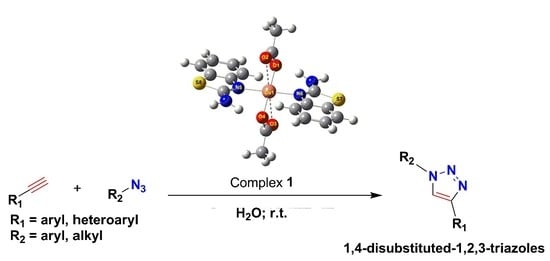
2-Aminobenzothiazole-Containing Copper(II) Complex as Catalyst in Click Chemistry: An Experimental and Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Studies
2.2. Comparison of Complex 1 with other Catalysts
2.3. Mechanistic Studies
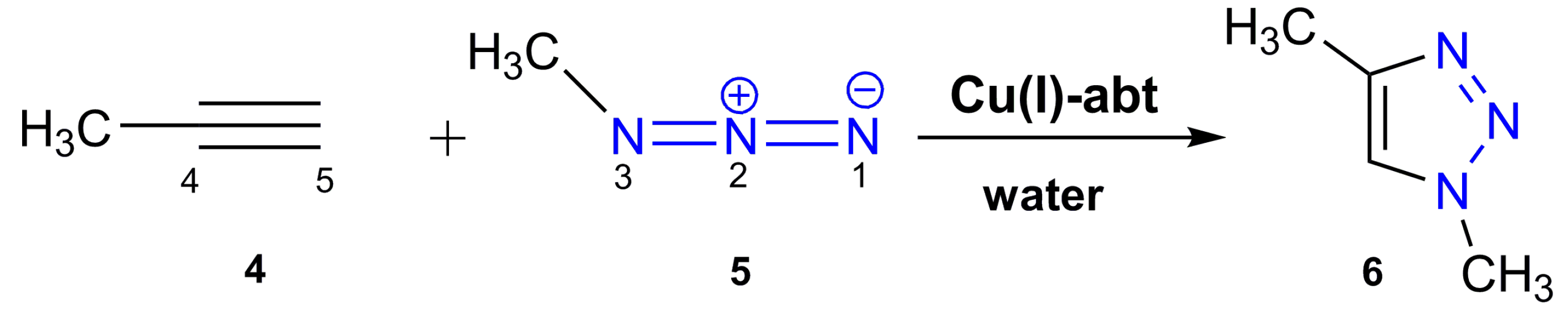
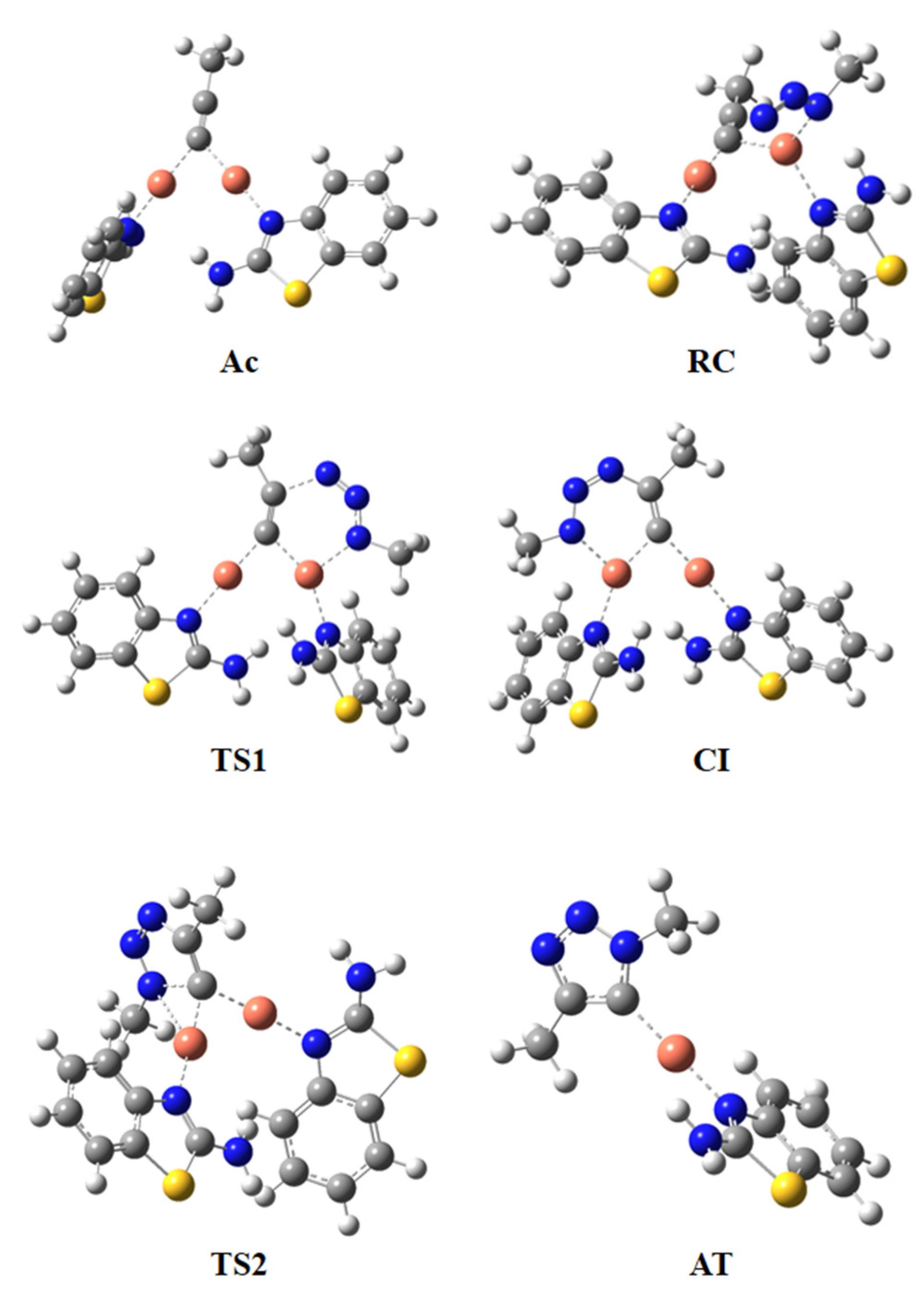
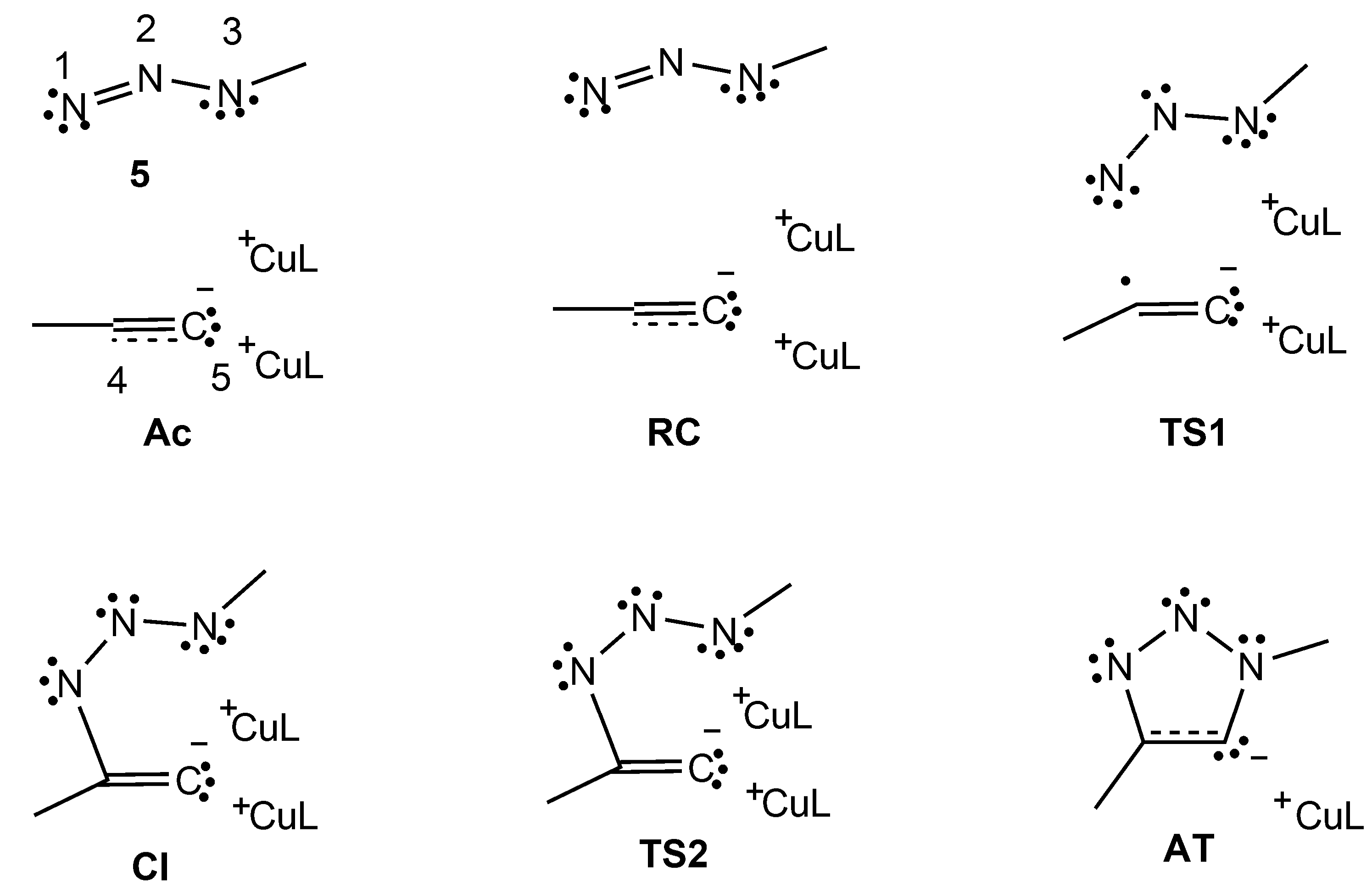
2.3.1. MEDT Study
2.3.2. ELF Topological Analysis
3. Materials and Methods
3.1. Materials
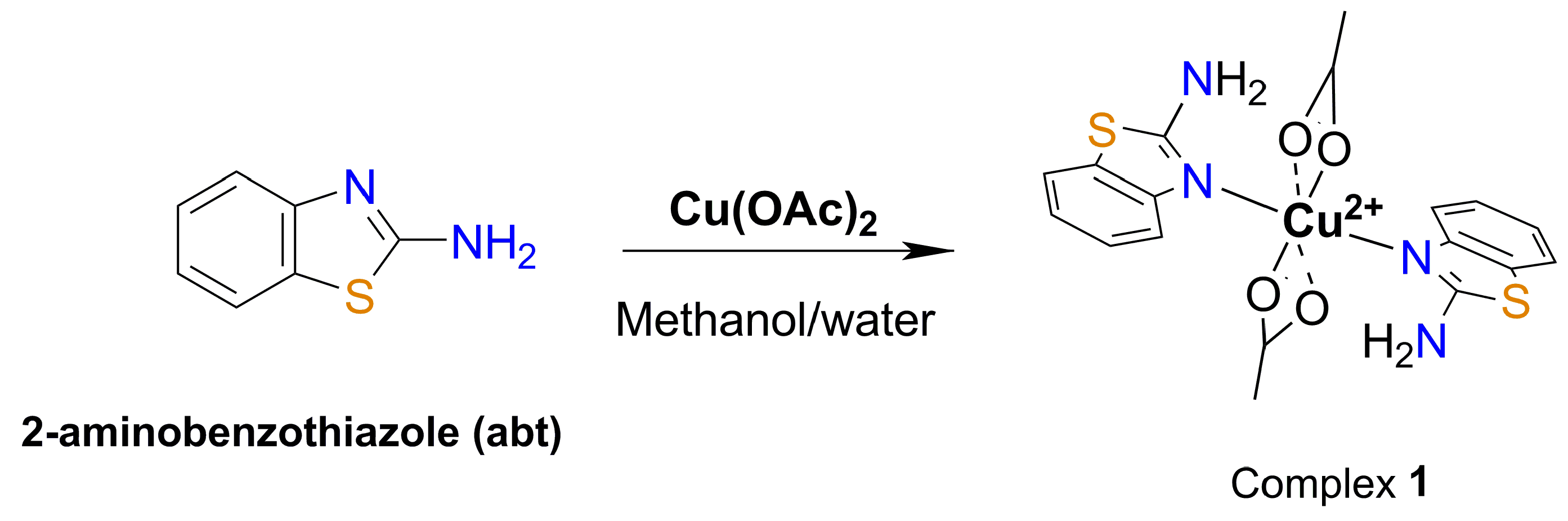
3.2. Synthesis of [Cu(abt)2(OOCCH3)2] (1)
3.3. General Procedure for the CuAAC Reaction
3.3.1. Synthesis of 1-Benzyl-4-phenyl-1H-1,2,3-triazole (3a)
3.3.2. Synthesis of 1- Benzyl-4-p-Tolyl-1H-1,2,3-Triazole (3b)
3.3.3. Synthesis of 1-(4-Methylbenzyl)-4-Phenyl-1H-1,2,3-Triazole (3c)
3.3.4. Synthesis of 1-Benzyl-4-(Phenoxymethyl)-1H-1,2,3-Triazole (3d)
3.3.5. Synthesis of 1-(4-Fluoro-Benzyl)-4-p-Tolyl-1H-[1,2,3]Triazole (3e)
3.3.6. Synthesis of 1-(4-Fluorobenzyl)-4-(4-Fluorophenyl)-1H-1,2,3-Triazole (3f)
3.3.7. Synthesis of (1-(4-Isopropylphenyl)-1H-1,2,3-Triazol-4-yl)Methyl Benzoate (3g)
3.3.8. Synthesis of Methyl 1-(4-Isopropylphenyl)-1H-1,2,3-Triazole-4-Carboxylate (3h)
3.3.9. Synthesis of 1-(4-Isopropylphenyl)-4-(Phenylthiomethyl)-1H-1,2,3-Triazole (3i)
3.3.10. Synthesis of (1-(4-Methoxyphenyl)-1H-1,2,3-Triazol-4-yl)Methyl Benzoate (3j)
3.3.11. Synthesis of Naphthalen-2-yl 1-Benzyl-1H-1,2,3-Triazole-4-Carboxylate (3k)
3.3.12. Synthesis of Naphthalen-2-yl 1-(4-Methoxyphenyl)-1H-1,2,3-Triazole-4-Carboxylate (3l)
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lutz, J.-F. Nanotechnology for Life Science Research Group 1,3-dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. Engl. 2007, 46, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem.–Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and reaction mechanisms: Selected examples from the experience of forty years. Pure Appl. Chem. 1989, 61, 613–628. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Nandivada, H.; Jiang, X.; Lahann, J. Click Chemistry: Versatility and Control in the Hands of Materials Scientists. Adv. Mater. 2007, 19, 2197–2208. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Costa, M.S.; Boechat, N.; Rangel, É.A.; de C da Silva, F.; de Souza, A.M.T.; Rodrigues, C.R.; Castro, H.C.; Junior, I.N.; Lourenço, M.C.S.; Wardell, S.M.S.V.; et al. Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives. Bioorg. Med. Chem. 2006, 14, 8644–8653. [Google Scholar] [CrossRef]
- Wu, P.; Fokin, V.V. Catalytic azide-alkyne cycloaddition: Reactivity and applications. Aldrichimica Acta 2007, 40, 7–17. [Google Scholar] [CrossRef]
- Rodionov, V.O.; Presolski, S.I.; Díaz Díaz, D.; Fokin, V.V.; Finn, M.G. Ligand-Accelerated Cu-Catalyzed Azide−Alkyne Cycloaddition: A Mechanistic Report. J. Am. Chem. Soc. 2007, 129, 12705–12712. [Google Scholar] [CrossRef]
- Bahsis, L.; Ben El Ayouchia, H.; Anane, H.; Ramirez de Arellano, C.; Bentama, A.; El Hadrami, E.M.; Julve, M.; Domingo, L.R.; Stiriba, S.-E. Clicking Azides and Alkynes with Poly(pyrazolyl)borate-Copper(I) Catalysts: An Experimental and Computational Study. Catalysts 2019, 9, 687. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Yang, C.; Flynn, J.P.; Niu, J. Facile Synthesis of Sequence-Regulated Synthetic Polymers Using Orthogonal SuFEx and CuAAC Click Reactions. Angew. Chem. Int. Ed. 2018, 57, 16194–16199. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef] [PubMed]
- Candelon, N.; Lastécouères, D.; Diallo, A.K.; Aranzaes, J.R.; Astruc, D.; Vincent, J.-M. A highly active and reusable copper(I)-tren catalyst for the “click” 1,3-dipolar cycloaddition of azides and alkynes. Chem. Commun. 2008, 741–743. [Google Scholar] [CrossRef]
- Bahsis, L.; Ben El Ayouchia, H.; Anane, H.; Triki, S.; Julve, M.; Stiriba, S.-E. Copper(II)-dipicolinate-mediated clickable azide–alkyne cycloaddition in water as solvent. J. Coord. Chem. 2018, 71, 633–643. [Google Scholar] [CrossRef]
- Cano, I.; Nicasio, M.C.; Pérez, P.J. Copper(I) complexes as catalysts for the synthesis of N-sulfonyl-1,2,3-triazoles from N-sulfonylazides and alkynes. Org. Biomol. Chem. 2010, 8, 536–538. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Liu, P.; Xie, J.; Dai, B. 2-Pyrrolecarbaldiminato–Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. RSC Adv. 2014, 5, 6661–6665. [Google Scholar] [CrossRef]
- Özçubukçu, S.; Ozkal, E.; Jimeno, C.; Pericàs, M.A. A Highly Active Catalyst for Huisgen 1,3-Dipolar Cycloadditions Based on the Tris(triazolyl)methanol−Cu(I) Structure. Org. Lett. 2009, 11, 4680–4683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzyme Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef]
- Alang, G.; Kaur, R.; Kaur, G.; Sïngh, A.; Sïngla, P. Synthesis and antibacterial activity of some new benzothiazole derivatives. ACTA Pharm. Sci. 2010, 52, 213–218. [Google Scholar]
- Catalano, A.; Carocci, A.; Defrenza, I.; Muraglia, M.; Carrieri, A.; Van Bambeke, F.; Rosato, A.; Corbo, F.; Franchini, C. 2-Aminobenzothiazole derivatives: Search for new antifungal agents. Eur. J. Med. Chem. 2013, 64, 357–364. [Google Scholar] [CrossRef]
- Manjula, S.N.; Malleshappa Noolvi, N.; Vipan Parihar, K.; Manohara Reddy, S.A.; Ramani, V.; Gadad, A.K.; Singh, G.; Gopalan Kutty, N.; Mallikarjuna Rao, C. Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef]
- Kadirova, S.A.; Tursunova, M.R.; Ishankhodzhaeva, M.M.; Parpiev, N.A.; Karimov, Z.; Tozhiboev, A.; Tashkhodzhaev, B. Synthesis and structure of complexes of Co(II) acetate and Zn(II) chloride with 2-aminobenzothiazole. Russ. J. Gen. Chem. 2007, 77, 1807–1810. [Google Scholar] [CrossRef]
- Dojer, B.; Pevec, A.; Breznik, K.; Jagličić, Z.; Gyergyek, S.; Kristl, M. Structural and thermal properties of new copper and nickel single-source precursors. J. Mol. Struct. 2019, 1194, 171–177. [Google Scholar] [CrossRef]
- Shabana, A.A.; Butler, I.S.; Gilson, D.F.R.; Jean-Claude, B.J.; Mouhri, Z.S.; Mostafa, M.M.; Mostafa, S.I. Synthesis, characterization, anticancer activity and DNA interaction studies of new 2-aminobenzothiazole complexes; crystal structure and DFT calculations of [Ag(Habt)2]ClO4. Inorg. Chim. Acta 2014, 423, 242–255. [Google Scholar] [CrossRef]
- BELMAR, J. New liquid crystals containing the benzothiazol unit: Amides and azo compounds. Liq. Cryst. 1999, 26, 389–396. [Google Scholar] [CrossRef]
- İlkimen, H. A Review on Biological Properties and Mixed Ligand Metal Complexes Of 2-Aminobenzothiazoles. Pamukkale Univ. J. Eng. Sci. 2018, 24, 1360–1369. [Google Scholar] [CrossRef]
- Reddy, K.R.; Rajgopal, K.; Kantam, M.L. Copper(II)-Promoted Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles in Water. Synlett 2006, 2006, 957–959. [Google Scholar] [CrossRef]
- Harmand, L.; Lescure, M.-H.; Candelon, N.; Duttine, M.; Lastécouères, D.; Vincent, J.-M. Huisgen click cycloadditions from a copper(II)-tren precatalyst without external sacrificial reductant. Tetrahedron Lett. 2012, 53, 1417–1420. [Google Scholar] [CrossRef]
- Tale, R.H.; Gopula, V.B.; Toradmal, G.K. ‘Click’ ligand for ‘click’ chemistry: (1-(4-methoxybenzyl)-1-H-1,2,3-triazol-4-yl)methanol (MBHTM) accelerated copper-catalyzed [3+2] azide–alkyne cycloaddition (CuAAC) at low catalyst loading. Tetrahedron Lett. 2015, 56, 5864–5869. [Google Scholar] [CrossRef]
- Han, B.; Xiao, X.; Wang, L.; Ye, W.; Liu, X. Highly active binuclear Cu(II) catalyst bearing an unsymmetrical bipyridine-pyrazole-amine ligand for the azide-alkyne cycloaddition reaction. Chin. J. Catal. 2016, 37, 1446–1450. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Bayrami, A.; Kia, R.; Amini, M.; Cook, L.J.K.; Raithby, P.R. Two new copper(II) complexes with chelating N,O-type bidentate ligands: Synthesis, characterization, crystal structure and catalytic activity in azide–alkyne cycloaddition reaction. Inorg. Chim. Acta 2017, 466, 398–404. [Google Scholar] [CrossRef]
- Amini, M.; Tekantappeh, S.B.; Eftekhari-Sis, B.; Derakhshandeh, P.G.; Hecke, K.V. Synthesis, characterization and catalytic properties of a copper-containing polyoxovanadate nanocluster in azide–alkyne cycloaddition. J. Coord. Chem. 2017, 70, 1564–1572. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Zhu, L. Chelation-Assisted, Copper(II)-Acetate-Accelerated Azide−Alkyne Cycloaddition. J. Org. Chem. 2010, 75, 6540–6548. [Google Scholar] [CrossRef]
- Brotherton, W.S.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Dalal, N.S.; Zhu, L. Apparent Copper(II)-Accelerated Azide−Alkyne Cycloaddition. Org. Lett. 2009, 11, 4954–4957. [Google Scholar] [CrossRef]
- Iacobucci, C.; Reale, S.; Gal, J.-F.; De Angelis, F. Dinuclear Copper Intermediates in Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Directly Observed by Electrospray Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2015, 54, 3065–3068. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.; Mayer, P.; Straub, B.F. Isolation of a Copper(I) Triazolide: A “Click” Intermediate. Angew. Chem. Int. Ed. 2007, 46, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.; Aronica, L.A. Acyl Sonogashira Cross-Coupling: State of the Art and Application to the Synthesis of Heterocyclic Compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide–alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef]
- Jin, L.; Tolentino, D.R.; Melaimi, M.; Bertrand, G. Isolation of bis(copper) key intermediates in Cu-catalyzed azide-alkyne “click reaction”. Sci. Adv. 2015, 1, e1500304. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A DFT analysis of the participation of zwitterionic TACs in polar [3+2] cycloaddition reactions. Tetrahedron 2014, 70, 4519–4525. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. Theochem. 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Bahsis, L.; Ben El Ayouchia, H.; Anane, H.; Pascual-Álvarez, A.; Munno, G.D.; Julve, M.; Stiriba, S.-E. A reusable polymer-supported copper(I) catalyst for triazole click reaction on water: An experimental and computational study. Appl. Organomet. Chem. 2019, 33, e4669. [Google Scholar] [CrossRef]
- Ben El Ayouchia, H.; Bahsis, L.; Anane, H.; Domingo, L.R.; Stiriba, S.-E. Understanding the mechanism and regioselectivity of the copper(I) catalyzed [3 + 2] cycloaddition reaction between azide and alkyne: A systematic DFT study. RSC Adv. 2018, 8, 7670–7678. [Google Scholar] [CrossRef]
- Silvi, B. The synaptic order: A key concept to understand multicenter bonding. J. Mol. Struct. 2002, 614, 3–10. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Molecular Electron Density Theory Study of the Reactivity of Azomethine Imine in [3+2] Cycloaddition Reactions. Molecules 2017, 22, 750. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef]
- Yang, H.; Wong, M.W. Oxyanion hole stabilization by C-H···O interaction in a transition state--a three-point interaction model for Cinchona alkaloid-catalyzed asymmetric methanolysis of meso-cyclic anhydrides. J. Am. Chem. Soc. 2013, 135, 5808–5818. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
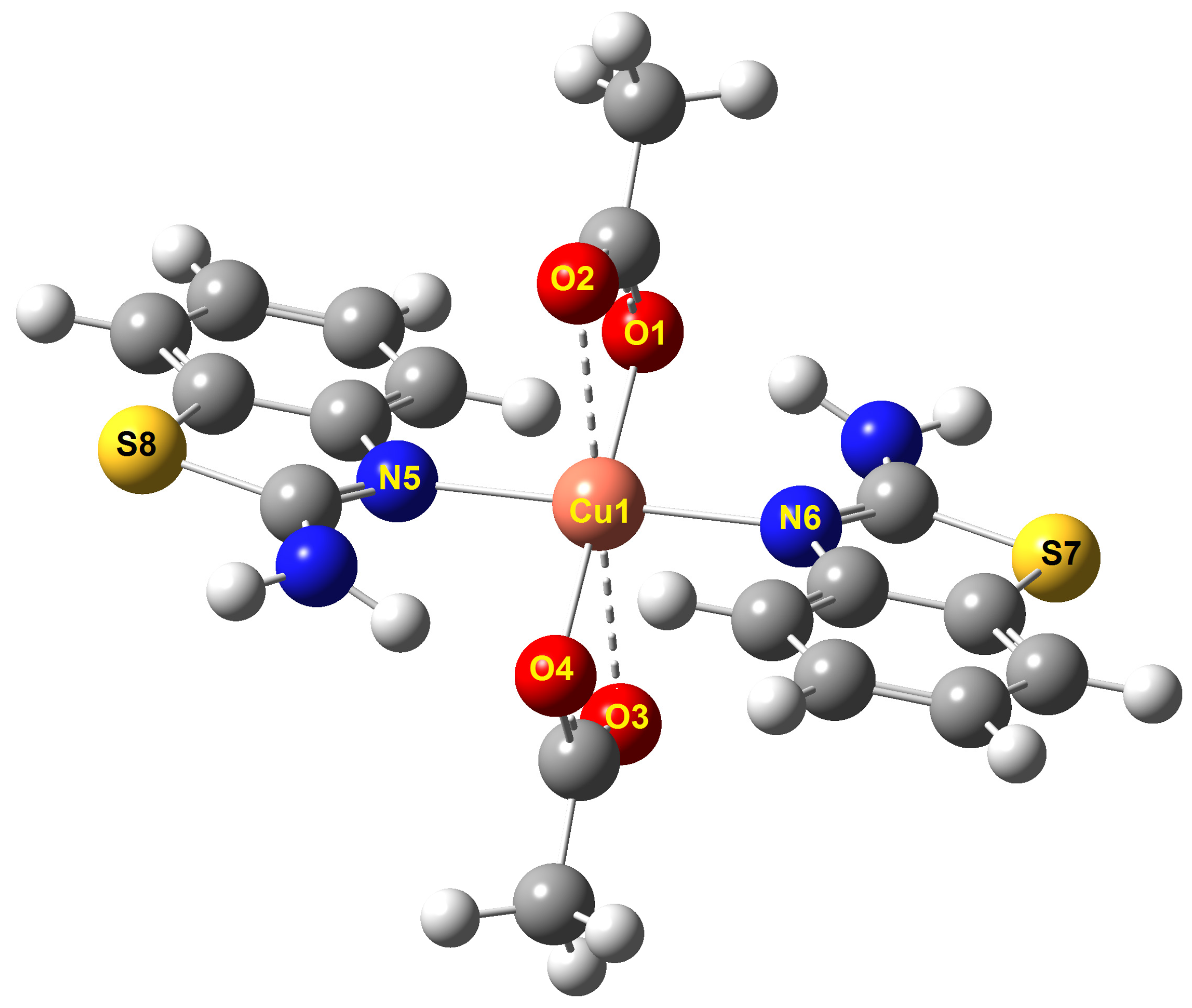
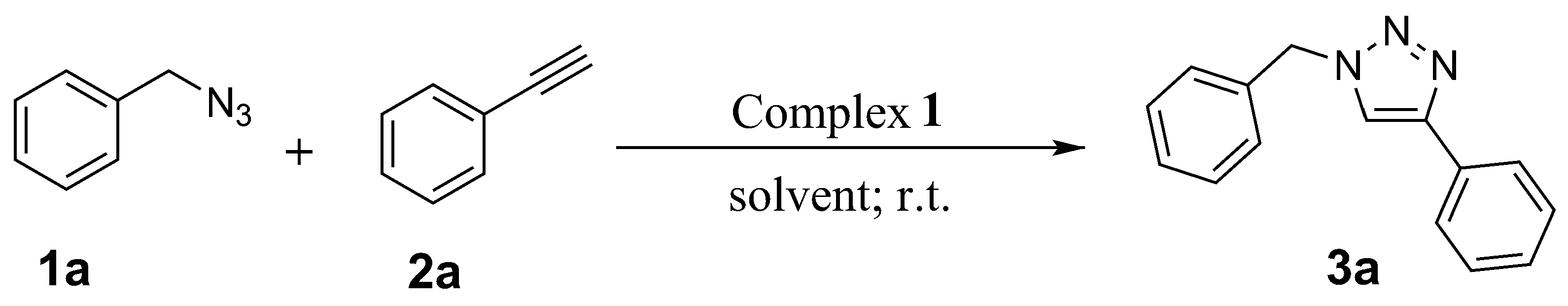


| Calculated | Experimental a | |
|---|---|---|
| Bond Lengths (Å) | ||
| Cu1—O1 | 1.981 | 1.961 |
| Cu1—O2 | 2.554 | 2.548 |
| Cu1—O4 | 1.980 | 1.961 |
| Cu1—O3 | 2.554 | 2.548 |
| Cu1—N5 | 2.130 | 2.018 |
| Cu1—N6 | 2.130 | 2.018 |
| Bond Angles (°) | ||
| O4—Cu1—N5 | 89.668 | 88.715 |
| O3—Cu1—N5 | 92.640 | 91.256 |
| O4—Cu1—N6 | 90.332 | 91.285 |
| O3—Cu1—N6 | 87.359 | 88.743 |
| O1—Cu1—N5 | 90.332 | 91.285 |
| O2—Cu1—N5 | 87.359 | 88.744 |
| O1—Cu1—N6 | 89.668 | 88.714 |
| O2—Cu1—N6 | 92.640 | 91.256 |
| N5—Cu1—N6 | 179.999 | 180.000 |
| Entry | Catalyst | Catalyst Loading (mol%) | Solvent | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | abt | - | Water | 24 | Traces |
| 2 | Cu(OAc)2 | 5 | Water | 24 | 40 |
| 3 | CuSO4 | 5 | Water | 24 | 30 |
| 4 | CuSO4 | 5 | Water | 12 | 85 c |
| 5 | 1 | 5 | Water | 12 | 98 |
| 6 | 1 | 3 | Water | 4 | 96 |
| 7 | 1 | 2 | Water | 4 | 92 |
| 8 | 1 | 1 | Water | 4 | 74 |
| 9 | 1 | 2 | Hexane | 4 | 40 |
| 10 | 1 | 2 | Toluene | 4 | 51 |
| 11 | 1 | 2 | Ethanol | 4 | 67 |
| 12 | 1 | 2 | Acetonitrile | 4 | 64 |
| 13 | 1 | 2 | Acetone | 4 | 56 |
| 14 | 1 | 2 | Ethanol/water | 4 | 62 |
 | |||||
| Entry | Alkyne | Azide | Time (h) | Product | Yield b (%) |
| 1 |  | 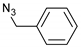 | 4 | 3a | 92 |
| 2 | 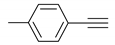 | 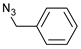 | 4 | 3b | 94 |
| 3 | 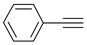 | 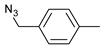 | 4 | 3c | 93 |
| 4 | 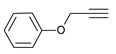 | 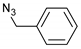 | 4 | 3d | 92 |
| 5 | 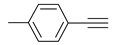 | 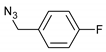 | 8 | 3e | 86 |
| 6 | 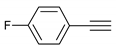 | 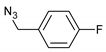 | 8 | 3f | 79 |
| 7 | 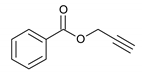 | 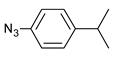 | 8 | 3g | 84 |
| 8 | 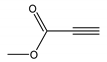 | 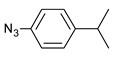 | 8 | 3h | 78 |
| 9 | 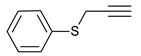 | 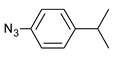 | 8 | 3i | 81 |
| 10 | 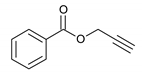 |  | 8 | 3j | 89 |
| 11 |  | 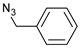 | 12 | 3k | 90 |
| 12 | 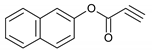 | 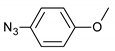 | 12 | 3l | 88 |
| Entry | Cu Loading (mol %) | Additives | Solvent | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Complex 1 | 2 | - | H2O | r.t. | 4 | 92 | This work |
| Cu(NO3)2·3H2O | 20 | - | H2O | r.t. | 20 | 13 | [32] |
| Cu(II)-tren | 0.2 | - | n-Octane | r.t. | 24 | 84 | [33] |
| Cu(II)-MBHTM | 1 | Sodium-ascorbate | DMSO/H2O | r.t. | 4 | 94 | [34] |
| Cu(II)-BPPA | 0.2 | Sodium-ascorbate | MeOH/N2 | r.t. | 16 | 99 | [35] |
| [Cu(II)(Phox)2] | 1.4 | - | H2O | 70 | 12 | 75 | [36] |
| [(C2H5)4N]4[V8Cu2O24] | 0.34 | - | H2O | 70 | 4 | 88 | [37] |
| Species | µ | η | ω | N |
|---|---|---|---|---|
| Propyne (4) | −2.68 | 8.71 | 0.42 | 2.06 |
| Methyl azide (5) | −3.87 | 6.16 | 1.22 | 2.15 |
| Cu(I)-acetylide (Ac) | −3.42 | 5.17 | 1.14 | 3.11 |
| Structures | 5 | Ac | RC | TS1 | Cl | TS2 | AT |
|---|---|---|---|---|---|---|---|
| V(N1,N2) | 1.74 | - | 1.80 | 2.32 | 2.00 | 1.89 | 1.85 |
| V′(N1,N2) | 2.22 | - | 1.83 | - | - | - | - |
| V(N2) | - | - | - | 2.60 | 2.84 | 2.82 | 3.34 |
| V(N2,N3) | 2.51 | - | 2.51 | 1.65 | 1.78 | 2.01 | 1.58 |
| V(C4,C5) | - | 2.38 | 2.41 | 1.96 | 3.35 | 2.90 | 3.09 |
| V′(C4,C5) | - | 2.39 | 2.34 | 1.98 | - | - | - |
| V(N3) | 3.53 | - | 3.58 | 1.99 | 1.85 | 2.99 | 0.79 |
| Vʹ(N3) | - | - | - | 1.63 | 1.67 | - | 0.76 |
| V(C4) | - | - | - | 0.39 | - | - | - |
| V(C5) | - | 3.37 | 3.45 | 2.34 | 3.00 | 2.21 | 3.21 |
| V(N1,C4) | - | - | - | - | 1.97 | 2.17 | 2.11 |
| V(N3,C5) | - | - | - | - | - | - | 2.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahsis, L.; Hrimla, M.; Ben El Ayouchia, H.; Anane, H.; Julve, M.; Stiriba, S.-E. 2-Aminobenzothiazole-Containing Copper(II) Complex as Catalyst in Click Chemistry: An Experimental and Theoretical Study. Catalysts 2020, 10, 776. https://doi.org/10.3390/catal10070776
Bahsis L, Hrimla M, Ben El Ayouchia H, Anane H, Julve M, Stiriba S-E. 2-Aminobenzothiazole-Containing Copper(II) Complex as Catalyst in Click Chemistry: An Experimental and Theoretical Study. Catalysts. 2020; 10(7):776. https://doi.org/10.3390/catal10070776
Chicago/Turabian StyleBahsis, Lahoucine, Meryem Hrimla, Hicham Ben El Ayouchia, Hafid Anane, Miguel Julve, and Salah-Eddine Stiriba. 2020. "2-Aminobenzothiazole-Containing Copper(II) Complex as Catalyst in Click Chemistry: An Experimental and Theoretical Study" Catalysts 10, no. 7: 776. https://doi.org/10.3390/catal10070776
APA StyleBahsis, L., Hrimla, M., Ben El Ayouchia, H., Anane, H., Julve, M., & Stiriba, S.-E. (2020). 2-Aminobenzothiazole-Containing Copper(II) Complex as Catalyst in Click Chemistry: An Experimental and Theoretical Study. Catalysts, 10(7), 776. https://doi.org/10.3390/catal10070776