Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells
Abstract
1. Introduction
2. Results and Discussion
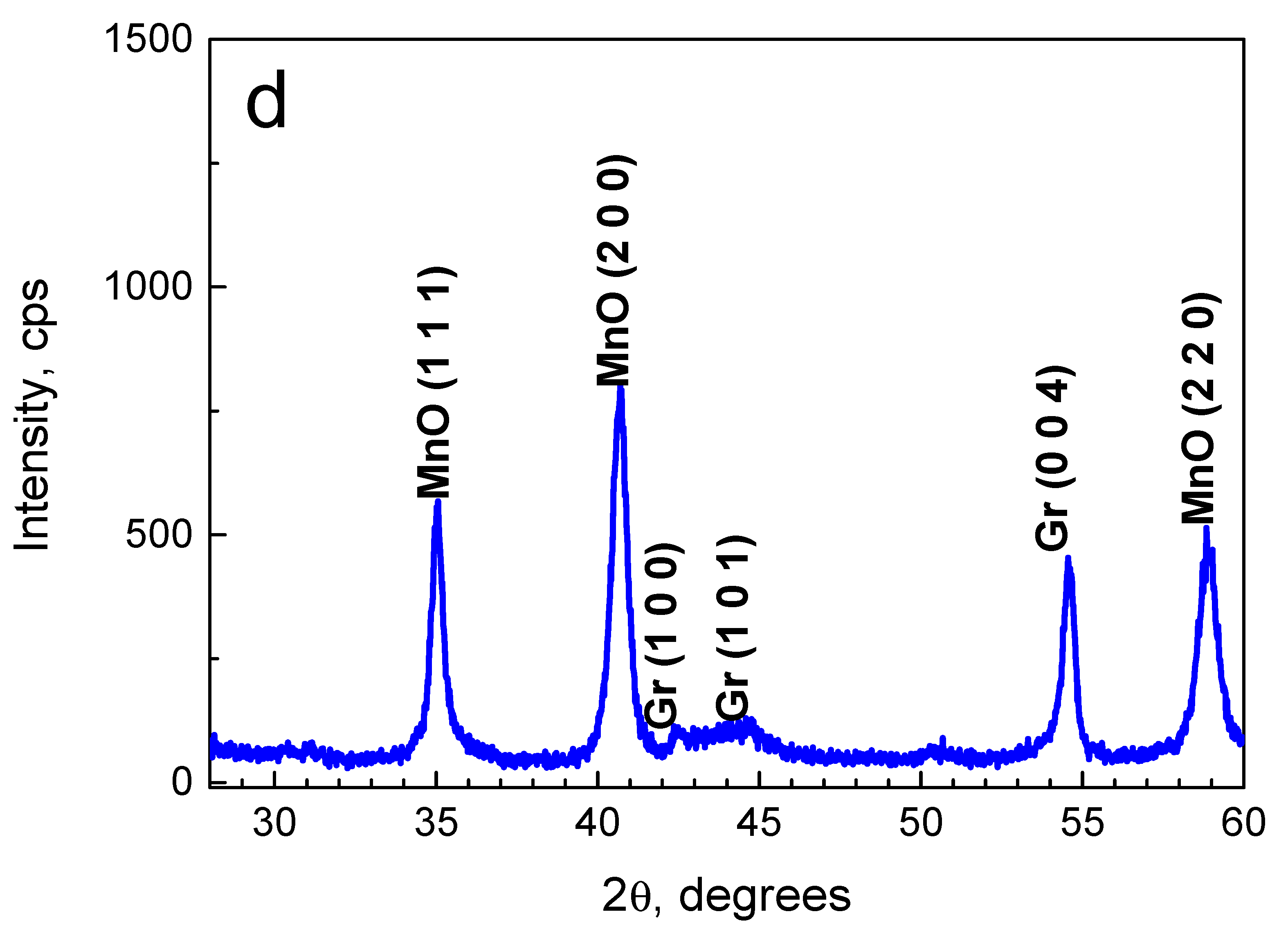
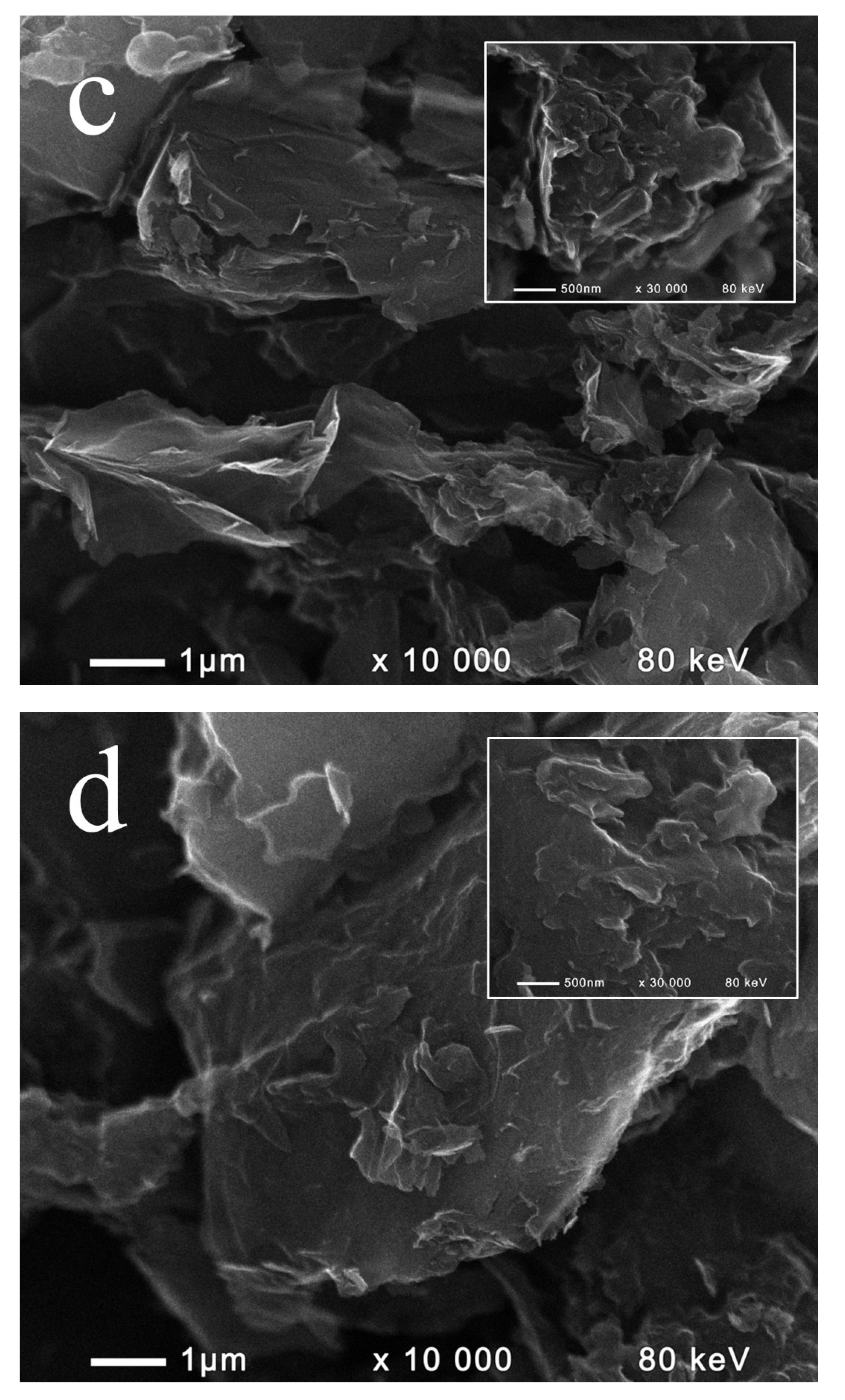
2.1. Composition and Morphology of the Fabricated Materials
2.2. Electrochemical Performance of the Composite Materials in Neutral Medium
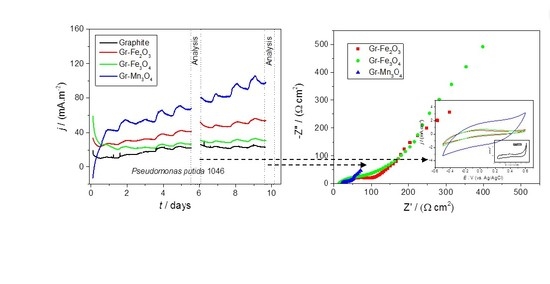
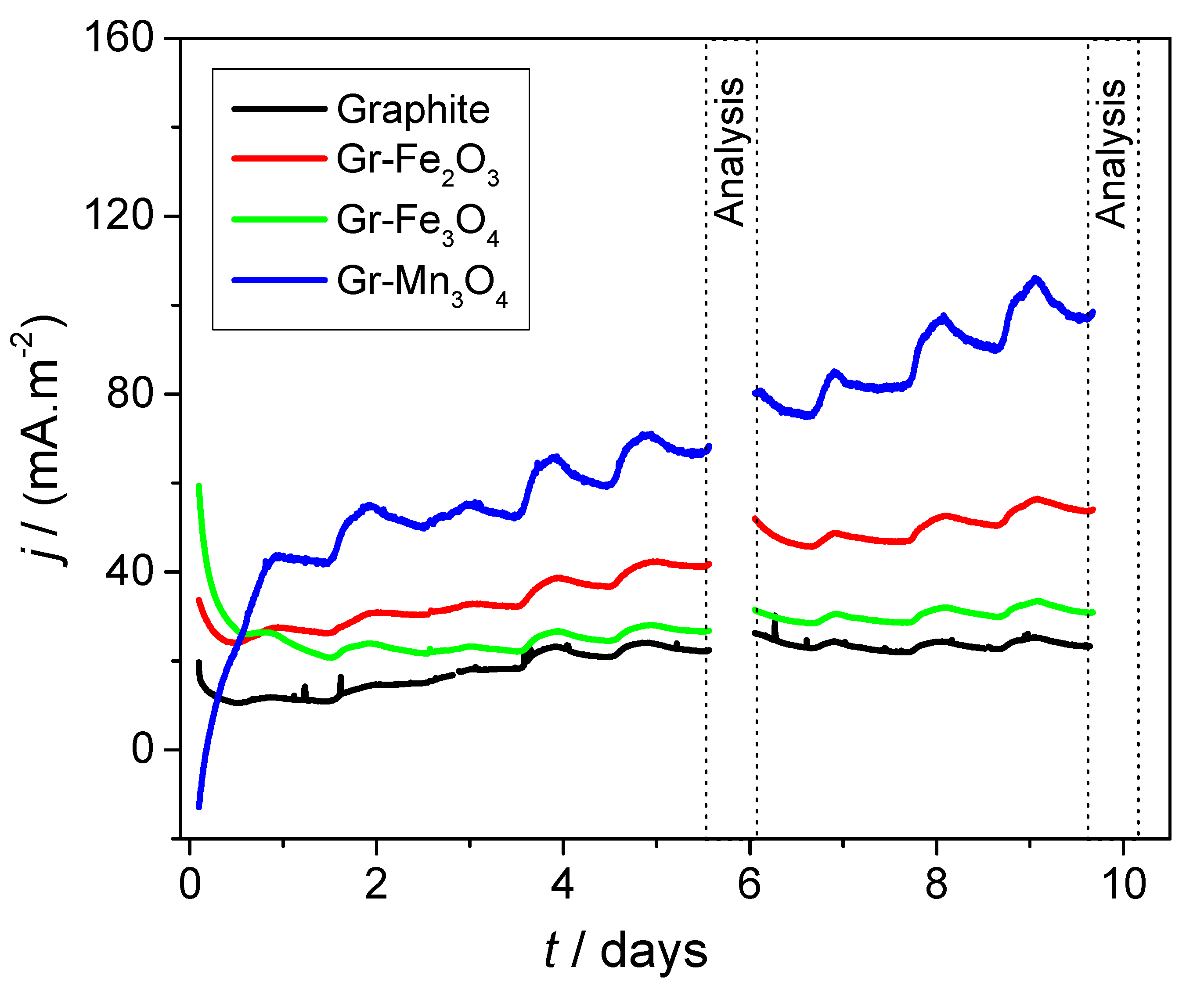
2.3. Electrochemical Analyses of Modified Electrodes in the Presence of Bacteria
3. Materials and Methods
3.1. Production and Characterization of Gr–MO Composites
3.2. Examination of Developed Electrodes in a Biotic Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Baicha, Z.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Labjar, N.; Lotfi, E.; Elmahi, M. A critical review on microalgae as an alternative source for bioenergy production: A promising low cost substrate for microbial fuel cells. Fuel Process. Technol. 2016, 154, 104–116. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Yuan, H.; Hou, Y.; Abu-Reesh, I.M.; Chen, J.; He, Z. Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater. Horiz. 2016, 3, 382–401. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, D.; Pedrycz, W.; Zhu, Y.; Guo, Y. Models for Microbial Fuel Cells: A critical review. J. Power Sources 2018, 373, 119–131. [Google Scholar] [CrossRef]
- Ilamathi, R.; Jayapriya, J. Microbial fuel cells for dye decolorization. Environ. Chem. Lett. 2018, 16, 239–250. [Google Scholar] [CrossRef]
- Hubenova, Y.V.; Rashkov, R.S.; Buchvarov, V.D.; Arnaudova, M.H.; Babanova, S.M.; Mitov, M.Y. Improvement of Yeast−Biofuel Cell Output by Electrode Modifications. Ind. Eng. Chem. Res. 2011, 50, 557–564. [Google Scholar] [CrossRef]
- Hubenova, Y.; Rashkov, R.; Buchvarov, V.; Babanova, S.; Mitov, M. Nanomodified NiFe- and NiFeP-carbon felt as anode electrocatalysts in yeast-biofuel cell. J. Mater. Sci. 2011, 46, 7074–7081. [Google Scholar] [CrossRef]
- Manickam, S.S.; Karra, U.; Huang, L.; Bui, N.-N.; Li, B.; McCutcheon, J.R. Activated carbon nanofiber anodes for microbial fuel cells. Carbon 2013, 53, 19–28. [Google Scholar] [CrossRef]
- Jayapriya, J.; Ramamurthy, V. Use of non-native phenazines to improve the performance of Pseudomonas aeruginosa MTCC 2474 catalysed fuel cells. Bioresour. Technol. 2012, 124, 23–28. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel Mode of Microbial Energy Metabolism: Organic Carbon Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef]
- Aiyer, S.M.; Zahnow, R.; Mazerolle, L.A. Developmental transitions during adulthood and neighborliness: A multilevel cluster analysis. J. Commun. Psychol. 2020, 48, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Schobert, H.H. Viscous sintering of coal ashes. 2. Sintering behavior at short residence times in a drop tube furnace. Energy Fuels 1992, 6, 59–68. [Google Scholar] [CrossRef]
- Nel, M.V.; Strydom, C.A.; Schobert, H.H.; Beukes, J.P.; Bunt, J.R. Comparison of sintering and compressive strength tendencies of a model coal mineral mixture heat-treated in inert and oxidizing atmospheres. Fuel Process. Technol. 2011, 92, 1042–1051. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Mui, M.; Gardner, G.; Zhang, Z.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical Water Oxidation by Crystalline Polymorphs of Manganese Oxides: Structural Requirements for Catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Talha, M.; Ma, Y.; Lin, Y.; Singh, A.; Liu, W.; Kong, X. Corrosion behaviour of austenitic stainless steels in phosphate buffer saline solution: Synergistic effects of protein concentration, time and nitrogen. New J. Chem. 2019, 43, 1943–1955. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, A. Electrochemical characterization of MnO2 as electrocatalytic energy material for fuel cell electrode. J. Fuel Chem. Technol. 2015, 43, 344–351. [Google Scholar] [CrossRef]
- Singh, N.; Geethika, M.; Eswarappa, S.M.; Mugesh, G. Manganese-Based Nanozymes: Multienzyme Redox Activity and Effect on the Nitric Oxide Produced by Endothelial Nitric Oxide Synthase. Chem. A Eur. J. 2018, 24, 8393–8403. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Poo, K.-M.; Ali Abdelkareem, M.; Talas, S.A.; Fadali, O.A.; Kim, H.Y.; Chae, K.-J. Fe/Fe2O3 nanoparticles as anode catalyst for exclusive power generation and degradation of organic compounds using microbial fuel cell. Chem. Eng. J. 2018, 349, 800–807. [Google Scholar] [CrossRef]
- Hubenova, Y.; Mitov, M. Mitochondrial origin of extracelullar transferred electrons in yeast-based biofuel cells. Bioelectrochemistry 2015, 106, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmy, B.R. Exploration of Electrochemcially Active Bacterial Strains for Microbial Fuel Cells: An Innovation in Bioelectricity Generation. J. Pure Appl. Microbiol. 2020, 14, 103–122. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Lovley, D.R. Electron Transport in the Dissimilatory Iron Reducer, GS-15. Appl. Environ. Microbiol. 1991, 57, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wu, H.; Desai, S.R. Sequential Oxygen Atom Chemisorption on Surfaces of Small Iron Clusters. Phys. Rev. Lett. 1996, 76, 4853–4856. [Google Scholar] [CrossRef]
- Hubenova, Y.; Hubenova, E.; Burdin, B.; Vladikova, D.; Mitov, M. Development of coupled redox active network in Ca-alginate polymer for immobilization of Pseudomonas putida 1046 on electrode surface. Electrochim. Acta 2019, 312, 432–440. [Google Scholar] [CrossRef]
- Sultana, S.T.; Babauta, J.T.; Beyenal, H. Electrochemical biofilm control: A review. Biofouling 2015, 31, 745–758. [Google Scholar] [CrossRef]
- Jiang, S.; Kim, D.-G.; Kim, J.-H.; Ko, S.-O. Characterization of the Biogenic Manganese Oxides Produced by Pseudomonas putida strain MnB1. Environ. Eng. Res. 2010, 15, 183–190. [Google Scholar] [CrossRef]
- Banh, A.; Chavez, V.; Doi, J.; Nguyen, A.; Hernandez, S.; Ha, V.; Jimenez, P.; Espinoza, F.; Johnson, H.A. Manganese (Mn) Oxidation Increases Intracellular Mn in Pseudomonas putida GB-1. PLoS ONE 2013, 8, e77835. [Google Scholar] [CrossRef]
- Thangasamy, P.; Sathish, M. Dwindling the re-stacking by simultaneous exfoliation of boron nitride and decoration of α-Fe2O3 nanoparticles using a solvothermal route. New J. Chem. 2018, 42, 5090–5095. [Google Scholar] [CrossRef]
- Li, P.; Yan, X.; Ji, J.; Wu, Y.; Hu, J.; Wang, Y.; Jiang, H.; Zhang, W. Monocrystalline hematite nanostructures: Three-dimensionally oriented aggregation synthesis and their comparative visible-light photocatalytic activities. CrystEngComm 2017, 19, 1926–1932. [Google Scholar] [CrossRef]
- Mani, D.; Mathivanan, D.; Chang, H.; Sakthivel, K.; Elangovan, E.; Sivakumar, T.; Arivanandhan, M.; Jayavel, R. A facile synthesis of novel ε-Fe2O3 grafted 2D h-BN nanostructures for enhanced visible active photocatalytic applications. New J. Chem. 2020. [Google Scholar] [CrossRef]
- Liu, H.; Tong, M.; Zhu, K.; Liu, H.; Chen, R. Preparation and photo-fenton degradation activity of α-Fe2O3 nanorings obtained by adding H2PO4−, SO42−, and citric acid. Chem. Eng. J. 2020, 382, 123010. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef] [PubMed]
- Nevárez-Martínez, M.C.; Kobylański, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Weng, T.-M.; Lu, M.-L.; Tan, W.-C.; Chen, J.-Y.; Chen, Y.-F. All Carbon-Based Photodetectors: An eminent integration of graphite quantum dots and two dimensional graphene. Sci. Rep. 2013, 3, 2694. [Google Scholar] [CrossRef]
- van der Horst, M.A.; Key, J.; Hellingwerf, K.J. Photosensing in chemotrophic, non-phototrophic bacteria: Let there be light sensing too. Trends Microbiol. 2007, 15, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Christy, M.; Kim, P.; Nahm, K.S. Enhanced electrical contact of microbes using Fe3O4/CNT nanocomposite anode in mediator-less microbial fuel cell. Biosens. Bioelectron. 2014, 58, 75–80. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Harnisch, F.; Kuhlicke, U.; Neu, T.R.; Schröder, U. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 2013, 93, 23–29. [Google Scholar] [CrossRef]





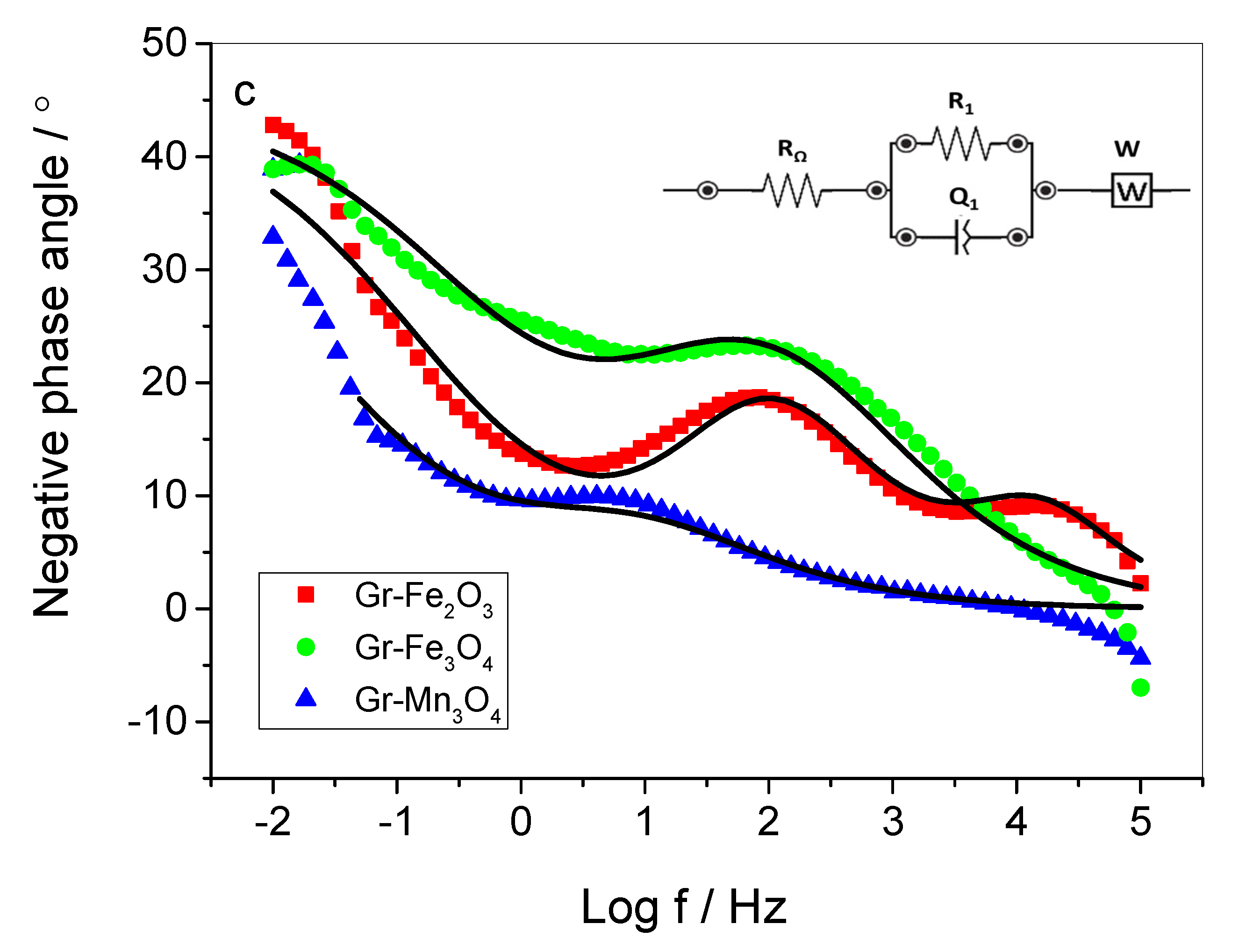
| Circuit Element/Unit | Gr–Fe2O3 | Gr–Fe3O4 | Gr–Mn3O4 |
|---|---|---|---|
| Equivalent Circuit Model | RΩ (R1Q1)(R2Q2)W | RΩ (R1Q1)W | RΩ (R1Q1)W |
| RΩ, Ω | 5.04 | 3.63 | 5.40 |
| R1, Ω | 11.93 | 13.96 | 3.50 |
| Q1, μT | 1450 | 5500 | 41,500 |
| n1, ɸ | 0.701 | 0.544 | 0.553 |
| R2, Ω | 2.18 | - | - |
| Q2, μT | 40.16 | - | - |
| n2, ɸ | 0.832 | - | - |
| W, Kσ | 0.014 | 0.025 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorbadzhiyska, E.; Bardarov, I.; Hubenova, Y.; Mitov, M. Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells. Catalysts 2020, 10, 796. https://doi.org/10.3390/catal10070796
Chorbadzhiyska E, Bardarov I, Hubenova Y, Mitov M. Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells. Catalysts. 2020; 10(7):796. https://doi.org/10.3390/catal10070796
Chicago/Turabian StyleChorbadzhiyska, Elitsa, Ivo Bardarov, Yolina Hubenova, and Mario Mitov. 2020. "Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells" Catalysts 10, no. 7: 796. https://doi.org/10.3390/catal10070796
APA StyleChorbadzhiyska, E., Bardarov, I., Hubenova, Y., & Mitov, M. (2020). Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells. Catalysts, 10(7), 796. https://doi.org/10.3390/catal10070796