.; Viswanathan, B.; Viswanath, R.P. Strong metal support interaction state in the Fe/TiO2 system—An XPS study. J. Mater. Sci. 1995, 30, 4980–4985. [Google Scholar] [CrossRef]
- van de Loosdrecht, J.; van der Kraan, A.M.; van Dillen, A.J.; Geus, J.W. Metal-support interaction: Titania-supported nickel-iron catalysts. Catal. Lett. 1996, 41, 27–34. [Google Scholar] [CrossRef]
- Junzhuo, D.; Dezheng, W.; Xuming, W.; Runsheng, Z.; Hongli, W. Interactions in the Fe/TiO2(110) system. Surf. Sci. 1991, 249, 213–222. [Google Scholar] [CrossRef]
- Bonanni, S.; Aït-Mansour, K.; Brune, H.; Harbich, W. Overcoming the Strong Metal−Support Interaction State: CO Oxidation on TiO2(110)-Supported Pt Nanoclusters. ACS Catal. 2011, 1, 385–389. [Google Scholar] [CrossRef]
- Bowker, M.; Stone, P.; Morrall, P.; Smith, R.; Bennett, R.; Perkins, N.; Kvon, R.; Pang, C.; Fourre, E.; Hall, M. Model catalyst studies of the strong metal–support interaction: Surface structure identified by STM on Pd nanoparticles on TiO2(110). J. Catal. 2005, 234, 172–181. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Chambers, S.A. Thermal stability and the role of oxygen vacancy defects in strong metal support interaction — Pt on Nb-doped TiO2(100). Surf. Sci. 1996, 365, 638–648. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M.; Nogami, G. Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater. Chem. Phys. 1999, 59, 254–261. [Google Scholar] [CrossRef]
- Gao, B.; Yang, C.; Chen, J.; Ma, Y.; Xie, J.; Zhang, H.; Wei, L.; Li, Q.; Du, J.; Xu, Q. Ferromagnetic photocatalysts of FeTiO3–Fe2O3 nanocomposites. RSC Adv. 2017, 7, 54594–54602. [Google Scholar] [CrossRef]
- Ren, Z.-S.; Hu, X.-j.; Li, S.-Y.; Xue, X.-X.; Chou, K.-C. Interdiffusion in the Fe2O3-TiO2 system. Int. J. Miner. Metall. Mater. 2013, 20, 273–278. [Google Scholar] [CrossRef]
- Afonin, N.N.; Logacheva, V.A. Interdiffusion and phase formation in the Fe–TiO2 thin-film system. Semiconductors 2017, 51, 1300–1305. [Google Scholar] [CrossRef]
- Chang, S.-M.; Liu, W.-S. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B 2014, 156–157, 466–475. [Google Scholar] [CrossRef]
- Li, X.; Yue, P.-L.; Kutal, C. Synthesis and photocatalytic oxidation properties of iron doped titanium dioxide nanosemiconductor particles. New J. Chem. 2003, 27, 1264–1269. [Google Scholar] [CrossRef]



| TiO2 | Fe-TiO2-375 | Fe-TiO2-750 | |
|---|---|---|---|
| Specific BET surface area (m2/g) | 15.1 | 13.8 | 11.8 |
| Acetaldehyde removal efficiency at 410 min (%) | Acetaldehyde removal efficiency at 590 min (%) | |
|---|---|---|
| AS-received TiO2 | 16.90 | 17.84 |
| Fe-TiO2-300 | 92.88 | 100 |
| Fe-TiO2-375 | 100 | 100 |
| Fe-TiO2-450 | 87.50 | 100 |
| Fe-TiO2-525 | 43.31 | 56.69 |
| Fe-TiO2-750 | 41.35 | 56.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Saqlain, S.; Cha, B.J.; Zhao, S.; Seo, H.O.; Kim, Y.D. Annealing Temperature-Dependent Effects of Fe-Loading on the Visible Light-Driven Photocatalytic Activity of Rutile TiO2 Nanoparticles and Their Applicability for Air Purification. Catalysts 2020, 10, 739. https://doi.org/10.3390/catal10070739
Kim SY, Saqlain S, Cha BJ, Zhao S, Seo HO, Kim YD. Annealing Temperature-Dependent Effects of Fe-Loading on the Visible Light-Driven Photocatalytic Activity of Rutile TiO2 Nanoparticles and Their Applicability for Air Purification. Catalysts. 2020; 10(7):739. https://doi.org/10.3390/catal10070739
Chicago/Turabian StyleKim, Soong Yeon, Shahid Saqlain, Byeong Jun Cha, Shufang Zhao, Hyun Ook Seo, and Young Dok Kim. 2020. "Annealing Temperature-Dependent Effects of Fe-Loading on the Visible Light-Driven Photocatalytic Activity of Rutile TiO2 Nanoparticles and Their Applicability for Air Purification" Catalysts 10, no. 7: 739. https://doi.org/10.3390/catal10070739
APA StyleKim, S. Y., Saqlain, S., Cha, B. J., Zhao, S., Seo, H. O., & Kim, Y. D. (2020). Annealing Temperature-Dependent Effects of Fe-Loading on the Visible Light-Driven Photocatalytic Activity of Rutile TiO2 Nanoparticles and Their Applicability for Air Purification. Catalysts, 10(7), 739. https://doi.org/10.3390/catal10070739






