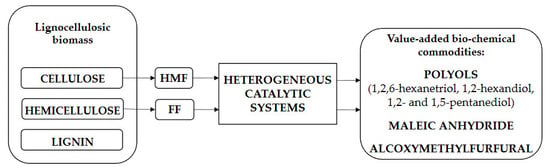
Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds
Abstract
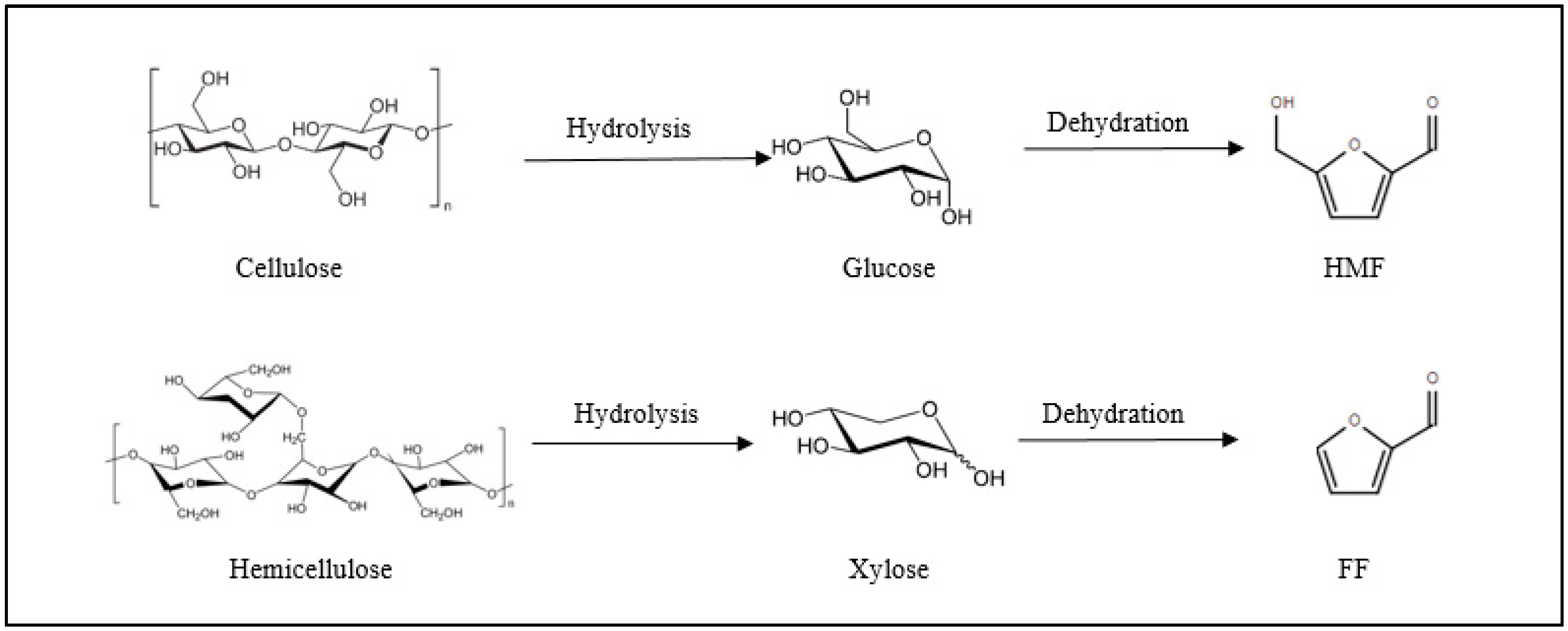
:1. Introduction
2. Polyols
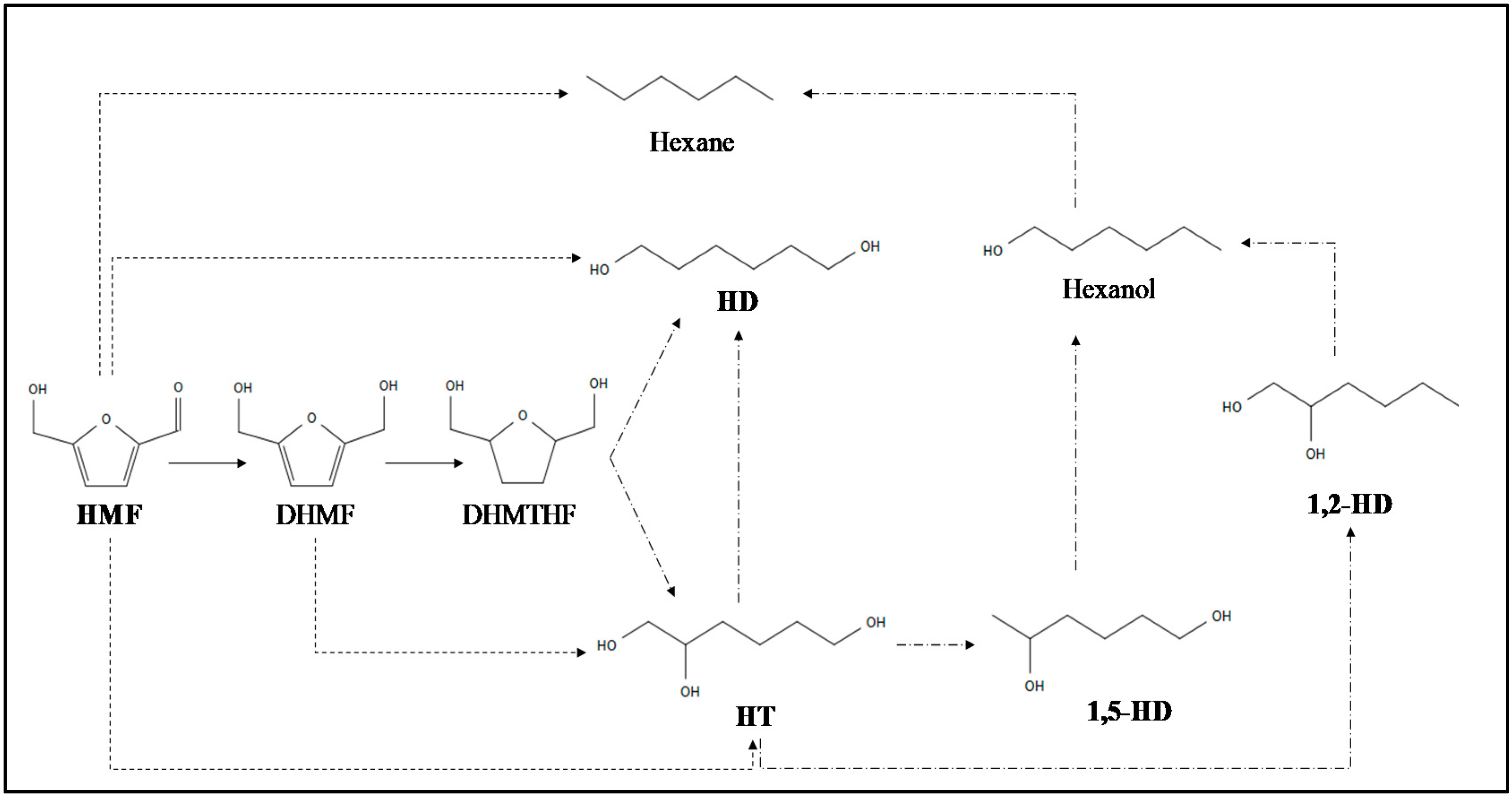
2.1. 1,2,6-Hexanetriol (HT) Production through Catalytic Conversion of HMF
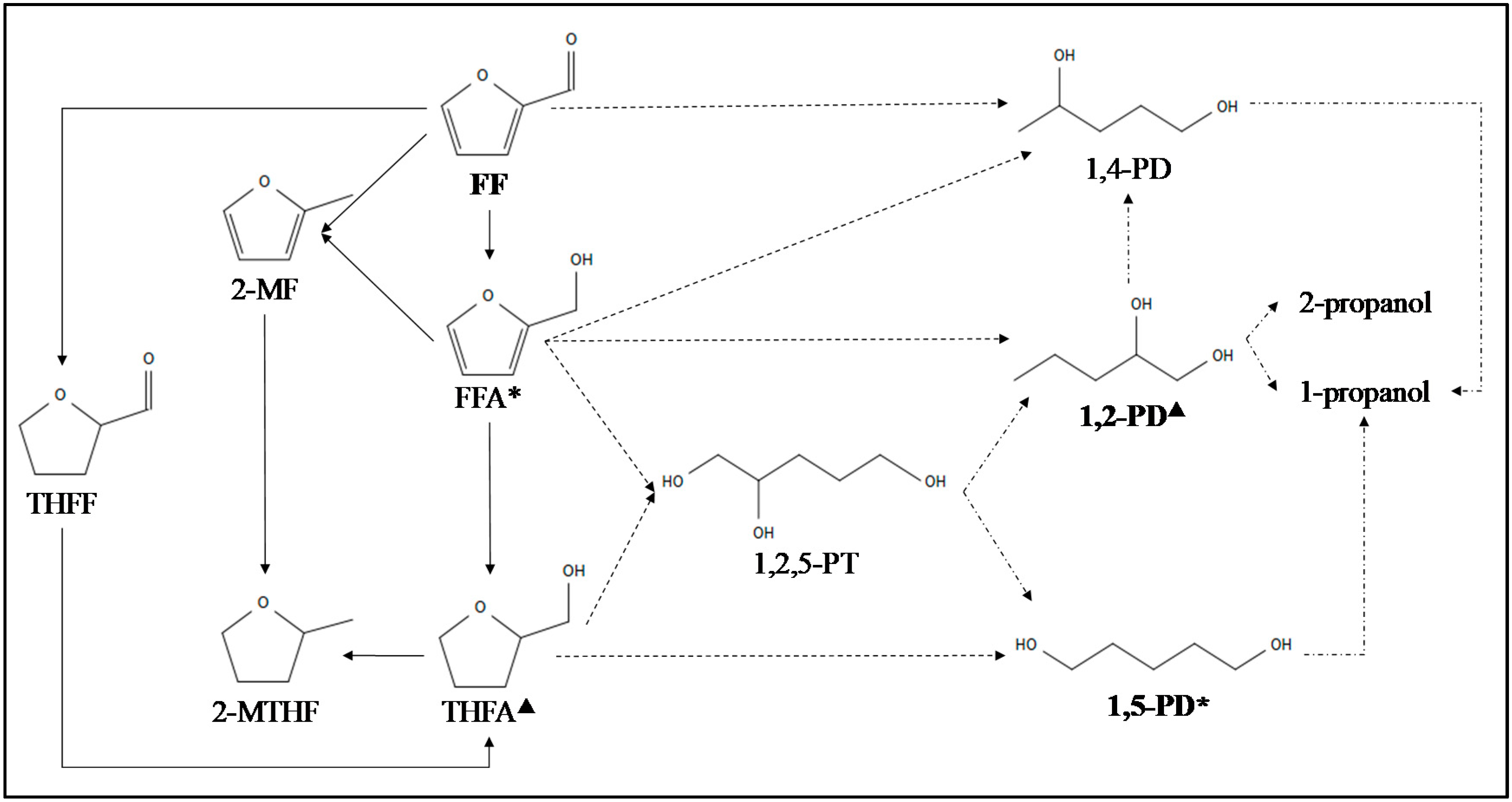
2.2. Diols from HMF and FF Using Metal Catalysts
2.2.1. HMF Transformation into 1,6-Hexanediol (HD)
2.2.2. 1,5- and 1,2-Pentanediol Synthesis from FF
3. Anhydride of Acids: Maleic Anhydride (MAN)
| Catalyst | T (°C) | Phase (Solvent) | Oxidating Agent | Raw Material | χ (%) | YMAN (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Vanadyl pyro- phosphate (VPP) | 340–360 | G | O2 | Butanol | 100 | 39 | [69] |
| VOx/SiO2 | 325 | G | O2 | Levulinic acid | 100 | 71 | [70] |
| VO(ACAC)2 | 90 | L(ACN) | O2 | HMF | 99 | 52 | [33] |
| H5PV2Mo10O40·xH2O | 90 | L (ACN) | O2 | HMF | >99 | 41.8 | [71] |
| V2O5 | 100 | L (HAc) | O2 (1 MPa) | HMF | 99 | 75 a (1.9:1) | [83] |
| VOHPO4 | 99 | 78 a (2.0:1 | |||||
| (VO)2P2O7 | 99 | 79 a (1.9:1) | |||||
| Mo9V3O8 | 97 | 69 a (2.6:1) | |||||
| V2O5 | 90 | L (HAc) | O2 (2 MPa) | HMF | 99.1 | 48.3 a (1:5.8) | [72,84] |
| VO(ACAC)2 | 99.8 | 55.2 a (1:3.8) | |||||
| (10%) V2O5/SiO2 | 99.8 | 64.5 a (1:4.2) | |||||
| VO–NH2-GO | 99.8 | 95.3 a (1:2.5) | |||||
| (V-GO) | 90 | L (GVL) | O2 (2 MPa) | HMF | 97.8 | 53.7 | [85] |
| α-MnO2/Cu(NO3)2 + K2S2O8 | 90 | L (H2O + MeCN) | O2 (1 MPa) | FMF | 100 | 89 | [86] |
| FeT(p-Cl)PP Cl | 90 | L (H2O/Org) | O2 (1.2 MPa) | FF | 95 | 44.0 | [80] |
| H3PMo12O40 x3H2O | 110 | L (TCE) | O2 (2 MPa) | FF | 50.4 | 34.5 | [87] |
| H5PV2Mo10O40 and Cu(CF3SO3)2 | 110 | L (HAc) | O2 (2 MPa) | FF | 98.5 | 54.0 | [76] |
| CaCu2P2O7 | 115 | L (H2O) | O2 (0.8 MPa) | FF | ≈55.0 | 37.3 | [88] |
| TS-1 | 50 | L (H2O) | H2O2 | FF | 100 | 70 | [74] |
| V2O5/γ-Al2O3 catalysts | 300 | G | O2 | FF | ≈100 | 70 | [31,89] |
| V2O5/γ-Al2O3 | 320 | G | O2 | FF | ≈100 | 50 | [90] |
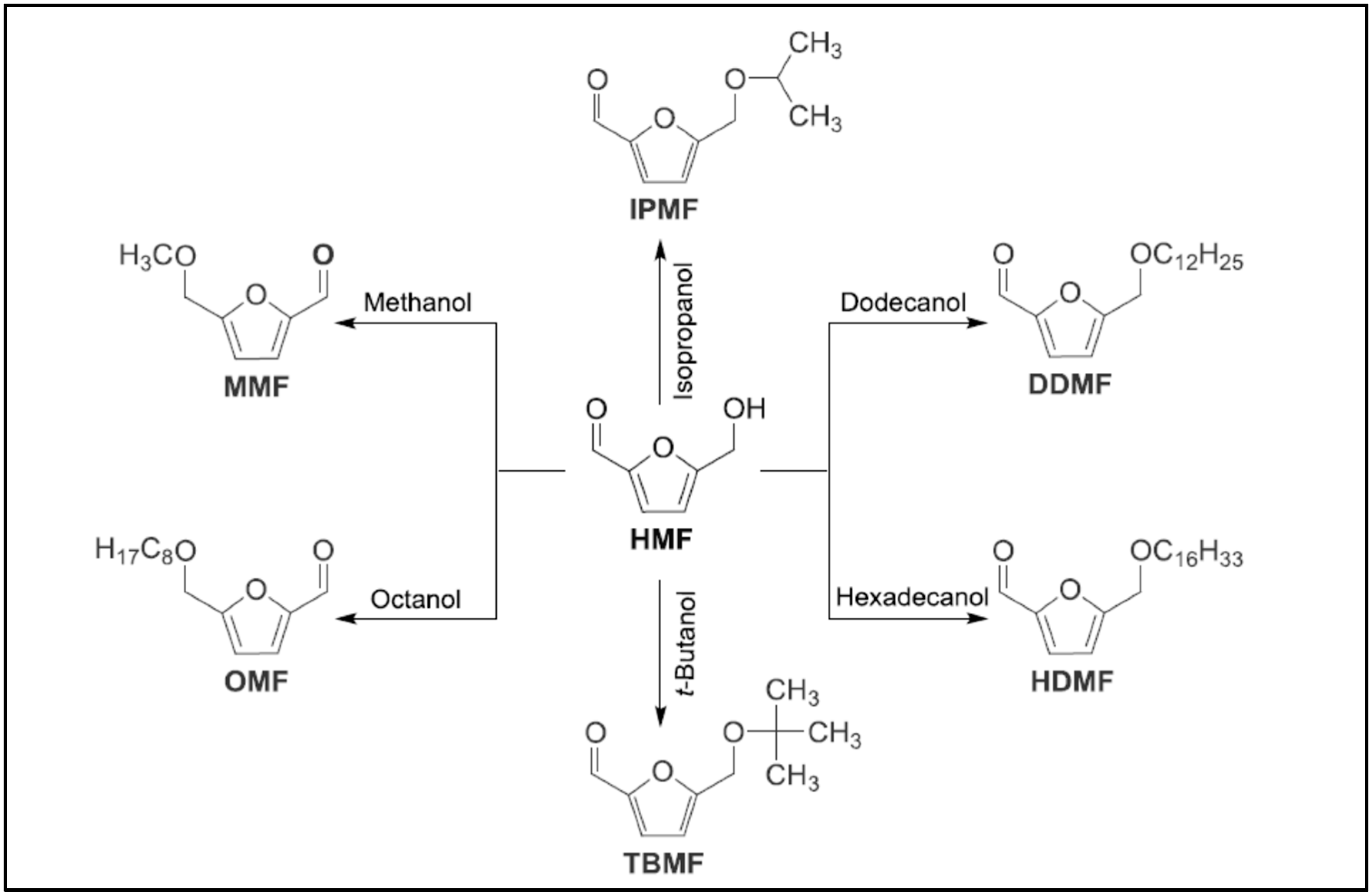
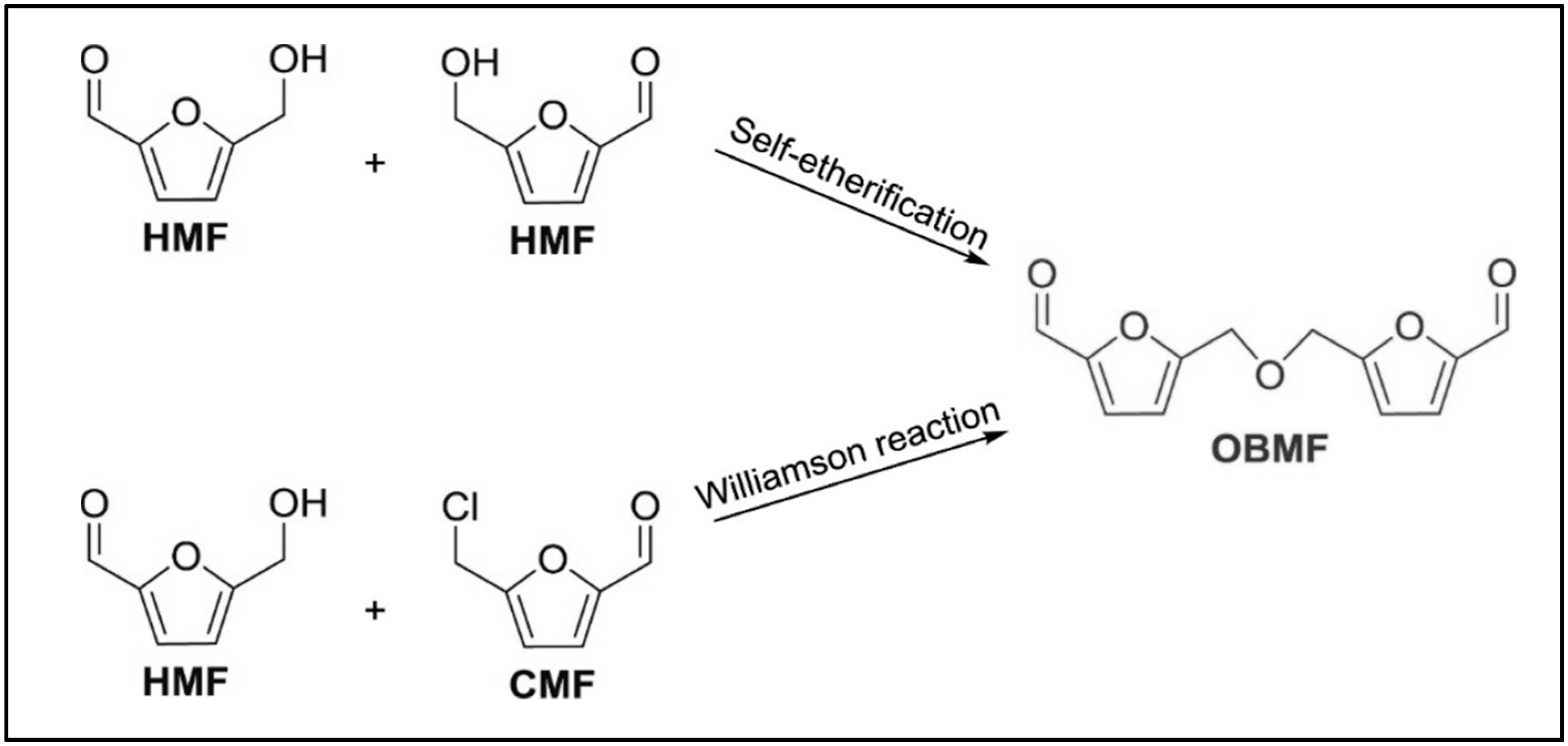
4. Alkoxymethylfurfurals (AMF) from HMF Etherification with Different Alcohols
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,2-HD | 1,2-hexanediol |
| 1,2-PD | 1,2-pentanediol |
| 1,2,5-HT | 1,2,5-hexanetriol |
| 1,2,5-PT | 1,2,5-pentanetriol |
| 1,4-PD | 1,4-pentanediol |
| 1,5-HD | 1,5-hexanediol |
| 1,5-PD | 1,5-pentanediol |
| 2-MF | 2-methylfuran |
| 2-MTHF | 2-methyltetrahydrofuran |
| AMF | alkoxymethylfurfural |
| DDMF | 5-dodecyloxymethylfurfural |
| DFF | 2,5-diformylfuran |
| DHMF | 2,5-dihydroxymethylfuran |
| DHMTHF | 2,5-dihydroxymethyltetrahydrofuran |
| DMSO | dimethyl sulfoxide |
| EMF | 5-ethoxymethylfurfural |
| FDCA | 2,5-furandicarboxylic acid |
| FF | furfural |
| FFA | furfuryl alcohol |
| FFCA | 5-formyl-2-furancarboxylic acid |
| FMF | [(formate)methyl]furfural |
| GO | Graphene oxide |
| GVL | γ-valerolactone |
| HD | 1,6-hexanediol |
| HDMF | 5-hexadecyloxymethylfurfural |
| HMF | 5-hydroxymethylfurfural |
| HMFCA | 5-hydroxymethyl-2-furancarboxylic acid |
| HT | 1,2,6-hexanetriol |
| IPMF | 5-isopropoxymethylfurfural |
| MAN | maleic anhydride |
| MIBK | methyl isobutyl ketone |
| MMF | methoximethylfurfural |
| OBMF | 5,5-oxybismethylene-2-furaldehyde |
| OMF | 5-octyloxymethylfurfural |
| TBMF | 5-tert-butoxymethylfurfural |
| THF | tetrahydrofuran |
| THFA | tetrahydrofurfuryl alcohol |
| THFF | tetrahydrofurfural |
| V-GO | graphene oxide supported vanadium catalyst |
References
- BP. BP Statistical Review of World Energy, 68th ed.; BP: London, UK, 2019. [Google Scholar]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. One-pot conversion of carbohydrates into 5-ethoxymethylfurfural and ethyl d-glucopyranoside in ethanol catalyzed by a silica supported sulfonic acid catalyst. RSC Adv. 2013, 3, 12313–12319. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. International Energy Outlook 2017; IEO2017; U.S. Energy Information Administration: Washington, DC, USA, 2017; p. 143. [Google Scholar]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Sella Kapu, N.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod. Biorefin. 2014, 8, 857–870. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Chen, Z.; Qi, Z.; Wang, X. Enhanced formation of 5-HMF from glucose using a highly selective and stable SAPO-34 catalyst. Chem. Eng. J. 2017, 307, 877–883. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Su, D. 5-Hydroxymethylfurfural: A key intermediate for efficient biomass conversion. J. Energy Chem. 2015, 24, 548–551. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Nhien, L.C.; Long, N.V.D.; Kim, S.; Lee, M. Design and optimization of intensified biorefinery process for furfural production through a systematic procedure. Biochem. Eng. J. 2016, 116, 166–175. [Google Scholar] [CrossRef]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Requies, J.; Agirre, I.; Iriondo, A. Production of Furanic Biofuels with Zeolite and Metal Oxide Bifunctional Catalysts for Energy- and Product-Driven Biorefineries. In Production of Biofuels and Chemicals with Bifunctional Catalysts; Fang, Z., Richard, L.S., Jr., Li, H., Eds.; Springer: Singapore, 2017; p. 504. ISBN 978-981-10-5136-4. [Google Scholar]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Lewis, D.; Chen, W.H.; Huang, H.W.; ALOthman, Z.A.; Yamauchi, Y.; Wu, K.C.W. Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Q.; Zhang, Q.; Chen, L.; Ma, L. A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies. Fuel Process. Technol. 2020, 208, 106485. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Tang, X.; Wei, J.; Ding, N.; Sun, Y.; Zeng, X.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels. Renew. Sustain. Energy Rev. 2017, 77, 287–296. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Supported metal catalysts for total hydrogenation of furfural and 5-hydroxymethylfurfural. J. Jpn. Pet. Inst. 2017, 60, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Buntara, T.; Melián-Cabrera, I.; Tan, Q.; Fierro, J.L.G.; Neurock, M.; De Vries, J.G.; Heeres, H.J. Catalyst studies on the ring opening of tetrahydrofuran-dimethanol to 1,2,6-hexanetriol. Catal. Today 2013, 210, 106–116. [Google Scholar] [CrossRef]
- Yao, S.; Wang, X.; Jiang, Y.; Wu, F.; Chen, X.; Mu, X. One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2,6-hexanetriol over ni-co-al mixed oxide catalysts under mild conditions. ACS Sustain. Chem. Eng. 2014, 2, 173–180. [Google Scholar] [CrossRef]
- Lohbeck, K.; Haferkorn, H.; Fuhrmann, W.; Fedtke, N. Maleic and Fumaric Acids; Ullmann’s Encyclopedia of Industrial Chemistry (Wiley): Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Resins, L.; Felthouse, T.R.; Burnett, J.C.; Horrell, B.; Mummey, M.J.; Kuo, Y.-J. Maleic Anhydride, Maleic Acid, and Fumaric Acid. Kirk-Othmer Encycl. Chem. Technol. 2001, 1–49. [Google Scholar] [CrossRef]
- Bridgwater, A.; Chinthapalli, R.; Smith, P. Identification and Market Analysis of Most Promising Added-Value Products to Be Co-Produced with the Fuels; Biored-Integ European Proyect, Aston University: Birmingham, UK, 2010. [Google Scholar]
- Alonso-Fagúndez, N.; Ojeda, M.; Mariscal, R.; Fierro, J.L.G.L.G.; López Granados, M. Gas phase oxidation of furfural to maleic anhydride on V2O5/Γ-Al2O3 catalysts: Reaction conditions to slow down the deactivation. J. Catal. 2017, 348, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Ghaznavi, T.; Neagoe, C.; Patience, G.S. Partial oxidation of d-xylose to maleic anhydride and acrylic acid over vanadyl pyrophosphate. Biomass Bioenergy 2014, 71, 285–293. [Google Scholar] [CrossRef]
- Du, Z.; Ma, J.; Wang, F.; Liu, J.; Xu, J. Oxidation of 5-hydroxymethylfurfural to maleic anhydride with molecular oxygen. Green Chem. 2011, 13, 554–557. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.; Hao, W.; Zeng, X.; Sun, Y.; Lei, T.; Lin, L. One-pot tandem conversion of fructose into biofuel components with in-situ generated catalyst system. J. Energy Chem. 2018, 27, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hu, C.; Abu-Omar, M.M. Conversion of glucose into furans in the presence of AlCl 3 in an ethanol-water solvent system. Bioresour. Technol. 2012, 116, 190–194. [Google Scholar] [CrossRef]
- Thombal, R.S.; Jadhav, V.H. Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF, dehydration of sorbitol to isosorbide, and esterification of fatty acids. Tetrahedron Lett. 2016, 57, 4398–4400. [Google Scholar] [CrossRef]
- Gupta, D.; Saha, B. Dual acidic titania carbocatalyst for cascade reaction of sugar to etherified fuel additives. Catal. Commun. 2018, 110, 46–50. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Climent, M.J.J.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef] [Green Version]
- Triebl, C.; Nikolakis, V.; Ierapetritou, M. Simulation and economic analysis of 5-hydroxymethylfurfural conversion to 2,5-furandicarboxylic acid. Comput. Chem. Eng. 2013, 52, 26–34. [Google Scholar] [CrossRef]
- He, J.; Burt, S.P.; Ball, M.; Zhao, D.; Hermans, I.; Dumesic, J.A.; Huber, G.W. Synthesis of 1,6-Hexanediol from Cellulose Derived Tetrahydrofuran-Dimethanol with Pt-WOx/TiO2Catalysts. ACS Catal. 2018, 8, 1427–1439. [Google Scholar] [CrossRef]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán-Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Li, N.; Huber, G.W. Aqueous-phase hydrodeoxygenation of sorbitol with Pt/SiO2-Al2O3: Identification of reaction intermediates. J. Catal. 2010, 270, 48–59. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; De Vries, J.G.; Heeres, H.J. Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Xiao, B.; Zheng, M.; Li, X.; Pang, J.; Sun, R.; Wang, H.; Pang, X.; Wang, A.; Wang, X.; Zhang, T. Synthesis of 1,6-hexanediol from HMF over double-layered catalysts of Pd/SiO2 + Ir-ReOx/SiO2in a fixed-bed reactor. Green Chem. 2016, 18, 2175–2184. [Google Scholar] [CrossRef]
- Iriondo, A.; Mendiguren, A.; Güemez, M.B.; Requies, J.; Cambra, J.F. 2,5-DMF production through hydrogenation of real and synthetic 5-HMF over transition metal catalysts supported on carriers with different nature. Catal. Today 2017, 279, 286–295. [Google Scholar] [CrossRef]
- Vilcocq, L.; Cabiac, A.; Especel, C.; Lacombe, S.; Duprez, D. New insights into the mechanism of Sorbitol transformation over an original bifunctional catalytic system. J. Catal. 2014, 320, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, J.; Satsuma, A. Reductive Conversion of 5-Hydroxymethylfurfural in Aqueous Solutions by Furan Ring Opening and Rearrangement. In Production of Biofuels and Chemicals with Bifunctional Catalysts; Fang, Z., Richard, L.S., Jr., Li, H., Eds.; Springer: Singapore, 2017; pp. 159–185. ISBN 9789811051371. [Google Scholar]
- Chen, J.; Ge, Y.; Guo, Y.; Chen, J. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using palladium catalyst supported on mesoporous graphitic carbon nitride. J. Energy Chem. 2018, 27, 283–289. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Burt, S.P.; Ball, M.R.; Hermans, I.; Dumesic, J.A.; Huber, G.W. Catalytic C-O bond hydrogenolysis of tetrahydrofuran-dimethanol over metal supported WOx/TiO2 catalysts. Appl. Catal. B Environ. 2019, 258, 117945. [Google Scholar] [CrossRef]
- Liu, F.; Audemar, M.; De Oliveira Vigier, K.; Clacens, J.M.; De Campo, F.; Jérôme, F. Palladium/carbon dioxide cooperative catalysis for the production of diketone derivatives from carbohydrates. ChemSusChem 2014, 7, 2089–2093. [Google Scholar] [CrossRef]
- Kataoka, H.; Kosuge, D.; Ogura, K.; Ohyama, J.; Satsuma, A. Reductive conversion of 5-hydroxymethylfurfural to 1,2,6-hexanetriol in water solvent using supported Pt catalysts. Catal. Today 2020, 352, 60–65. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Sang, S.; Gao, L.; Xiao, G. Supported Cu catalysts for the hydrogenation of furfural in aqueous phase: Effect of support. Asia-Pac. J. Chem. Eng. 2017, 12, 422–431. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Conversions of Furfural to Pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mori, K.; Chen, K.; Amada, Y.; Tamura, M.; Tomishige, K. Hydrogenolysis of CO bond over Re-modified Ir catalyst in alkane solvent. Appl. Catal. A Gen. 2013, 468, 418–425. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; De Vries, J.G.; Heeres, H.J. From 5-hydroxymethylfurfural (HMF) to polymer precursors: Catalyst screening studies on the conversion of 1,2,6-hexanetriol to 1,6-hexanediol. Top. Catal. 2012, 55, 612–619. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol. Catal. Sci. Technol. 2014, 4, 2535–2549. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Kang, H.; Xia, C.; Chen, J. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 700–710. [Google Scholar] [CrossRef]
- Musci, J.J.; Merlo, A.B.; Casella, M.L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts. Catal. Today 2017, 296, 43–50. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, X.; Ren, J.; Wang, Y.; Lu, G. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst. Chem. Commun. 2011, 47, 3924–3926. [Google Scholar] [CrossRef]
- Date, N.S.; Chikate, R.C.; Roh, H.S.; Rode, C.V. Bifunctional role of Pd/MMT-K 10 catalyst in direct transformation of furfural to 1,2-pentanediol. Catal. Today 2017, 309, 195–201. [Google Scholar] [CrossRef]
- Soghrati, E.; Choong, C.; Poh, C.K.; Kawi, S.; Borgna, A. Single-Pot Conversion of Tetrahydrofurfuryl Alcohol into Tetrahydropyran over a Ni/HZSM-5 Catalyst under Aqueous-Phase Conditions. ChemCatChem 2017, 9, 1402–1408. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-pot selective conversion of furfural into 1,5-pentanediol over a Pd-added Ir-ReOx/SiO2 bifunctional catalyst. Green Chem. 2014, 16, 617–626. [Google Scholar] [CrossRef]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct transformation of furfural to 1,2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Yeh, J.; Matsagar, B.M.; Chen, S.S.; Sung, H.; Tsang, D.C.W.; Li, Y.; Wu, K.C. Synergistic Effects of Pt-embedded, MIL-53-derived Pentanediol at Near-ambient Temperature. J. Catal. 2020. [Google Scholar] [CrossRef]
- Pisal, D.S.; Yadav, G.D. Single-Step Hydrogenolysis of Furfural to 1,2-Pentanediol Using a Bifunctional Rh/OMS-2 Catalyst. ACS Omega 2019, 4, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, Z.; Liu, L. Acid catalysis dominated suppression of xylose hydrogenation with increasing yield of 1,2-pentanediol in the acid-metal dual catalyst system. Appl. Catal. A Gen. 2018, 561, 41–48. [Google Scholar] [CrossRef]
- Pavarelli, G.; Velasquez Ochoa, J.; Caldarelli, A.; Puzzo, F.; Cavani, F.; Dubois, J.L. A New Process for Maleic Anhydride Synthesis from a Renewable Building Block: The Gas-Phase Oxidehydration of Bio-1-butanol. ChemSusChem 2015, 8, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, A.; Bond, J.Q. Oxidation of levulinic acid for the production of maleic anhydride: Breathing new life into biochemicals. Green Chem. 2015, 17, 4367–4376. [Google Scholar] [CrossRef]
- Lan, J.; Lin, J.; Chen, Z.; Yin, G. Transformation of 5-Hydroxymethylfurfural (HMF) to Maleic Anhydride by Aerobic Oxidation with Heteropolyacid Catalysts. ACS Catal. 2015, 5, 2035–2041. [Google Scholar] [CrossRef]
- Lv, G.; Chen, C.; Lu, B.; Li, J.; Yang, Y.; Chen, C.; Deng, T.; Zhu, Y.; Hou, X. Vanadium-oxo immobilized onto Schiff base modified graphene oxide for efficient catalytic oxidation of 5-hydroxymethylfurfural and furfural into maleic anhydride. RSC Adv. 2016, 6, 101277–101282. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Laserna, V.; Alba-Rubio, A.C.; Mengibar, M.; Heras, A.; Mariscal, R.; Granados, M.L. Poly-(styrene sulphonic acid): An acid catalyst from polystyrene waste for reactions of interest in biomass valorization. Catal. Today 2014, 234, 285–294. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Agirrezabal-Telleria, I.; Arias, P.L.; Fierro, J.L.G.; Mariscal, R.; Granados, M.L. Aqueous-phase catalytic oxidation of furfural with H2O2: High yield of maleic acid by using titanium silicalite-1. RSC Adv. 2014, 4, 54960–54972. [Google Scholar] [CrossRef] [Green Version]
- Alba-Rubio, A.C.; Fierro, J.L.G.; León-Reina, L.; Mariscal, R.; Dumesic, J.A.; López Granados, M. Oxidation of furfural in aqueous H2O2 catalysed by titanium silicalite: Deactivation processes and role of extraframework Ti oxides. Appl. Catal. B Environ. 2017, 202, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Chen, Z.; Lin, J.; Yin, G. Catalytic aerobic oxidation of renewable furfural to maleic anhydride and furanone derivatives with their mechanistic studies. Green Chem. 2014, 16, 4351–4358. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Metal-free oxidative synthesis of succinic acid from biomass-derived furan compounds using a solid acid catalyst with hydrogen peroxide. Appl. Catal. A Gen. 2013, 458, 55–62. [Google Scholar] [CrossRef]
- Hemant, C.; Shun, N.; Kohki, E. Highly Efficient Aqueous Oxidation of Furfural to Succinic Acid Using Reusable Heterogeneous Acid Catalyst with Hydrogen Peroxide. Chem. Lett. 2012, 41, 409–411. [Google Scholar]
- Li, X.; Lan, X.; Wang, T. Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst. Catal. Today 2016, 276, 97–104. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Yuan, W.; Xia, Y.; Liu, X.; Yang, H.; Wang, H. Catalytic Aerobic Oxidation of Biomass-based Furfural into Maleic Acid in Aqueous Phase with Metalloporphyrin Catalysts. J. Chin. Chem. Soc. 2017, 64, 786–794. [Google Scholar] [CrossRef]
- Shi, S.; Guo, H.; Yin, G. Synthesis of maleic acid from renewable resources: Catalytic oxidation of furfural in liquid media with dioxygen. Catal. Commun. 2011, 12, 731–733. [Google Scholar] [CrossRef]
- Gabriele, C.; van Santen, R.A. Conclusions, Perspectives and Roadmap. In Catalysis for Renewables; Wiley-Blackwell: Weinheim, Germany, 2007; pp. 387–411. ISBN 9783527621118. [Google Scholar]
- Li, X.; Zhang, Y. The conversion of 5-hydroxymethyl furfural (HMF) to maleic anhydride with vanadium-based heterogeneous catalysts. Green Chem. 2016, 18, 643–647. [Google Scholar] [CrossRef]
- Lv, G.; Deng, L.; Lu, B.; Li, J.; Hou, X.; Yang, Y. Efficient dehydration of fructose into 5-hydroxymethylfurfural in aqueous medium over silica-included heteropolyacids. J. Clean. Prod. 2017, 142, 2244–2251. [Google Scholar] [CrossRef]
- Chai, L.; Hou, X.; Cui, X.; Li, H.; Zhang, N.; Zhang, H.; Chen, C.; Wang, Y.; Deng, T. 5-Hydroxymethylfurfural oxidation to Maleic acid by O2 over graphene oxide supported vanadium: Solvent effects and reaction mechanism. Chem. Eng. J. 2020, 388, 124187. [Google Scholar] [CrossRef]
- Jia, W.; Si, Z.; Feng, Y.; Zhang, X.; Zhao, X.; Sun, Y.; Sun, Y.; Sun, Y.; Tang, X.; Zeng, X.; et al. Oxidation of 5-[(Formyloxy)methyl]-furfural to Maleic Anhydride with Atmospheric Oxygen Using α-MnO2/Cu(NO3)2 as Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 7901–7908. [Google Scholar] [CrossRef]
- Guo, H.; Yin, G. Catalytic aerobic oxidation of renewable furfural with phosphomolybdic acid catalyst: An alternative route to maleic acid. J. Phys. Chem. C 2011, 115, 17516–17522. [Google Scholar] [CrossRef]
- Soták, T.; Hronec, M.; Gál, M.; Dobročka, E.; Škriniarová, J. Aqueous-Phase Oxidation of Furfural to Maleic Acid Catalyzed by Copper Phosphate Catalysts. Catal. Lett. 2017, 147, 2714–2723. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Granados, M.L.; Mariscal, R.; Ojeda, M. Selective conversion of furfural to maleic anhydride and furan with VOx/Al2O3 catalysts. ChemSusChem 2012, 5, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Santander, P.; Bravo, L.; Pecchi, G.; Karelovic, A. The consequences of support identity on the oxidative conversion of furfural to maleic anhydride on vanadia catalysts. Appl. Catal. A Gen. 2020, 595, 117513. [Google Scholar] [CrossRef]
- Araji, N.; Madjinza, D.D.; Chatel, G.; Moores, A.; Jérôme, F.; De Oliveira Vigier, K. Synthesis of maleic and fumaric acids from furfural in the presence of betaine hydrochloride and hydrogen peroxide. Green Chem. 2017, 19, 98–101. [Google Scholar] [CrossRef]
- Saleem, F.; Müller, P.; Eränen, K.; Warnå, J.; Yu Murzin, D.; Salmi, T. Kinetics and modelling of furfural oxidation with hydrogen peroxide over a fibrous heterogeneous catalyst: Effect of reaction parameters on yields of succinic acid. J. Chem. Technol. Biotechnol. 2017, 92, 2206–2220. [Google Scholar] [CrossRef]
- Agirre, I.; Gandarias, I.; Granados, M.L.; Arias, P.L. Process design and techno-economic analysis of gas and aqueous phase maleic anhydride production from biomass-derived furfural. Biomass Convers. Biorefin. 2019. [Google Scholar] [CrossRef]
- Milas, N.A.; Walsh, W.L. Catalytic Oxidations. I. Oxidations in the Furan Series. J. Am. Chem. Soc. 1935, 57, 1389–1393. [Google Scholar] [CrossRef]
- Rajamani, K.; Subramanian, P.; Murthy, M.S. Kinetics and Mechanism of Vapor Phase Oxidation of Furfural over Tin Vanadate Catalyst. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 232–234. [Google Scholar] [CrossRef]
- Kläusli, T. AVA Biochem: Commercialising renewable platform chemical 5-HMF. Green Process. Synth. 2014, 3, 235–236. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Zhang, Z.C.; Mao, J.; Li, S.; Yin, J.; Zhou, J. A biodiesel additive: Etherification of 5-hydroxymethylfurfural with isobutene to tert-butoxymethylfurfural. Catal. Sci. Technol. 2015, 5, 4602–4612. [Google Scholar] [CrossRef]
- Sarin, R.; Kumar, R.; Srivastav, B.; Puri, S.K.; Tuli, D.K.; Malhotra, R.K.; Kumar, A. Biodiesel surrogates: Achieving performance demands. Bioresour. Technol. 2009, 100, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Peng, L.; Yu, X.; He, L. Magnetically recyclable cellulose-derived carbonaceous solid acid catalyzed the biofuel 5-ethoxymethylfurfural synthesis from renewable carbohydrates. Fuel 2018, 219, 344–352. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Lai, L.; Zhang, Y. The production of 5-hydroxymethylfurfural from fructose in isopropyl alcohol: A green and efficient system. ChemSusChem 2011, 4, 1745–1748. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Huang, K.; Fang, Z. Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3. Fuel 2013, 113, 625–631. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, B.; Zhou, Q.; Wang, S.; Deng, K. Catalytic conversion of fructose into furans using FeCl3 as catalyst. J. Ind. Eng. Chem. 2014, 20, 644–649. [Google Scholar] [CrossRef]
- Pereira, J.G.; Sousa, S.C.A.; Fernandes, A.C. Direct Conversion of Carbohydrates into 5-Ethoxymethylfurfural (EMF) and 5-Hydroxymethylfurfural (HMF) catalyzed by Oxomolybdenum complexes. ChemistrySelect 2017, 2, 4516–4521. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Hiraga, Y.; Aida, T.M.; Smith, R.L. Efficient conversion of fructose into 5-ethoxymethylfurfural with hydrogen sulfate ionic liquids as co-solvent and catalyst. Chem. Eng. J. 2017, 314, 508–514. [Google Scholar] [CrossRef]
- Guo, H.; Duereh, A.; Hiraga, Y.; Aida, T.M.; Qi, X.; Smith, R.L. Perfect recycle and mechanistic role of hydrogen sulfate ionic liquids as additive in ethanol for efficient conversion of carbohydrates into 5-ethoxymethylfurfural. Chem. Eng. J. 2017, 323, 287–294. [Google Scholar] [CrossRef]
- Yang, Y.; Abu-Omar, M.M.; Hu, C. Heteropolyacid catalyzed conversion of fructose, sucrose, and inulin to 5-ethoxymethylfurfural, a liquid biofuel candidate. Appl. Energy 2012, 99, 80–84. [Google Scholar] [CrossRef]
- Wang, H.; Deng, T.; Wang, Y.; Qi, Y.; Hou, X.; Zhu, Y. Efficient catalytic system for the conversion of fructose into 5-ethoxymethylfurfural. Bioresour. Technol. 2013, 136, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, B.; Wang, Y.; Ren, R.; Zhang, Z. Efficient one-pot synthesis of 5-ethoxymethylfurfural from fructose catalyzed by heteropolyacid supported on K-10 clay. Fuel 2014, 117, 68–73. [Google Scholar] [CrossRef]
- Li, H.; Govind, K.S.; Kotni, R.; Shunmugavel, S.; Riisager, A.; Yang, S. Direct catalytic transformation of carbohydrates into 5-ethoxymethylfurfural with acid-base bifunctional hybrid nanospheres. Energy Convers. Manag. 2014, 88, 1245–1251. [Google Scholar] [CrossRef]
- Morales, G.; Paniagua, M.; Melero, J.A.; Iglesias, J. Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent. Catal. Today 2017, 279, 305–316. [Google Scholar] [CrossRef]
- Wang, H.; Deng, T.; Wang, Y.; Cui, X.; Qi, Y.; Mu, X.; Hou, X.; Zhu, Y. Graphene oxide as a facile acid catalyst for the one-pot conversion of carbohydrates into 5-ethoxymethylfurfural. Green Chem. 2013, 15, 2379–2383. [Google Scholar] [CrossRef]
- Collignon, F.; Loenders, R.; Martens, J.A.; Jacobs, P.A.; Poncelet, G. Liquid phase synthesis of MTBE from methanol and isobutene over acid zeolites and amberlyst-15. J. Catal. 1999, 182, 302–312. [Google Scholar] [CrossRef]
- Guzmán, I.; Heras, A.; Güemez, M.B.; Iriondo, A.; Cambra, J.F.; Requies, J. Levulinic Acid Production Using Solid-Acid Catalysis. Ind. Eng. Chem. Res. 2016, 55, 5139–5144. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Fan, C.; Guan, H.; Zhang, H.; Wang, J.; Wang, S.; Wang, X. Conversion of fructose and glucose into 5-hydroxymethylfurfural catalyzed by a solid heteropolyacid salt. Biomass Bioenergy 2011, 35, 2659–2665. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Zhao, S.; Wang, X.; Wang, S. High selective production of 5-hydroymethylfurfural from fructose by a solid heteropolyacid catalyst. Fuel 2011, 90, 2289–2293. [Google Scholar] [CrossRef]
- Cheng, M.; Shi, T.; Wang, S.; Guan, H.; Fan, C.; Wang, X. Fabrication of micellar heteropolyacid catalysts for clean production of monosaccharides from polysaccharides. Catal. Commun. 2011, 12, 1483–1487. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.K.; Park, D.R.; Park, S.; Yi, J.; Song, I.K. Etherification of n-butanol to di-n-butyl ether over H3PMo12-XWxO40(x = 0, 3, 6, 9, 12) Keggin and H6P2Mo18-XWxO62(x = 0, 3, 9, 15, 18) Wells-Dawson heteropolyacid catalysts. Catal. Commun. 2011, 14, 48–51. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, W.; Xie, H.; Zhao, Z.K. An unexpected reaction between 5-hydroxymethylfurfural and imidazolium-based ionic liquids at high temperatures. Molecules 2011, 16, 8463–8474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.K.R.; Lund, C.R.F. Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
| Catalyst | T (°C) | P (MPa) | Time (h) | Solvent | Raw Material | Target Product (TP) | χ (%) | STP (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 0.3Ni2.7CoAl | 120 | 4 | 4 | MeOH | HMF | HT | 100 | 14.8 y | [27] |
| 0.5Ni2.5CoAl | 37.4 y | ||||||||
| 0.9Ni2.1CoAl | 23.3 y | ||||||||
| 0.5Ni2.5CoAl | 8 | 54.4 y | |||||||
| 0.5Ni2.5CoAl | 12 | 64.5 y | |||||||
| 0.5Ni2.5CoAl | 6 | 8 | ≈42.5 y | ||||||
| 0.5Ni2.5CoAl | 140 | 4 | 41.9 y | ||||||
| Rh-ReOx/SiO2 a | 120 | 8 | 4 | H2O | DHMTHF | HT | 31 | 84 | [26] |
| Rhb-ReOx/SiO2 a | 24 | 84 | |||||||
| Rh-ReOx/SiO2 c | 14 | 85 | |||||||
| Rh-ReOx/Nb2O5 | 17 | 95 | |||||||
| Rh-Pt/SiO2 a Rh-Pd/SiO2 a | 1 | 0 | |||||||
| 1 | 0 | ||||||||
| Rh-ReOx/SiO2 a | 20 | 53 | 76 | ||||||
| Rh-ReOx/SiO2 a | 180 | 88 | 34 | ||||||
| Pt-WOx/TiO2 | 160 | 5.5 | 8 | H2O | DHMTHF | HT | 23 | 95 | [41] |
| Unsupported Ru | 130 | 3 | 1 | H2O/ 1-BuOH | HMF | DHMTHF | 100 | 53 (41 e) | [42] |
| Unsupported Ru | 2 | 100 | 46 (37 e) | ||||||
| Ru/C | 2 | 100 | 56 (38 e) | ||||||
| Ru/CeO2 | 6 | 100 | 48 (15 e) | ||||||
| Ru/MgO-ZrO2 | 20 | 100 | 88 (3 e) | ||||||
| Ru/γ-Al2O3 | 12 | 100 | 89 (10 e) | ||||||
| Unsupported Ru d | n.a. | 100 | 75 (12 e) | ||||||
| Unsupported Ru | 2 | H2O | 100 | 22 (50 e) | |||||
| Unsupported Ru | 2 | THF/H2O | 100 | 50 (28 e) |
| Catalyst | T (°C) | P (MPa) | Time (h) | Solvent Reactor | Raw Material | Target Product | χ (%) | YHD (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pd-Ir-ReOx/SiO2 a | 100 | 3 | n.a. | H2O/THF FIXED BED | HMF | HD | 100 | 19.1 | [45] |
| Pd-Ir-ReOx/SiO2 b | 14.6 | ||||||||
| Ir-ReOx/SiO2 c | 15.2 | ||||||||
| Pd/SiO2 + Ir-ReOx/SiO2 d,g | 28.7 | ||||||||
| Pd/SiO2 + Ir-ReOx/SiO2 e,g | 39.4 | ||||||||
| Pd/SiO2 + Ir-ReOx/SiO2 f,g | 46.2 | ||||||||
| Pd/SiO2 + Ir-ReOx/SiO2 c,g | 46.0 | ||||||||
| Pd/SiO2 + Ir-ReOx/SiO2 c,g | 7 | 57.8 | |||||||
| 7%Pd/ZrP | 140 | - | 21 | FOA/EtOH BATCH | HMF | HD | 96.9 | 42.5 | [55] |
| Pd/HY | 70.4 | 27.4 | |||||||
| Pd/Nb2O5 | 93.3 | 20.7 | |||||||
| Pd/ZSM5 | 90.4 | 17.3 | |||||||
| Pt-WOx/TiO2 h | 160 | 5.5 | 8 | H2O BATCH | HMF | HD | ≈23 | 90 s | [41] |
| Pt-WOx/TiO2 i | 100 | 70 s | |||||||
| Pt-WOx/TiO2 i | 3.5 | H2O FIXED BED | ≈23 | 60 s | |||||
| Rh-ReOx/SiO2 | 180 | 8 | 3 | H2O BATCH | HT | HD | 17 | 73 s | [57] |
| Rh-ReOx/SiO2 + γ-Al2O3 | 22 | 69 s | |||||||
| Rh-ReOx/SiO2 − γ-Al2O3 | 7 | 66 s | |||||||
| Rh-ReOx/SiO2 − γ-Al2O3 | 20 | 20 | 76 s |
| Catalyst | T (°C) | Time (h) | P (MPa) | Solvent | Raw Material | Target Product | χ (%) | YPD (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pd(0.66)-Ir-ReOx/SiO2 | 40–100 | 8–72 | 6 | H2O | FF | 1,5-PD | 100 | 71.4 | [64] |
| 30–160 | 2–24 | 100 | 60.3 | ||||||
| 40–160 | 2–24 | 100 | 63.2 | ||||||
| 40–120 | 2–24 | 2 | 100 | 11.1 | |||||
| Rh(0.66)-Ir-ReOx/SiO2 | 30–100 | 8–24 | 6 | H2O | FF | 1,5-PD | 100 | 66.4 | [58] |
| 40–140 | 8–24 | 100 | 37.7 | ||||||
| 40–100 | 8–24 | 100 | 65.8 | ||||||
| 8 | 100 | 71.1 | |||||||
| Ni/HZSM-5 | 250 | 4 | 3.4 | H2O | THFA | 1,5-PD | 17 | 36.6 s | [63] |
| Ni/SiO2 | 7.9 | 33.1 s | |||||||
| Ni/Al2O3 | 6.1 | 14.7 s | |||||||
| 10Cu/Al2O3 | 140 | 6 | 8 | EtOH | FFA | 1,5-PD | 60.4 | 22.7 s (71.3 e) | [59] |
| 10Cu/Al2O3 a | 85.5 | 22.2 s (70 e) | |||||||
| Pt/hydrotalcite | 150 | 3 | 8 | 2-PrOH | FF | 1,2-PD | 100 | 73 s (81 e) | [65] |
| 100 | 100 | 43 s (56 e) | |||||||
| 150 | H2O | 100 | 18 s (24 e) | ||||||
| EtOH | 100 | 71 s (80 e) | |||||||
| Pt/Al2O3 + NaBH4 | 45 | 8 | 0.45 | H2O | FF | 1,5-PD | 100 | 18.0 | [66] |
| MIL-53-AI-NH2 + NaBH4 | 30.1 | ||||||||
| Pt/Al2O3-MIL-53-AI-NH2 + NaBH4 | 75.2 | ||||||||
| 3Pd/MMT | 220 | 3.5 | 5 | IPA | FF | 1,2-PD | 100 | 66 s (68 f) | [62] |
| Ru/OMS-2 | 165 | 8 | 3 | MeOH | FF | 1,2-PD | ≥99 | 87 | [67] |
| Pd/OMS-2 | 76 | ||||||||
| NbPO (synthesized at pH = 7) | 150 | 3 | 4 | H2O/ GVL-Cyclo | xylose | 1,2-PD | 100 | 19.1 s (25.6 f) | [68] |
| NbPO (synthesized at pH = 5) | 100 | 17.5 s (22.6 f) | |||||||
| Nb2O5 | 100 | 6.5 s (8.8 f) |
| Catalyst | Solvent | Feed | T (°C) | YEMF (%) | Ref. |
|---|---|---|---|---|---|
| Z-SBA-15 | THF | HMF | 140 | 76 | [115] |
| H3PW12O40 | THF | HMF | 130 | 76 | [107] |
| Phosphotungstic acid | DMSO | Fructose | 140 | 64 | [108] |
| Silica sulfate | DMSO | HMF | 100 | 83.8 | [3] |
| Silica sulfate | Fructose | 63.1 | |||
| Silica sulfate | Sucrose | 34.9 | |||
| Pr-SO3H-SBA-15 | DMSO | HMF | 116 | 63.4 | [111] |
| Graphene oxides | DMSO | HMF | 100 | 92 | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo, A.; Agirre, I.; Viar, N.; Requies, J. Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds. Catalysts 2020, 10, 895. https://doi.org/10.3390/catal10080895
Iriondo A, Agirre I, Viar N, Requies J. Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds. Catalysts. 2020; 10(8):895. https://doi.org/10.3390/catal10080895
Chicago/Turabian StyleIriondo, Aitziber, Ion Agirre, Nerea Viar, and Jesús Requies. 2020. "Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds" Catalysts 10, no. 8: 895. https://doi.org/10.3390/catal10080895
APA StyleIriondo, A., Agirre, I., Viar, N., & Requies, J. (2020). Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds. Catalysts, 10(8), 895. https://doi.org/10.3390/catal10080895