Catalytic Performance of Calcium Titanate for Catalytic Decomposition of Waste Polypropylene to Carbon Nanotubes in a Single-Stage CVD Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. Effect of Support Composition and Content on the Microstructure, Yield, and Quality of CNTs
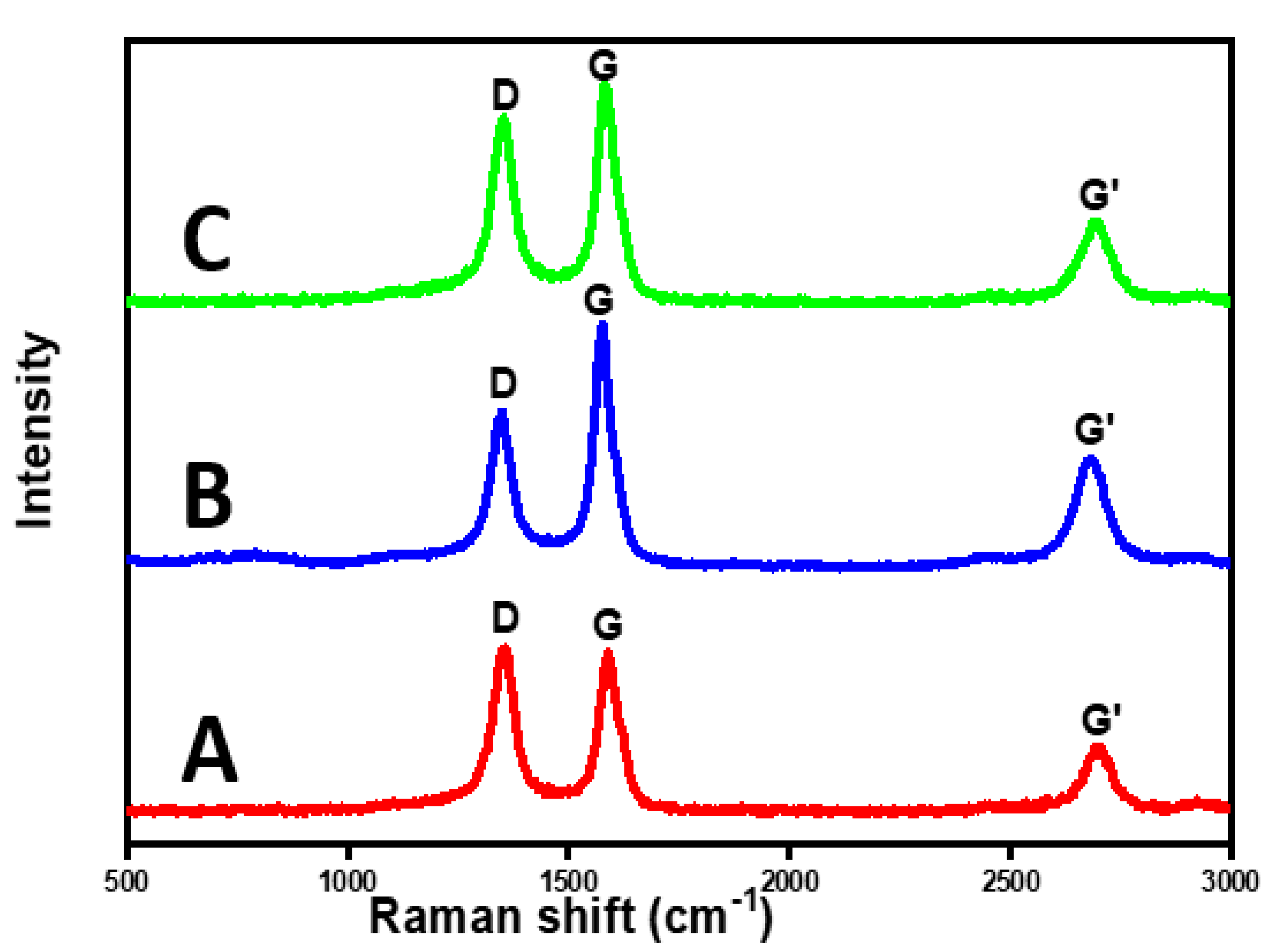
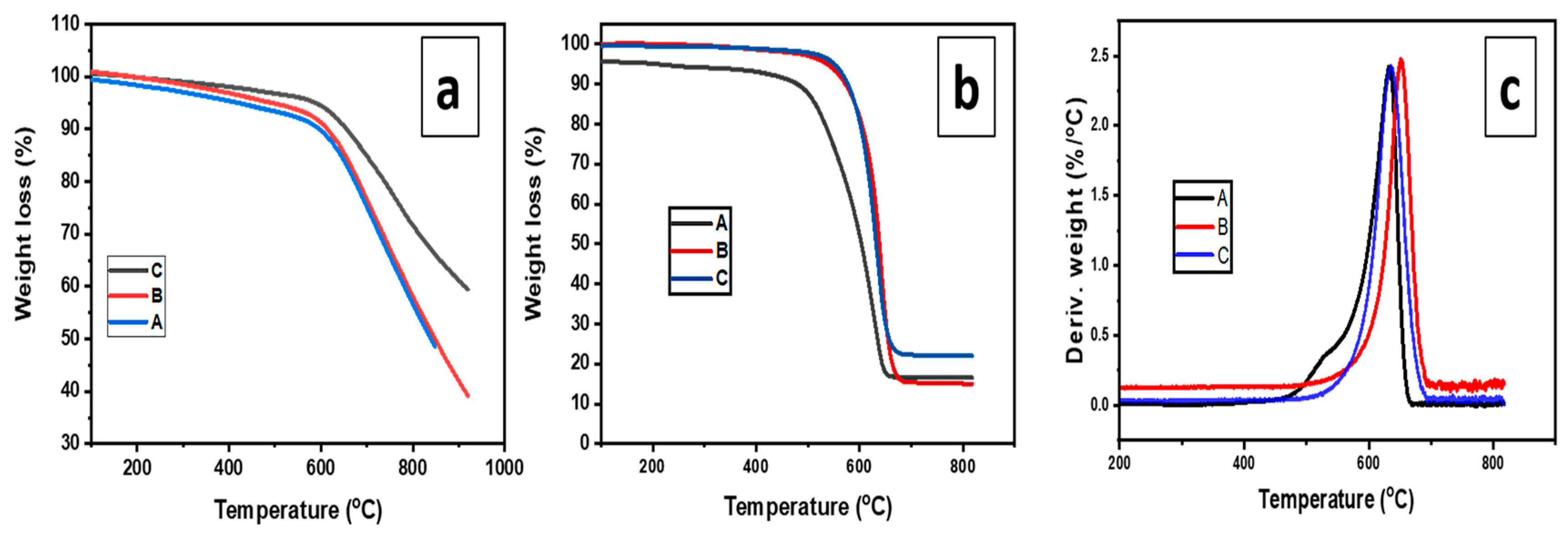
2.3. The Effect of Calcination Temperatures on the Yield and Quality of CNTs
3. Materials and Methods
3.1. Materials and Catalyst Preparation
3.2. Production of CNTs from Waste PP
3.3. Catalysts and CNTs Characterization
3.3.1. Morphological Analysis
3.3.2. Microstructure Analysis
3.3.3. X-Ray Diffraction
3.3.4. Surface Area and Pore Volume Measurement
3.3.5. Temperature-Programmed Reduction (TPR)
3.3.6. Thermogravimetric Analysis (TGA)
3.3.7. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mubarak, N.M.; Abdullah, E.C.; Jayakumar, N.S.; Sahu, J.N. An overview on methods for the production of carbon nanotubes. J. Ind. Eng. Chem. 2014, 20, 1186–1197. [Google Scholar] [CrossRef]
- Pang, J.; Bachmatiuk, A.; Ibrahim, I.; Fu, L.; Placha, D.; Martynkova, G.S.; Trzebicka, B.; Gemming, T.; Eckert, J.; Rümmeli, M.H. CVD growth of 1D and 2D sp2 carbon nanomaterials. J. Mater. Sci. 2016, 51, 640–667. [Google Scholar] [CrossRef]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Borsodi, N.; Szentes, A.; Miskolczi, N.; Wu, C.; Liu, X. Carbon nanotubes synthetized from gaseous products of waste polymer pyrolysis and their application. J. Anal. Appl. Pyrolysis 2016, 120, 304–313. [Google Scholar] [CrossRef]
- Alves, J.O.; Zhuo, C.; Levendis, Y.A.; Tenório, J.A.S. Catalytic conversion of wastes from the bioethanol production into carbon nanomaterials. Appl. Catal. B Environ. 2011, 106, 433–444. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Veksha, A.; Oh, W.-D.; Giannis, A.; Udayanga, W.D.C.; Lin, S.-X.; Ge, L.; Lisak, G. Plastic derived carbon nanotubes for electrocatalytic oxygen reduction reaction: Effects of plastic feedstock and synthesis temperature. Electrochem. Commun. 2019, 101, 11–18. [Google Scholar] [CrossRef]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic. Process Saf. Environ. Prot. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Yilmaz, S.; Selamet, Y. The effects of catalyst pretreatment, growth atmosphere and temperature on carbon nanotube synthesis using Co-Mo/MgO catalyst. Diam. Relat. Mater. 2015, 60, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Nag, A.; Mukhopadhyay, S.C.; Xu, Y. Carbon nanotubes and its gas-sensing applications: A review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Esteves, L.M.; Oliveira, H.A.; Passos, F.B. Carbon nanotubes as catalyst support in chemical vapor deposition reaction: A review. J. Ind. Eng. Chem. 2018, 65, 1–12. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. The role of the hydrocarbon source on the growth of carbon materials. Carbon 2012, 50, 3376–3398. [Google Scholar] [CrossRef]
- Chung, Y.; Jou, S. Carbon nanotubes from catalytic pyrolysis of polypropylene. Mater. Chem. Phys. 2005, 92, 256–259. [Google Scholar] [CrossRef]
- Bajad, G.S.; Tiwari, S.K.; Vijayakumar, R.P. Synthesis and characterization of CNTs using polypropylene waste as precursor. Mater. Sci. Eng. B 2015, 194, 68–77. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, R.; Bi, W.; Lu, J.; Tang, T. Polypropylene as a carbon source for the synthesis of multi-walled carbon nanotubes via catalytic combustion. Carbon 2007, 45, 449–458. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B Environ. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Lan, M.; Kang, D.; Wu, C. Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: The influence of catalyst and reaction pressure. Catal. Today 2020, 351, 50–57. [Google Scholar] [CrossRef]
- Yao, D.; Wang, C.H. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe- and Ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, X.B.; Tao, X.Y.; Xu, J.M.; Huang, W.Z.; Luo, J.H.; Luo, Z.Q.; Li, T.; Liu, F.; Bao, Y.; et al. Mass production of high-quality multi-walled carbon nanotube bundles on a Ni/Mo/MgO catalyst. Carbon 2005, 43, 295–301. [Google Scholar] [CrossRef]
- Signoretto, M.; Menegazzo, F.; Di Michele, A.; Fioriniello, E. Effects of support and synthetic procedure for sol-immobilized Au nanoparticles. Catalysts 2016, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Doustan, F.; Pasha, M.A. Growth of carbon nanotubes over Fe-Co and Ni-Co catalysts supported on different phases of TiO2 substrate by thermal CVD. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 25–33. [Google Scholar] [CrossRef]
- Rashid, H.U.; Yu, K.; Umar, M.N.; Anjum, M.N.; Khan, K.; Ahmad, N.; Jan, M.T. Catalyst role in chemical vapor deposition (CVD) process: A review. Rev. Adv. Mater. Sci. 2015, 40, 235–248. [Google Scholar]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The role of metal nanoparticles in the catalytic production of single-walled carbon nanotubes—A review. J. Phys. Condens. Matter 2003, 15, S3011–S3035. [Google Scholar] [CrossRef]
- Lobiak, E.V.; Shlyakhova, E.V.; Bulusheva, L.G.; Plyusnin, P.E.; Shubin, Y.V.; Okotrub, A.V. Ni—Mo and Co—Mo alloy nanoparticles for catalytic chemical vapor deposition synthesis of carbon nanotubes. J. Alloys Compd. 2015, 621, 351–356. [Google Scholar] [CrossRef]
- Xu, X.; Huang, S.; Yang, Z.; Zou, C.; Jiang, J.; Shang, Z. Controllable synthesis of carbon nanotubes by changing the Mo content in bimetallic Fe-Mo/MgO catalyst. Mater. Chem. Phys. 2011, 127, 379–384. [Google Scholar] [CrossRef]
- Yeoh, W.M.; Lee, K.Y.; Chai, S.P.; Lee, K.T.; Mohamed, A.R. The role of molybdenum in Co-Mo/MgO for large-scale production of high quality carbon nanotubes. J. Alloys Compd. 2010, 493, 539–543. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, W.; Zheng, B.; Gao, L. Ni—Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B Environ. 2016, 181, 769–778. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Nahil, M.A.; Williams, P.T.; Wu, C. Development of Ni- and Fe- based catalysts with different metal particle sizes for the production of carbon nanotubes and hydrogen from thermo-chemical conversion of waste plastics. J. Anal. Appl. Pyrolysis 2017, 125, 32–39. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, D.; Kumar, A.; Al-Muhtaseb, A.H.; Pathania, D.; Naushad, M.; Mola, G.T. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Hasegawa, K.; Sugime, H.; Kakehi, K.; Zhang, Z.; Maruyama, S.; Yamaguchi, Y. Millimeter-thick single-walled carbon nanotube forests: Hidden role of catalyst support. Jpn. J. Appl. Phys. Part 2 Lett. 2007, 46, 399–401. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Mamo, M.; Moothi, K.; Daramola, M.O. Synthesis of bimetallic NiMo/MgO catalyst for catalytic conversion of waste plastics (polypropylene) to carbon nanotubes (CNTs) via chemical vapour deposition method. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Perego, C.; Villa, P. Chapter 3—Catalyst preparation methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Yang, N.; Li, M.; Patscheider, J.; Youn, S.K.; Park, H.G. A forest of sub-1.5-nm-wide single-walled carbon nanotubes over an engineered alumina support. Sci. Rep. 2017, 7, 46725. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, D.; Bakhshi, N.N.; Dalai, A.K.; Adjaye, J. Synthesis, characterization and performance of NiMo catalysts supported on titania modified alumina for the hydroprocessing of different gas oils derived from Athabasca bitumen. Appl. Catal. B Environ. 2007, 72, 118–128. [Google Scholar] [CrossRef]
- Gonzalez-Cortes, S.L.; Aray, I.; Rodulfo-Baechler, S.M.A.; Lugo, C.A.; Castillo, H.L.D.; Loaiza-Gil, A.; Imbert, F.E.; Figueroa, H.; Pernia, W.; Rodriguez, A.; et al. On the structure and surface properties of NiO/MgO–La2O3 catalyst: Influence of the support composition and preparation method. J. Mater. Sci. 2007, 42, 6532–6540. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.M.A.W.; Hayashi, J. A review on methane transformation to hydrogen and nanocarbon: Relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 2017, 76, 743–767. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Mostafa, M.S.; Aboul-enein, A.A.; Hanafi, S.A. Hydrogen production via methane decomposition over Al2O3–TiO2 binary oxides supported Ni catalysts: Effect of Ti content on the catalytic efficiency. Fuel 2014, 129, 68–77. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Investigation of coke formation on Ni-Mg-Al catalyst for hydrogen production from the catalytic steam pyrolysis-gasification of polypropylene. Appl. Catal. B Environ. 2010, 96, 198–207. [Google Scholar] [CrossRef]
- Lanfredi, S.; Storti, F.; Simões, L.P.M.; Djurado, E.; Nobre, M.A.L. Synthesis and structural characterization of calcium titanate by spray pyrolysis method. Mater. Lett. 2017, 201, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Evans, I.R.; Howard, J.A.K.; Sreckovic, T.; Ristic, M.M. Variable temperature in situ X-ray diffraction study of mechanically activated synthesis of calcium titanate, CaTiO3. Mater. Res. Bull. 2003, 38, 1203–1213. [Google Scholar] [CrossRef]
- Mallik, P.K.; Biswal, G.; Patnaik, S.C.; Senapati, S.K. Characterisation of sol-gel synthesis of phase pure CaTiO3 nano powders after drying. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012005. [Google Scholar] [CrossRef]
- Veksha, A.; Giannis, A.; Oh, W.D.; Lisak, G. Catalytic processing of non-condensable pyrolysis gas from plastics: Effects of calcium supports on nickel-catalyzed decomposition of hydrocarbons and HCl sorption. Chem. Eng. Sci. 2018, 189, 311–319. [Google Scholar] [CrossRef]
- Kaneko, E.Y.; Pulcinelli, S.H.; Teixeira, V.; Santilli, C.V. Sol–gel synthesis of titania—Alumina catalyst supports. Appl. Catal. A Gen. 2002, 235, 71–78. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Hamid, S.B.A. Titanium dioxide catalyst support in heterogenous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, G. Synthesis of calcium titanate powders by the sol-gel process. Chem. Mater. 1994, 6, 58–62. [Google Scholar] [CrossRef]
- Gargori, C.; Cerro, S.; Galindo, R.; García, A.; Llusar, M.; Badenes, J.; Monrós, G. New vanadium doped calcium titanate ceramic pigment. Ceram. Int. 2011, 37, 3665–3670. [Google Scholar] [CrossRef]
- Yahya, N.Y.; Ngadi, N.; Lani, N.S.; Ali, M.W. Pilot evaluation of calcium titanate catalyst for biodiesel production from waste cooking oil. Chem. Eng. Trans. 2017, 56, 595–600. [Google Scholar] [CrossRef]
- Baker, E.G.; Summit, N.J. Titanium Dioxide-Calcium Oxide Catalyst for Cracking Hydrocarbons. U.S. Patent 2,886,513, 12 May 1959. [Google Scholar]
- Pudukudy, M.; Yaakob, Z.; Kadier, A.; Takriff, M.S.; Hassan, N.S.M. One-pot sol-gel synthesis of Ni/TiO2 catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes. Int. J. Hydrogen Energy 2017, 42, 16495–16513. [Google Scholar] [CrossRef]
- Salinas, D.A.; Marchena, C.L.; Pierella, L.B.; Pecchi, G. Catalytic oxidation of 2-(methylthio)-benzothiazole on alkaline earth titanates, ATiO3 (A = Ca, Sr, Ba). Mol. Catal. 2017, 438, 76–85. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Adel-Rahman, H.; Haggar, A.M.; Awadallah, A.E. Simple method for synthesis of carbon nanotubes over Ni-Mo/Al2O3 catalyst via pyrolysis of polyethylene waste using a two-stage process. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 211–222. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Preparing catalytic materials by the sol-gel method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Li, L.-J.; Chen, Y. Effect of different catalyst supports on the (n,m) selective growth of single-walled carbon nanotube from Co–Mo catalyst. J. Mater. Sci. 2009, 44, 3285–3295. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, L.; Ling, H.; Qian, W.Z.; Luo, G.H.; Fei, W. Synthesis of thin-walled carbon nanotubes from methane by changing the Ni/Mo ratio in a Ni/Mo/MgO catalyst. New Carbon Mater. 2008, 23, 319–325. [Google Scholar] [CrossRef]
- Yeoh, W.M.; Lee, K.Y.; Chai, S.P.; Lee, K.T.; Mohamed, A.R. Effective synthesis of carbon nanotubes via catalytic decomposition of methane: Influence of calcination temperature on metal-support interaction of Co-Mo/MgO catalyst. J. Phys. Chem. Solids 2013, 74, 1553–1559. [Google Scholar] [CrossRef]
- Zhuo, C.; Hall, B.; Richter, H.; Levendis, Y. Synthesis of carbon nanotubes by sequential pyrolysis and combustion of polyethylene. Carbon 2010, 48, 4024–4034. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E.; Abdel-Rahman, A.A.-H.; Haggar, A.M. Synthesis of multi-walled carbon nanotubes via pyrolysis of plastic waste using a two-stage process. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 443–450. [Google Scholar] [CrossRef]
- Dikio, E.D.; Shooto, N.D.; Thema, F.T.; Farah, A.M. Raman and TGA study of carbon nanotubes synthesized over Mo/Fe catalyst on aluminium oxide, calcium carbonate and magnesium oxide support. Chem. Sci. Trans. 2013, 2, 1160–1173. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Das, G.; Ansaldo, A.; Genovese, A.; Malerba, M.; Povia, M.; Ricci, D.; Fabrizio, E.D.; Zitti, E.D.; Sharon, M.; et al. Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J. Anal. Appl. Pyrolysis 2012, 94, 91–98. [Google Scholar] [CrossRef]
- Bajad, G.; Jain, R.; Harhare, W.; Vijayakumar, R.P.; Bose, S. Synthesis of fuel oil and carbon nanotubes in an autoclave using plastic waste as precursor. Mater. Manuf. Process. 2017, 32, 495–500. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Liu, B.; Li, X.; Li, Y.; Chai, Y.; Liu, C. Effect of calcination temperature of unsupported NiMo catalysts on the hydrodesulfurization of dibenzothiophene. Energy Fuels 2014, 28, 2429–2436. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of steam input to the pyrolysis-gasification of waste plastics for improved production of hydrogen or carbon nanotubes. Appl. Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Fan, X.; Liu, D.; Zhao, Z.; Li, J.; Liu, J. Influence of Ni/Mo ratio on the structure-performance of ordered mesoporous Ni-Mo-O catalysts for oxidative dehydrogenation of propane. Catal. Today 2020, 339, 67–78. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; He, J. Synthesis of carbon nanotubes and nanospheres with controlled morphology using different catalyst precursors. Nanotechnol. Rev. 2008, 19, 325607. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling Waste Plastics into Carbon Nanomaterials: A Review. J. Appl. Polym. Sci. 2014, 131, 39931. [Google Scholar] [CrossRef]
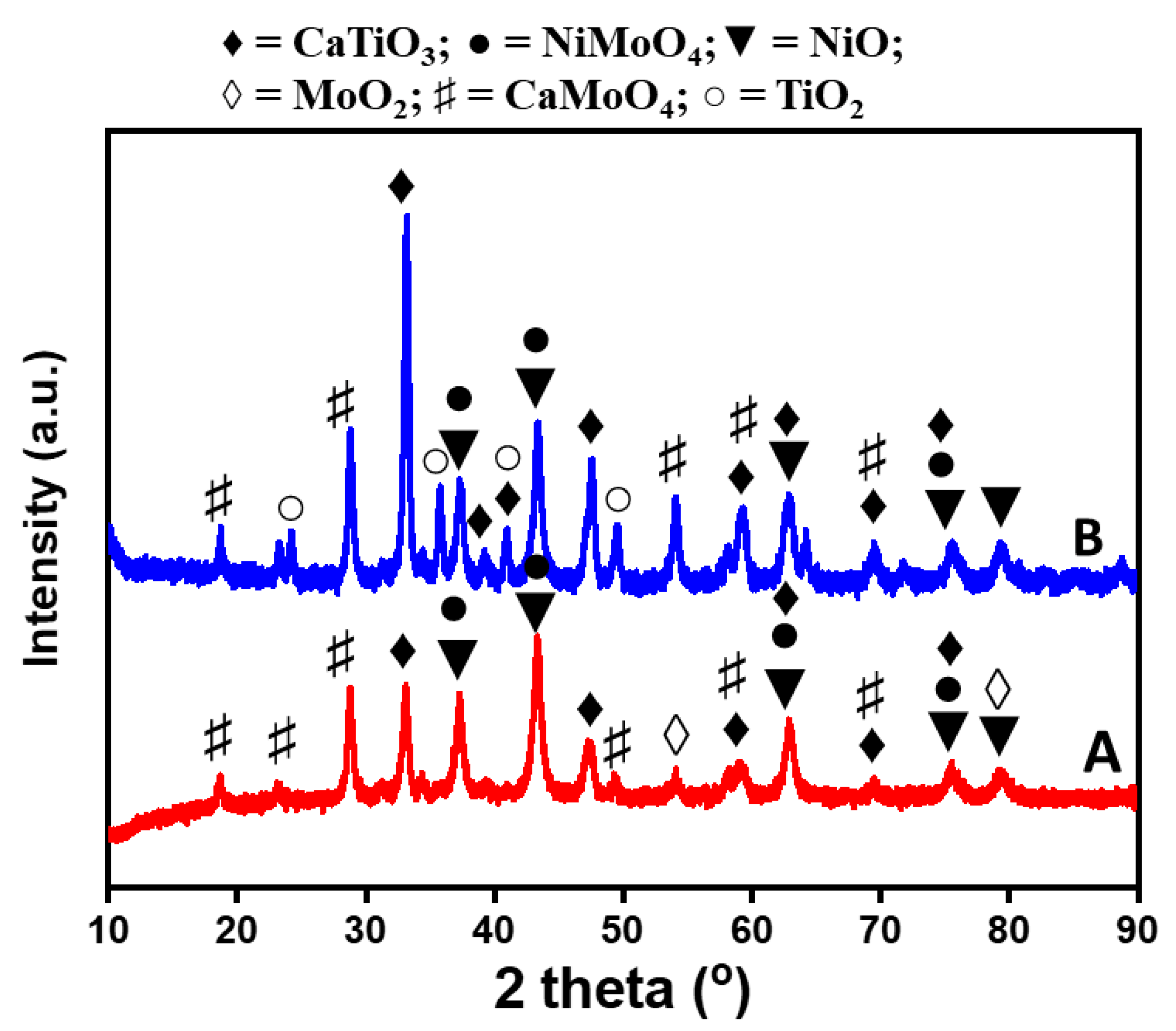
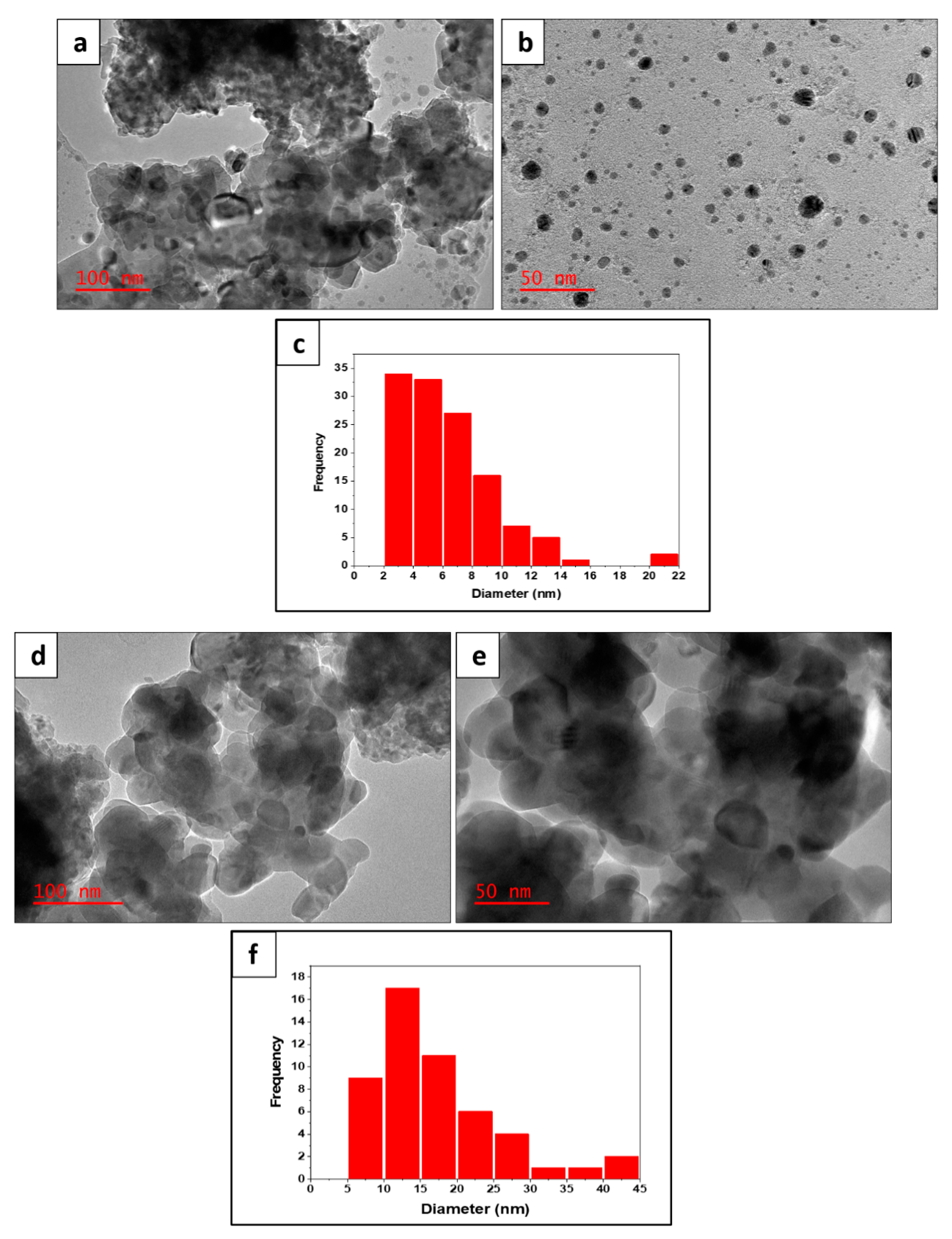
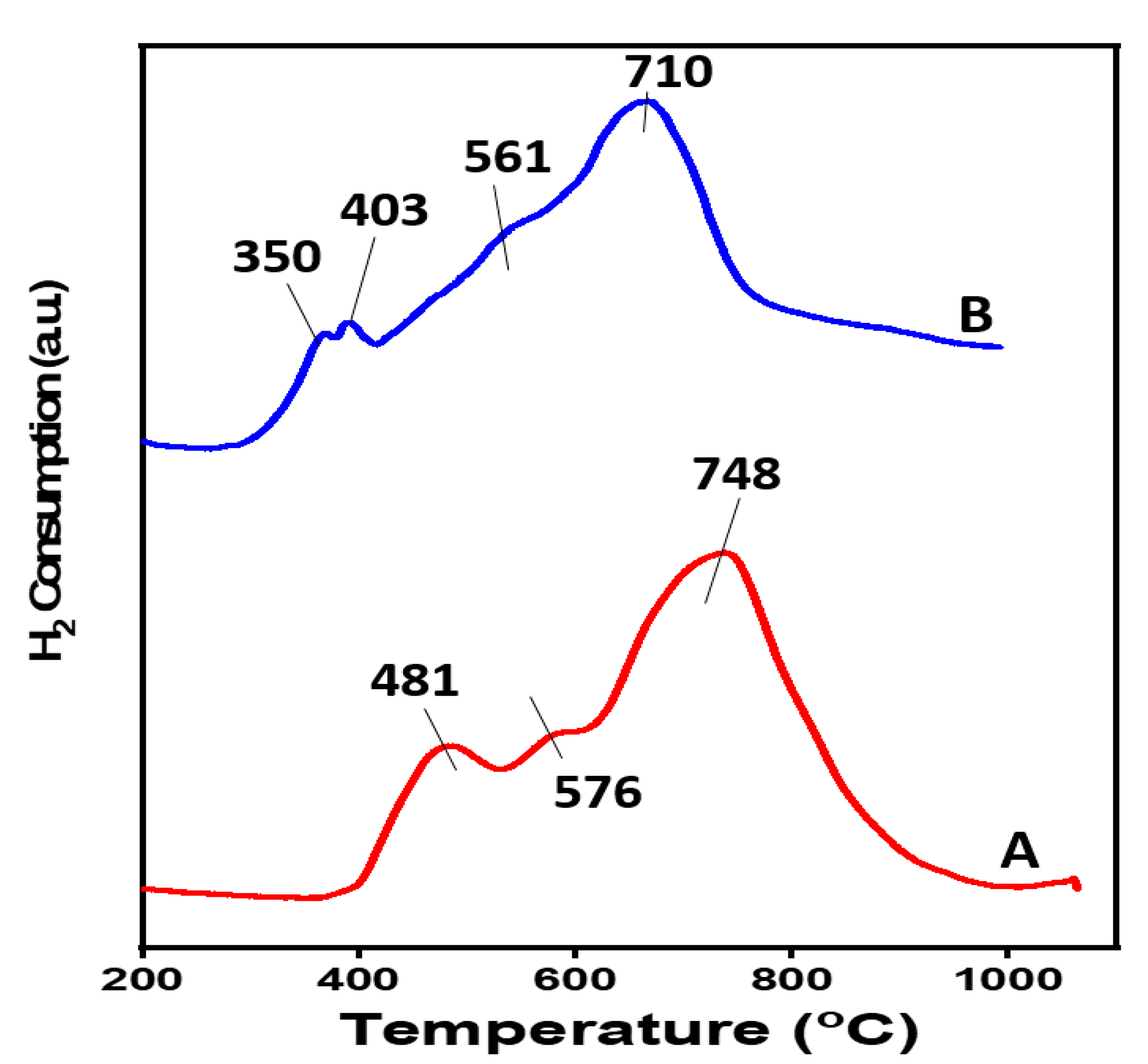
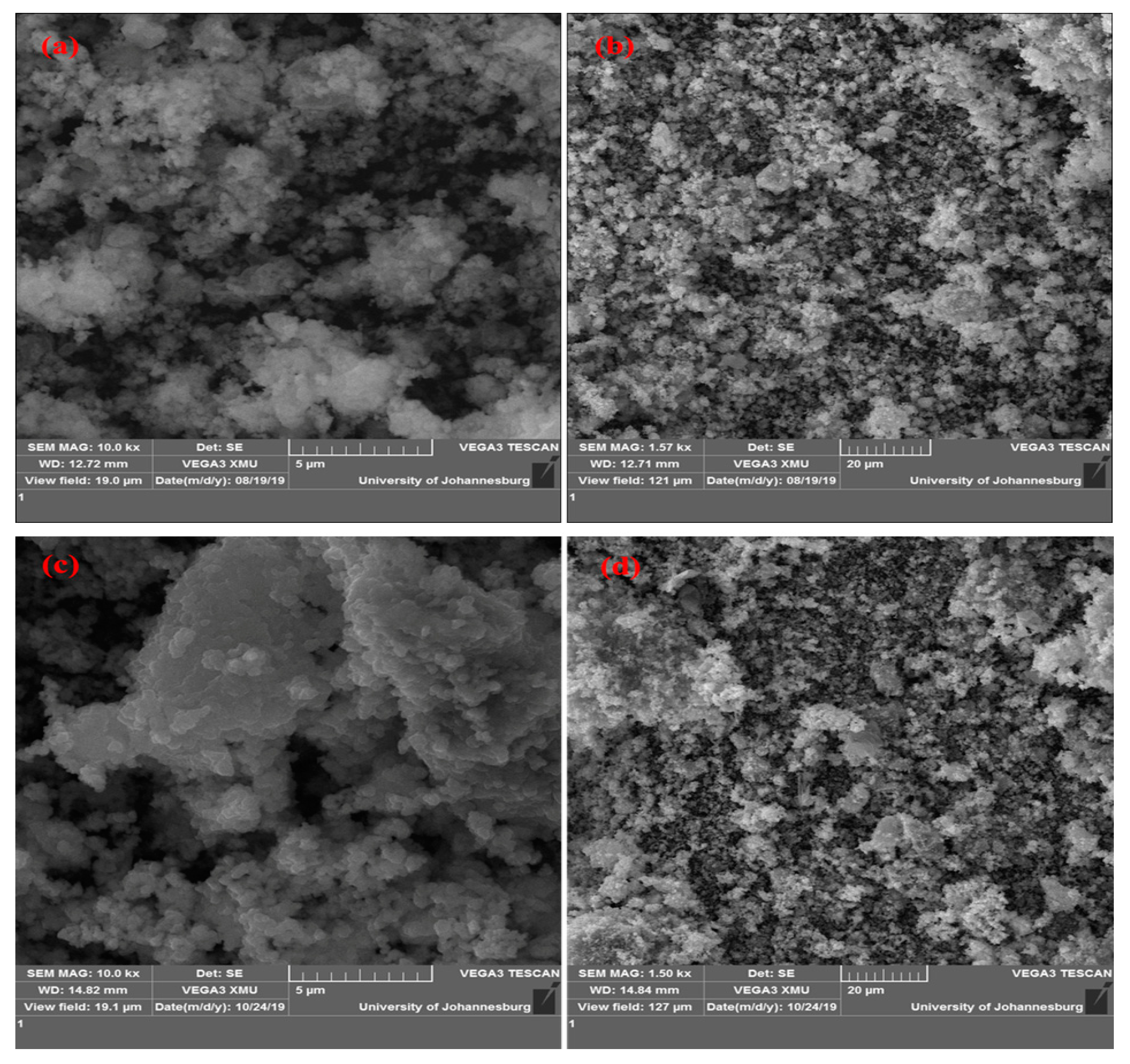
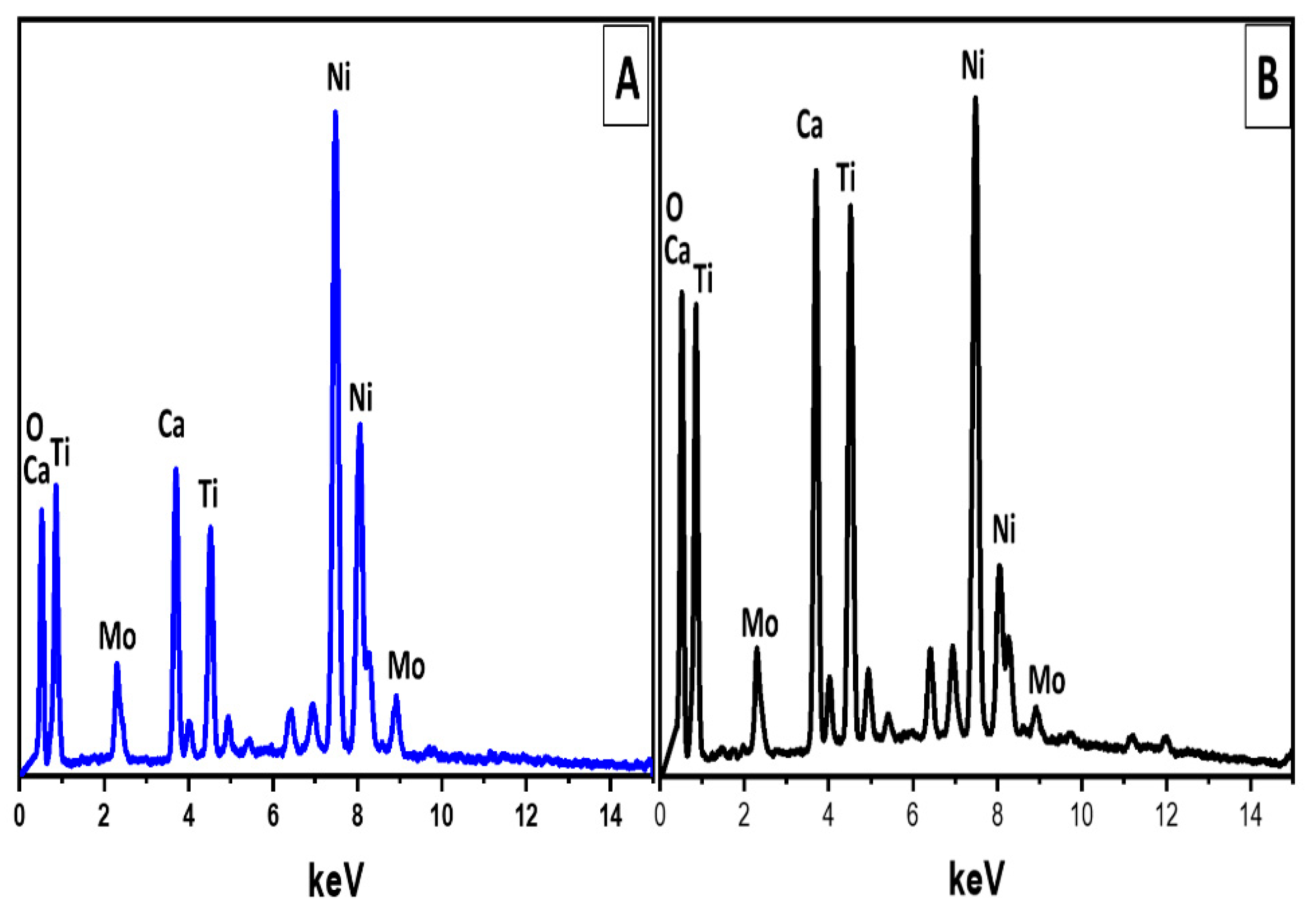
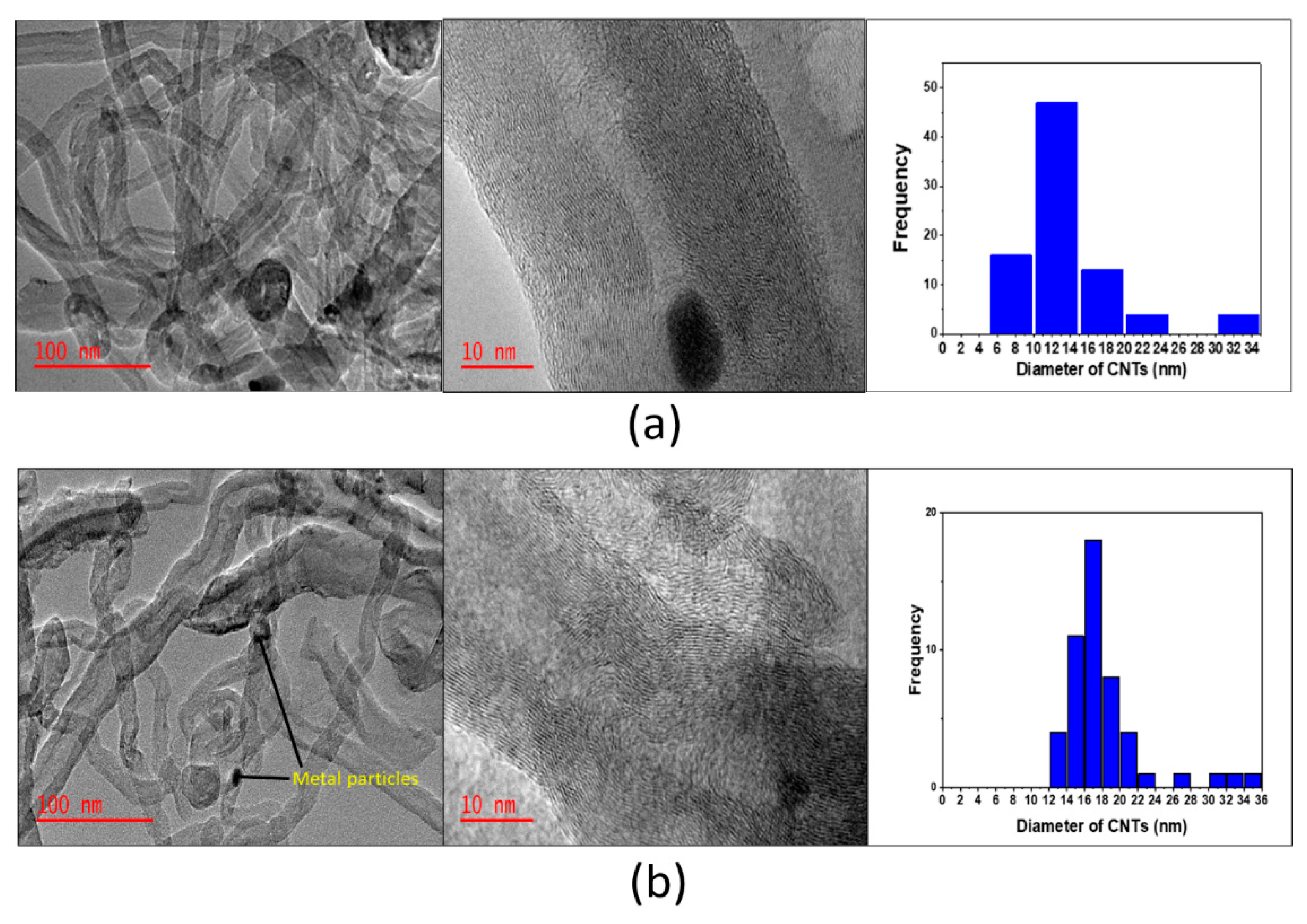
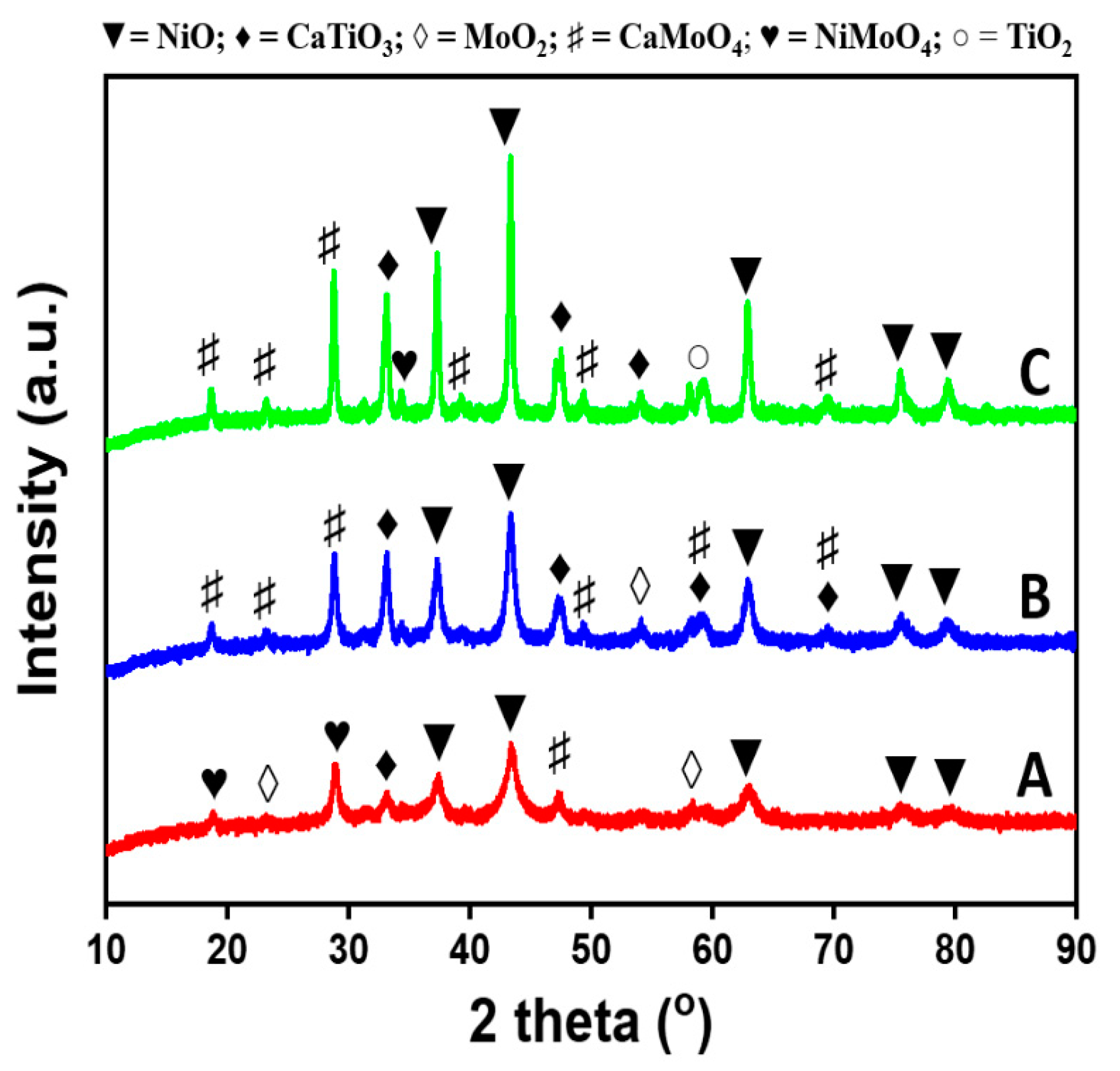

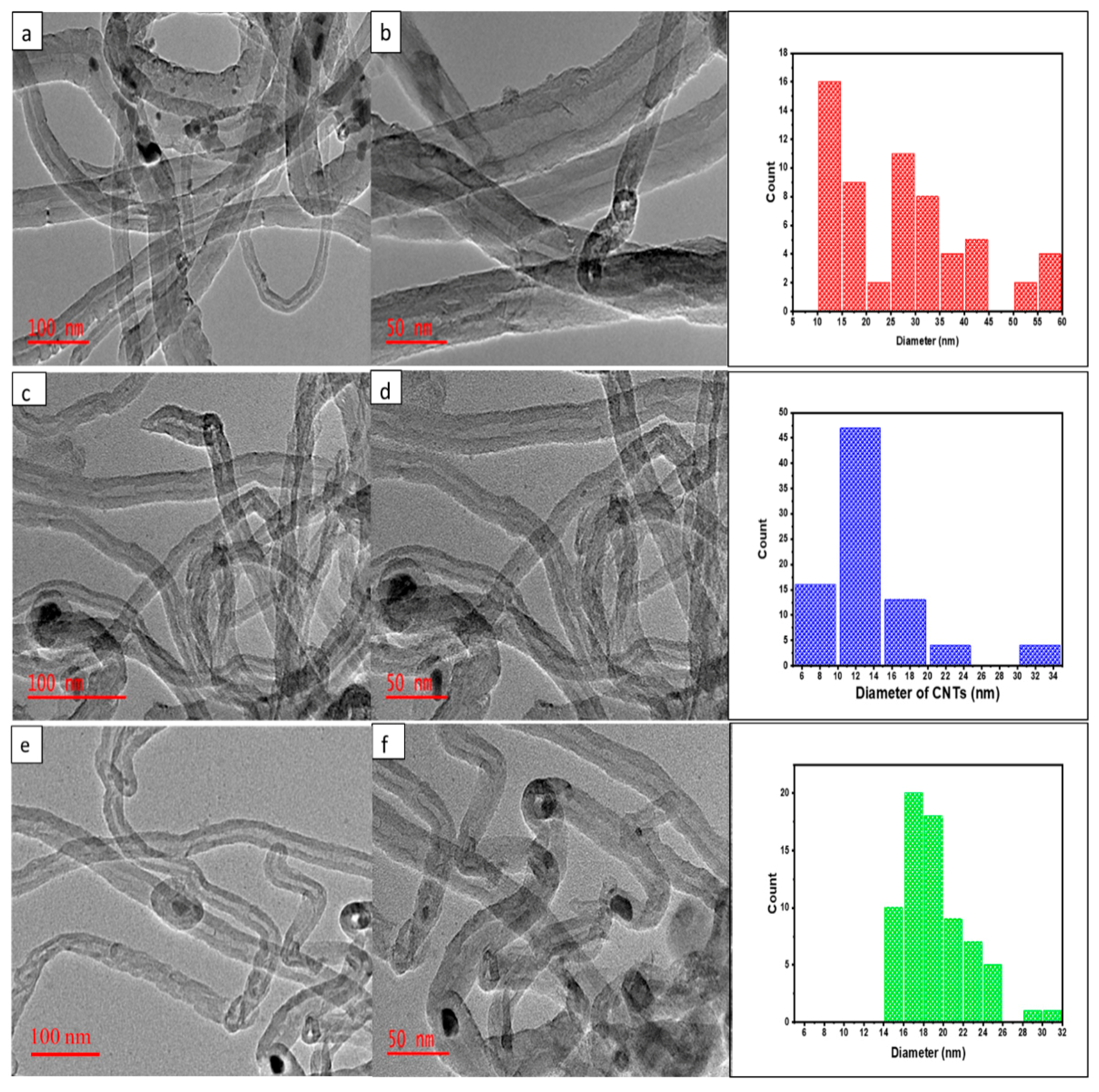
| Calcination Temperature (°C) | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| 600 | 17.43 | 0.0974 |
| 700 | 13.46 | 0.0805 |
| 800 | 7.74 | 0.0476 |
| Calcination Temperature (°C) | NiO Crystallite Size (nm) | CaTiO3/Ca3Ti2O7 Crystallite Size (nm) |
|---|---|---|
| 600 | 5.9 | 4.4 |
| 700 | 9.1 | 10.1 |
| 800 | 45.1 | 23.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modekwe, H.U.; Mamo, M.A.; Daramola, M.O.; Moothi, K. Catalytic Performance of Calcium Titanate for Catalytic Decomposition of Waste Polypropylene to Carbon Nanotubes in a Single-Stage CVD Reactor. Catalysts 2020, 10, 1030. https://doi.org/10.3390/catal10091030
Modekwe HU, Mamo MA, Daramola MO, Moothi K. Catalytic Performance of Calcium Titanate for Catalytic Decomposition of Waste Polypropylene to Carbon Nanotubes in a Single-Stage CVD Reactor. Catalysts. 2020; 10(9):1030. https://doi.org/10.3390/catal10091030
Chicago/Turabian StyleModekwe, Helen Uchenna, Messai Adenew Mamo, Michael Olawale Daramola, and Kapil Moothi. 2020. "Catalytic Performance of Calcium Titanate for Catalytic Decomposition of Waste Polypropylene to Carbon Nanotubes in a Single-Stage CVD Reactor" Catalysts 10, no. 9: 1030. https://doi.org/10.3390/catal10091030







