Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview
Abstract
:1. Introduction and Scope of Review
2. Possible Routes for Hydrogen Production via Processing of Methane
3. Oxy-Steam Reforming of the Methane Reaction Mechanism
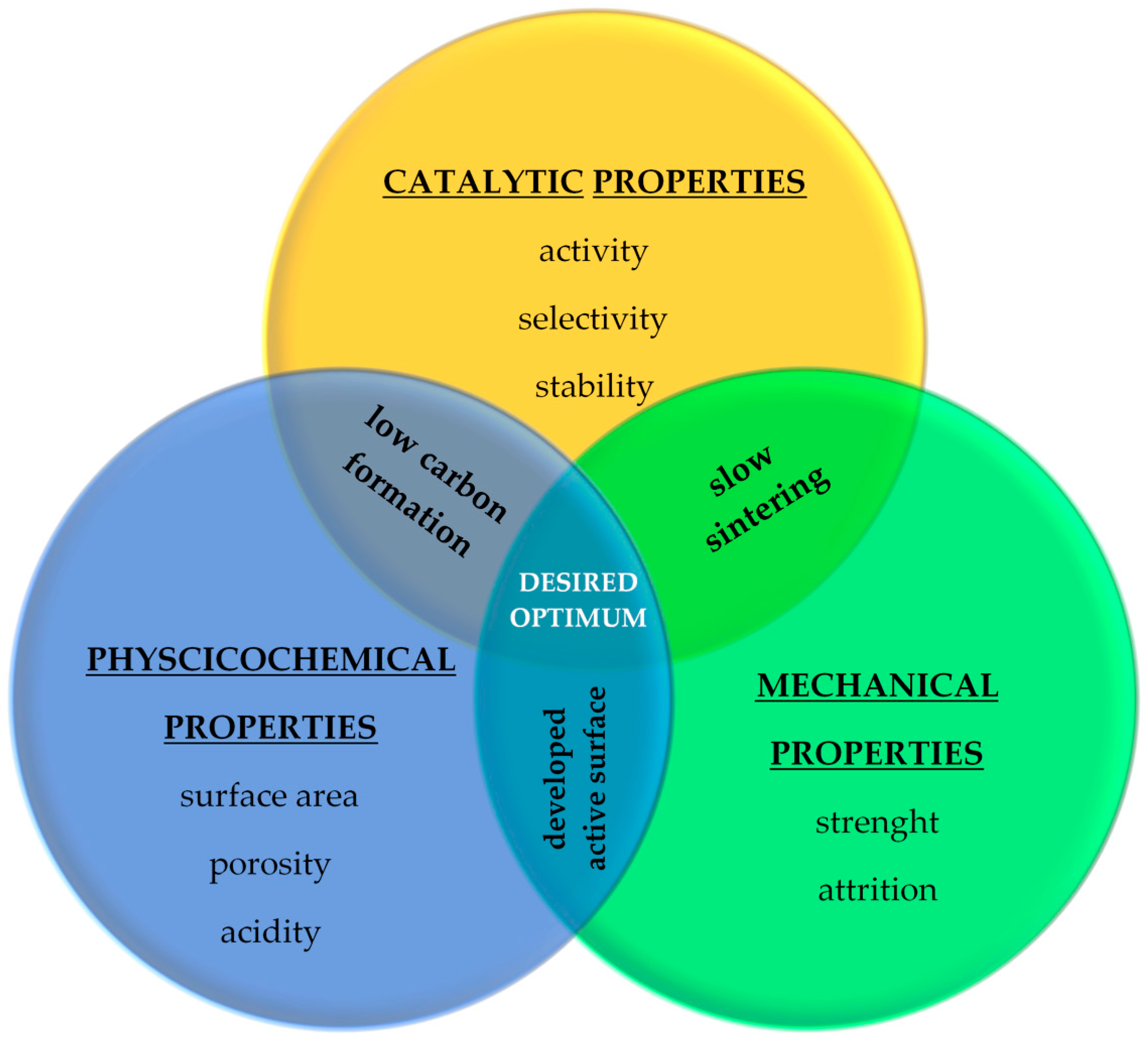
4. The Catalytic Material Used in the Oxy-Steam Reforming of Methane/LNG Process
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Madej-Lachowska, M.; Kulawska, M.; Hamryszak, L. Kinetic studies on steam reforming of methanol over Cu/Zn/Zr/Ce/Cr catalyst. Przem. Chem. 2016, 95, 2281–2284. [Google Scholar] [CrossRef]
- Deczyński, J.; Żółtowski, B. WodÓr Jako Paliwo Alternatywne Do Zasilania SilnikÓw Ze Spalaniem WewnĘtrznym. Studia Mater. Pol. Stowarzyszenia Zarz. Wiedza/Stud. Proc. Pol. Assoc. Knowl. Manag. 2014, 69, 18–31. [Google Scholar]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Mierczynski, P. Comparative Studies of Bimetallic Ru–Cu, Rh–Cu, Ag–Cu, Ir–Cu Catalysts Supported on ZnO–Al2O3, ZrO2–Al2O3 Systems. Catal. Lett. 2016, 146, 1825–1837. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P.; Vasilev, K. MWCNTs as a catalyst in oxy-steam reforming of methanol. Rsc Adv. 2016, 6, 81408–81413. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Nowosielska, M.; Kubicki, J.; Maniukiewicz, W.; Czylkowska, A.; Maniecki, T.P. Monometallic copper catalysts supported on multi-walled carbon nanotubes for the oxy-steam reforming of methanol. React. Kinet. Mech. Catal. 2016, 117, 675–691. [Google Scholar] [CrossRef]
- Appleby, A.J. Fuel cell technology: Status and future prospects. Energy 1996, 21, 521–653. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.; Kim, S.; Oh, J.; Choi, S.; Bae, M.; Kang, I.; Katikaneni, S.P. Liquid fuel processing for hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 19990–20022. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Reversible solid oxide fuel cells (R-SOFCs) with chemically stable proton-conducting oxides. Solid State Ion. 2015, 275, 101–105. [Google Scholar] [CrossRef]
- Cheekatamarla, P.K.; Finnerty, C.M. Reforming catalysts for hydrogen generation in fuel cell applications. J. Power Sources 2006, 160, 490–499. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Vanbroekhoven, K.; Pant, D. Techno-productive potential of photosynthetic microbial fuel cells through different configurations. Renew. Sustain. Energy Rev. 2014, 39, 617–627. [Google Scholar] [CrossRef]
- Eveloy, V. Numerical analysis of an internal methane reforming solid oxide fuel cell with fuel recycling. Appl. Energy 2012, 93, 107–115. [Google Scholar] [CrossRef]
- Fu, X.-Z.; Melnik, J.; Low, Q.-X.; Luo, J.-L.; Chuang, K.T.; Sanger, A.R.; Yang, Q.-M. Surface modified Ni foam as current collector for syngas solid oxide fuel cells with perovskite anode catalyst. Int. J. Hydrog. Energy 2010, 35, 11180–11187. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S. Bio-ethanol, a suitable fuel to produce hydrogen for a molten carbonate fuel cell. J. Power Sources 2007, 173, 200–209. [Google Scholar] [CrossRef]
- Geissler, K.; Newson, E.; Vogel, F.; Truong, T.-B.; Hottinger, P.; Wokaun, A. Autothermal methanol reforming for hydrogen production in fuel cell applications. Phys. Chem. Chem. Phys. 2001, 3, 289–293. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Zheng, T.; Liao, M.; Shao, Z. Nickel zirconia cerate cermet for catalytic partial oxidation of ethanol in a solid oxide fuel cell system. Int. J. Hydrog. Energy 2012, 37, 8603–8612. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J. Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications. Int. J. Hydrog. Energy 2001, 26, 923–933. [Google Scholar] [CrossRef]
- Rosen, M.A. Thermodynamic investigation and comparison of selected production processes for hydrogen and hydrogen-derived fuels. Energy 1996, 21, 1079–1094. [Google Scholar] [CrossRef]
- Demusiak, G. Otrzymywanie paliwa wodorowego metodą reformowania gazu ziemnego dla ogniw paliwowych małej mocy. Naft. Gaz 2012, 68, 661–673. [Google Scholar]
- Muritala, I.K.; Guban, D.; Roeb, M.; Sattler, C. High temperature production of hydrogen: Assessment of non-renewable resources technologies and emerging trends. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Sustainable hydrogen production options and the role of IAHE. Int. J. Hydrog. Energy 2012, 37, 16266–16286. [Google Scholar] [CrossRef]
- Park, S.; Yoo, J.; Han, S.J.; Song, J.H.; Lee, E.J.; Song, I.K. Steam reforming of liquefied natural gas (LNG) for hydrogen production over nickel–boron–alumina xerogel catalyst. Int. J. Hydrog. Energy 2017, 42, 15096–15106. [Google Scholar] [CrossRef]
- Ma, L.; Geng, J.; Li, W.; Liu, P.; Li, Z. The development of natural gas as an automotive fuel in China. Energy Policy 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Bernatík, A.; Senovsky, P.; Pitt, M. LNG as a potential alternative fuel—Safety and security of storage facilities. J. Loss Prev. Process Ind. 2011, 24, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Tejada, J.L.; Llera-Sastresa, E.; Scarpellini, S. LNG: An alternative fuel for road freight transport in Europe. In Sustainable Development; Wessex Institute of Technology: Southampton, UK, 2015; pp. 235–246. [Google Scholar] [CrossRef] [Green Version]
- Atsonios, K.; Samlis, C.; Manou, K.; Nikolopoulos, A.; Sfetsioris, K.; Mitsotakis, A.; Grammelis, P. Technical assessment of LNG based polygeneration systems for non-interconnected island cases using SOFC. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Strantzali, E.; Aravossis, K.; Livanos, G.A. Evaluation of future sustainable electricity generation alternatives: The case of a Greek island. Renew. Sustain. Energy Rev. 2017, 76, 775–787. [Google Scholar] [CrossRef]
- Bang, Y.; Park, S.; Han, S.J.; Yoo, J.; Song, J.H.; Choi, J.H.; Kang, K.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni/Al2O3 catalyst prepared by an EDTA-assisted impregnation method. Appl. Catal. B Environ. 2016, 180, 179–188. [Google Scholar] [CrossRef]
- Yoo, J.; Bang, Y.; Han, S.J.; Kang, T.H.; Lee, J.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous alkaline earth metal-promoted nickel-alumina xerogel catalysts. J. Mol. Catal. A Chem. 2013, 380, 28–33. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Song, I.K. Hydrogen production by steam reforming of LNG over Ni/Al2O3–ZrO2 catalysts: Effect of Al2O3–ZrO2 supports prepared by a grafting method. J. Mol. Catal. A Chem. 2007, 268, 9–14. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells. In Fuel Cell Technology: Reaching Towards Commercialization; Sammes, N., Ed.; Springer: London, UK, 2006; pp. 27–51. [Google Scholar]
- Park, S.; Bang, Y.; Han, S.J.; Yoo, J.; Song, J.H.; Song, J.C.; Lee, J.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous nickel–iron–alumina catalyst. Int. J. Hydrog. Energy 2015, 40, 5869–5877. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Hou, Q. Proposal and thermodynamic performance study of a novel LNG-fueled SOFC-HAT-CCHP system with near-zero CO2 emissions. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Bang, Y.; Seo, J.G.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni–La–Al2O3 aerogel catalysts: Effect of La content. Int. J. Hydrog. Energy 2011, 36, 8307–8315. [Google Scholar] [CrossRef]
- Mukainakano, Y.; Yoshida, K.; Kado, S.; Okumura, K.; Kunimori, K.; Tomishige, K. Catalytic performance and characterization of Pt–Ni bimetallic catalysts for oxidative steam reforming of methane. Chem. Eng. Sci. 2008, 63, 4891–4901. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Miyao, T.; Naito, S.; Okumura, K.; Kunimori, K.; Tomishige, K. Catalytic performance and characterization of Pd/Ni0.2Mg0.8Al2O4 in oxidative steam reforming of methane under atmospheric and pressurized conditions. Appl. Catal. A Gen. 2007, 325, 154–162. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Hashemipour rafsanjani, H.; Talebizadeh, A.R. Methane oxy-steam reforming over a highly efficient Ni/Al2O3 nanocatalyst prepared by microwave-assisted impregnation method. Iran. J. Chem. Eng. (Ijche) 2017, 14, 3–16. [Google Scholar]
- Tomishige, K. Oxidative Steam Reforming of Methane over Ni Catalysts Modified with Noble Metals. J. Jpn. Pet. Inst. 2007, 50, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Park, S.-E.; Baek, Y.-S. Highly stable Ni catalyst supported on Ce–ZrO2 for oxy-steam reforming of methane. Catal. Lett. 2001, 74, 31–36. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Yoo, J.; Choi, J.H.; Lee, J.K.; Song, J.H.; Lee, J.; Song, I.K. Hydrogen production by steam reforming of simulated liquefied natural gas (LNG) over nickel catalyst supported on mesoporous phosphorus-modified alumina xerogel. Appl. Catal. B Environ. 2014, 148, 269–280. [Google Scholar] [CrossRef]
- Bang, Y.; Park, S.; Han, S.J.; Yoo, J.; Choi, J.H.; Kang, T.H.; Lee, J.; Song, I.K. Hydrogen Production by Steam Reforming of Liquefied Natural Gas (LNG) Over Nickel-Phosphorus-Alumina Xerogel Catalyst Prepared by a Carbon-Templating Epoxide-Driven Sol-Gel Method. J. Nanosci. Nanotechnol. 2016, 16, 4605–4611. [Google Scholar] [CrossRef]
- Pino, L.; Recupero, V.; Beninati, S.; Shukla, A.; Hegde, M.; Bera, P. Catalytic partial-oxidation of methane on a ceria-supported platinum catalyst for application in fuel cell electric vehicles. Appl. Catal. A Gen. 2002, 225, 63–75. [Google Scholar] [CrossRef]
- Boudjeloud, M.; Boulahouache, A.; Rabia, C.; Salhi, N. La-doped supported Ni catalysts for steam reforming of methane. Int. J. Hydrog. Energy 2019, 44, 9906–9913. [Google Scholar] [CrossRef]
- Al-Ubaid, A.; Wolf, E.E. Steam reforming of methane on reduced non-stoichiometric nickel aluminate catalysts. Appl. Catal. 1988, 40, 73–85. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Methane steam reforming over Ni/Ce–ZrO2 catalyst: Influences of Ce–ZrO2 support on reactivity, resistance toward carbon formation, and intrinsic reaction kinetics. Appl. Catal. A Gen. 2005, 290, 200–211. [Google Scholar] [CrossRef]
- de Lima, S.M.; da Cruz, I.O.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Pt/CeZrO2 catalyst. J. Catal. 2008, 257, 356–368. [Google Scholar] [CrossRef]
- Yang, X.; Da, J.; Yu, H.; Wang, H. Characterization and performance evaluation of Ni-based catalysts with Ce promoter for methane and hydrocarbons steam reforming process. Fuel 2016, 179, 353–361. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Nascente, P.A.P.; Assaf, E.M. Partial oxidation of methane on NiO–MgO–ZrO2 catalysts. Fuel 2012, 97, 630–637. [Google Scholar] [CrossRef]
- Eriksson, S.; Rojas, S.; Boutonnet, M.; Fierro, J.L.G. Effect of Ce-doping on Rh/ZrO2 catalysts for partial oxidation of methane. Appl. Catal. A Gen. 2007, 326, 8–16. [Google Scholar] [CrossRef]
- Dong, W.-S.; Jun, K.-W.; Roh, H.-S.; Liu, Z.-W.; Park, S.-E. Comparative Study on Partial Oxidation of Methane over Ni/ZrO2, Ni/CeO2 and Ni/Ce–ZrO2 Catalysts. Catal. Lett. 2002, 78, 215–222. [Google Scholar] [CrossRef]
- Lødeng, R.; Bjørgum, E.; Enger, B.C.; Eilertsen, J.L.; Holmen, A.; Krogh, B.; Rønnekleiv, M.; Rytter, E. Catalytic partial oxidation of CH4 to H2 over cobalt catalysts at moderate temperatures. Appl. Catal. A Gen. 2007, 333, 11–23. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Ran, R.; Park, H.J.; Kwak, C.; Shao, Z. Physically mixed LiLaNi–Al2O3 and copper as conductive anode catalysts in a solid oxide fuel cell for methane internal reforming and partial oxidation. Int. J. Hydrog. Energy 2011, 36, 5632–5643. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş.; Özensoy, E.; Aksoylu, A.E. The effect of impregnation strategy on methane dry reforming activity of Ce promoted Pt/ZrO2. Int. J. Hydrog. Energy 2009, 34, 9711–9722. [Google Scholar] [CrossRef] [Green Version]
- Bachiller-Baeza, B.; Mateos-Pedrero, C.; Soria, M.A.; Guerrero-Ruiz, A.; Rodemerck, U.; Rodríguez-Ramos, I. Transient studies of low-temperature dry reforming of methane over Ni-CaO/ZrO2-La2O3. Appl. Catal. B Environ. 2013, 129, 450–459. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Hassani Rad, S.J.; Haghighi, M.; Alizadeh Eslami, A.; Rahmani, F.; Rahemi, N. Sol–gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrog. Energy 2016, 41, 5335–5350. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; La Parola, V.; Venezia, A.M.; Collard, X.; Aprile, C.; Liotta, L.F. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MOx (M=Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B Environ. 2014, 156–157, 350–361. [Google Scholar] [CrossRef]
- Vella, L.D.; Specchia, S. Alumina-supported nickel catalysts for catalytic partial oxidation of methane in short-contact time reactors. Catal. Today 2011, 176, 340–346. [Google Scholar] [CrossRef]
- Vella, L.D.; Villoria, J.A.; Specchia, S.; Mota, N.; Fierro, J.L.G.; Specchia, V. Catalytic partial oxidation of CH4 with nickel–lanthanum-based catalysts. Catal. Today 2011, 171, 84–96. [Google Scholar] [CrossRef]
- Maniecki, T.P.; Bawolak, K.; Gebauer, D.; Mierczynski, P.; Jozwiak, W.K. Catalytic activity and physicochemical properties of Ni-Au/Al3CrO6 system for partial oxidation of methane to synthesis gas. Kinet. Catal. 2009, 50, 138–144. [Google Scholar] [CrossRef]
- Maniecki, T.P.; Bawolak, K.; Mierczyński, P.; Jozwiak, W.K. Development of Stable and Highly Active Bimetallic Ni–Au Catalysts Supported on Binary Oxides CrAl3O6 for POM Reaction. Catal. Lett. 2008, 128, 401. [Google Scholar] [CrossRef]
- Maniecki, T.; Bawolak, K.; Mierczynski, P.; Kaczorowski, P.; Jozwiak, W. The effect of the nature of the support on catalytic properties of ruthenium supported catalysts in partial oxidation of methane to syn-gas. Kinet. Catal. 2011, 52, 711–715. [Google Scholar] [CrossRef]
- Pańczyk, M.; Borowiecki, T. Otrzymywanie i zastosowanie gazu syntezowego. W: Adsorbenty i katalizatory: Wybrane technologie a środowisko. Red. J. Univ. Rzesz. 2012, 275–287. [Google Scholar]
- Wiącek, D. Wodór jako paliwo przyszłości. Autobusy Tech. Eksploat. Syst. Transp. 2011, 12, 446–452. [Google Scholar]
- Pino, L.; Vita, A.; Cordaro, M.; Recupero, V.; Hegde, M.S. A comparative study of Pt/CeO2 catalysts for catalytic partial oxidation of methane to syngas for application in fuel cell electric vehicles. Appl. Catal. A Gen. 2003, 243, 135–146. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. In Catalysis: Science and Technology Volume 5; Anderson, J.R., Boudart, M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1984; pp. 1–117. [Google Scholar]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Millot, Y.; Valentin, L.; Casale, S.; Dzwigaj, S. Fischer-Tropsch reaction on Co-containing microporous and mesoporous Beta zeolite catalysts: The effect of porous size and acidity. Catal. Today 2019. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt based catalysts supported on two kinds of beta zeolite for application in fischer-tropsch synthesis. Catalysts 2019, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Mierczynski, P.; Dawid, B.; Maniukiewicz, W.; Mosinska, M.; Zakrzewski, M.; Ciesielski, R.; Kedziora, A.; Dubkov, S.; Gromov, D.; Rogowski, J.; et al. Fischer–Tropsch synthesis over various Fe/Al2O3–Cr2O3 catalysts. React. Kinet. Mech. Catal. 2018, 124, 545–561. [Google Scholar] [CrossRef] [Green Version]
- Chalupka, K.A.; Maniukiewicz, W.; Mierczynski, P.; Maniecki, T.; Rynkowski, J.; Dzwigaj, S. The catalytic activity of Fe-containing SiBEA zeolites in Fischer–Tropsch synthesis. Catal. Today 2015, 257 Pt 1, 117–121. [Google Scholar] [CrossRef]
- Maniecki, T.; Stadnichenko, A.; Maniukiewicz, W.; Bawolak, K.; Mierczynski, P.; Boronin, A.; Jozwiak, W. An active phase transformation on surface of Ni-Au/Al2O3 catalyst during partial oxidation of methane to synthesis gas. Kinet. Catal. 2010, 51, 573–578. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In Situ Infrared Study of Methanol Synthesis from H2/CO over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1998, 178, 153–173. [Google Scholar] [CrossRef]
- Abbaslou, R.M.M.; Tavassoli, A.; Soltan, J.; Dalai, A.K. Iron catalysts supported on carbon nanotubes for Fischer–Tropsch synthesis: Effect of catalytic site position. Appl. Catal. A Gen. 2009, 367, 47–52. [Google Scholar] [CrossRef]
- Albuquerque, J.S.; Costa, F.O.; Barbosa, B.V.S. Fischer–Tropsch Synthesis: Analysis of Products by Anderson–Schulz–Flory Distribution Using Promoted Cobalt Catalyst. Catal. Lett. 2019, 149, 831–839. [Google Scholar] [CrossRef]
- Bahome, M.C.; Jewell, L.L.; Hildebrandt, D.; Glasser, D.; Coville, N.J. Fischer–Tropsch synthesis over iron catalysts supported on carbon nanotubes. Appl. Catal. A Gen. 2005, 287, 60–67. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W.; Van der Kraan, A.M.; Van Dijk, W.L.; Van der Baan, H.S. Behavior of metallic iron catalysts during Fischer-Tropsch synthesis studied with Moessbauer spectroscopy, x-ray diffraction, carbon content determination, and reaction kinetic measurements. J. Phys. Chem. 1980, 84, 3363–3370. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch Synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Iablokov, V.; Kruse, N. Discovery of a Fischer-Tropsch Hybrid Reaction: Hydrogenation of Methylformate to Long-Chain Hydrocarbons with Anderson-Schulz-Flory Chain Length Distribution. ChemCatChem 2019, 11, 1200–1204. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Mitigation of Climate Change: Working Group III Contribution to the IPCC Fifth Assessment Report; Cambridge University Press: Cambridge, UK, 2015.
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Piña, J.; El Solh, T.; de Lasa, H.I. Coke Formation over a Nickel Catalyst under Methane Dry Reforming Conditions: Thermodynamic and Kinetic Models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts I. Catalyst characterization and activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Liu, Z.-W.; Jun, K.-W.; Park, S.-E. Hydrogen Production from Methane Reforming Reactions over Ni/MgO Catalyst. Bull. Korean Chem. Soc. 2001, 22, 1323–1327. [Google Scholar]
- Peela, N.R.; Kunzru, D. Oxidative steam reforming of ethanol over Rh based catalysts in a micro-channel reactor. Int. J. Hydrog. Energy 2011, 36, 3384–3396. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Zaborowski, M.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska, M.I.; Maniecki, T.P. Novel Pd-Cu/ZnAl2O4-ZrO2 Catalysts for Methanol Synthesis. Catal. Lett. 2014, 144, 723–735. [Google Scholar] [CrossRef]
- Bell, A.T. Molecular Design of Highly Active Methanol Synthesis Catalysts. In Studies in Surface Science and Catalysis; Iglesia, E., Spivey, J.J., Fleisch, T.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 136, pp. 13–19. [Google Scholar]
- Fan, L.; Fujimoto, K. Reaction Mechanism of Methanol Synthesis from Carbon Dioxide and Hydrogen on Ceria-Supported Palladium Catalysts with SMSI Effect. J. Catal. 1997, 172, 238–242. [Google Scholar] [CrossRef]
- Larrubia Vargas, M.A.; Busca, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Patrono, P.; Pinzari, F.; Ramis, G. An IR study of methanol steam reforming over ex-hydrotalcite Cu–Zn–Al catalysts. J. Mol. Catal. A Chem. 2007, 266, 188–197. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, Q.; Li, W.; Xu, H. Methanol synthesis from sulfur-containing syngas over Pd/CeO2 catalyst. Appl. Catal. B Environ. 2009, 90, 99–104. [Google Scholar] [CrossRef]
- Pasupulety, N.; Driss, H.; Alhamed, Y.A.; Alzahrani, A.A.; Daous, M.A.; Petrov, L. Studies on Au/Cu–Zn–Al catalyst for methanol synthesis from CO2. Appl. Catal. A Gen. 2015, 504, 308–318. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Liu, X.; Pan, X.; Pei, G.; Huang, Y.; Wang, X.; Zhang, T.; Geng, H. Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst structure dependence of methanol selectivity. Appl. Catal. A Gen. 2016, 514, 51–59. [Google Scholar] [CrossRef]
- Jens, R.-N. Concepts in Syngas Manufacture; World Scientific: Haldor Topsoe A/S, Denmark, 2011; Volume 10. [Google Scholar] [CrossRef]
- Makvandi, S.; Alavi, S. COx free hydrogen production by catalytic decomposition of methane over porous Ni/Al2O3 catalysts. Iran. J. Chem. Eng. 2011, 8, 24–33. [Google Scholar]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Palma, S.; Ivanova, S.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Steam reforming of methanol over supported Ni and Ni–Sn nanoparticles. Int. J. Hydrog. Energy 2013, 38, 6646–6656. [Google Scholar] [CrossRef]
- Kurzina, I.A.; Reshetnikov, S.I.; Karakchieva, N.I.; Kurina, L.N. Direct synthesis of dimethyl ether from synthesis gas: Experimental study and mathematical modeling. Chem. Eng. J. 2017, 329, 135–141. [Google Scholar] [CrossRef]
- Dong, X.; Liang, X.-L.; Li, H.-Y.; Lin, G.-D.; Zhang, P.; Zhang, H.-B. Preparation and characterization of carbon nanotube-promoted Co–Cu catalyst for higher alcohol synthesis from syngas. Catal. Today 2009, 147, 158–165. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Liu, Z.-M.; Lin, G.-D.; Zhang, H.-B. Pd/CNT-promoted CuZrO2/HZSM-5 hybrid catalysts for direct synthesis of DME from CO2/H2. Appl. Catal. A Gen. 2013, 451, 28–35. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B Environ. 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Mosinska, M.; Stępińska, N.; Maniukiewicz, W.; Rogowski, J.; Mierczynska-Vasilev, A.; Vasilev, K.; Szynkowska, M.I.; Mierczynski, P. Hydrogen Production on Cu-Ni Catalysts via the Oxy-Steam Reforming of Methanol. Catalysts 2020, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Haynes, D.J.; Shekhawat, D. Chapter 6—Oxidative Steam Reforming. In Fuel Cells: Technologies for Fuel Processing; Shekhawat, D., Spivey, J.J., Berry, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 129–190. [Google Scholar]
- Rabenstein, G.; Hacker, V. Hydrogen for fuel cells from ethanol by steam-reforming, partial-oxidation and combined auto-thermal reforming: A thermodynamic analysis. J. Power Sources 2008, 185, 1293–1304. [Google Scholar] [CrossRef]
- Neiva, A.; Gama, A. A study on the characteristics of the reforming of methane: A review. Braz. J. Pet Gas 2010, 4. [Google Scholar] [CrossRef] [Green Version]
- Herrera Delgado, K.; Maier, L.; Tischer, S.; Zellner, A.; Stotz, H.; Deutschmann, O. Surface Reaction Kinetics of Steam- and CO2-Reforming as Well as Oxidation of Methane over Nickel-Based Catalysts. Catalysts 2015, 5, 871–904. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Maruyama, K.; Nurunnabi, M.; Kunimori, K.; Tomishige, K. Temperature profiles of alumina-supported noble metal catalysts in autothermal reforming of methane. Appl. Catal. A Gen. 2004, 275, 157–172. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Performance of Pt/CeO2 catalyst for propane oxidative steam reforming. Appl. Catal. A Gen. 2006, 306, 68–77. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Passos, F.B.; Bueno, J.M.C.; Souza-Aguiar, E.F.; Mattos, L.V.; Noronha, F.B. Syngas production by autothermal reforming of methane on supported platinum catalysts. Appl. Catal. A Gen. 2008, 334, 259–267. [Google Scholar] [CrossRef]
- Souza, A.E.A.M.; Maciel, L.J.L.; Cavalcanti-Filho, V.O.; Filho, N.M.L.; Abreu, C.A.M. Kinetic-Operational Mechanism to Autothermal Reforming of Methane. Ind. Eng. Chem. Res. 2011, 50, 2585–2599. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Weng, W.-Z.; Zhang, Q.; Huang, C.-J.; Wan, H.-L. Synthesis gas production from partial oxidation of methane over highly dispersed Pd/SiO2 catalyst. Fuel 2013, 103, 1032–1038. [Google Scholar] [CrossRef]
- Li, C.; Ying, W.; Cao, F.; Zhang, H.; Fang, D. Effects of Impregnation Solvents on Catalytic Performance of Co-Ru-ZrO2/γ-Al2O3 Catalyst for Fischer-Tropsch Synthesis. Pet. Sci. Technol. 2008, 26, 704–716. [Google Scholar] [CrossRef]
- Negrier, F.; Marceau, É.; Che, M.; de Caro, D. Role of ethylenediamine in the preparation of alumina-supported Ni catalysts from [Ni(en)2(H2O)2](NO3)2: From solution properties to nickel particles. Comptes Rendus Chim. 2003, 6, 231–240. [Google Scholar] [CrossRef]
- Bentaleb, F.; Che, M.; Dubreuil, A.-C.; Thomazeau, C.; Marceau, E. Influence of organic additives on the properties of impregnation solutions and on nickel oxide particle size for Ni/Al2O3 catalysts. Catal. Today 2014, 235, 250–255. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158, 190–201. [Google Scholar] [CrossRef]
- Morales-Cano, F.; Lundegaard, L.F.; Tiruvalam, R.R.; Falsig, H.; Skjøth-Rasmussen, M.S. Improving the sintering resistance of Ni/Al2O3 steam-reforming catalysts by promotion with noble metals. Appl. Catal. A Gen. 2015, 498, 117–125. [Google Scholar] [CrossRef]
- Moradi, P.; Parvari, M. Preparation of Lanthanum-Nickel-Aluminium Perovskite Systems And Their Application In Methane-Reforming Reactions. Iran. J. Chem. Eng. 2006, 3, 29–43. [Google Scholar]
- Pérez-Hernández, R.; Mondragón-Galicia, G.; Allende Maravilla, A.; Palacios, J. Nano-dimensional CeO2 nanorods for high Ni loading catalysts: H2 production by autothermal steam reforming of methanol reaction. Phys. Chem. Chem. Phys. 2013, 15, 12702–12708. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Chang, J.-S.; Park, S.-E.; Joe, Y.-I. Highly active and stable Ni/Ce–ZrO2 catalyst for H2 production from methane. J. Mol. Catal. A Chem. 2002, 181, 137–142. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Yu, C.; Liu, Y.; Shen, S. Mechanistic investigations on the partial oxidation of methane to synthesis gas over a nickel-on-alumina catalyst. Appl. Catal. A Gen. 1998, 174, 121–128. [Google Scholar] [CrossRef]
- Santos, A.C.S.F.; Damyanova, S.; Teixeira, G.N.R.; Mattos, L.V.; Noronha, F.B.; Passos, F.B.; Bueno, J.M.C. The effect of ceria content on the performance of Pt/CeO2/Al2O3 catalysts in the partial oxidation of methane. Appl. Catal. A Gen. 2005, 290, 123–132. [Google Scholar] [CrossRef]
- Cherian, M.; Gupta, R.; Someswara Rao, M.; Deo, G. Effect of Modifiers on the Reactivity of Cr2O3/Al2O3 and Cr2O3/TiO2 Catalysts for the Oxidative Dehydrogenation of Propane. Catal. Lett. 2003, 86, 179–189. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, X.; Yao, L.; Shen, J.; Tong, D.; Hu, C. The promoting effect of La, Mg, Co and Zn on the activity and stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrog. Energy 2011, 36, 7094–7104. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane To Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Ziegler, C.; Zapf, R.; Hessel, V.; Kolb, G. Characterization of Cu/CeO2/γ-Al2O3 Thin Film Catalysts by Thermal Desorption Spectroscopy. Catal. Lett. 2005, 105, 35–40. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Zapf, R.; Hessel, V.; Ziegler, C. Parallel screening of Cu/CeO2/γ-Al2O3 catalysts for steam reforming of methanol in a 10-channel micro-structured reactor. Catal. Commun. 2004, 5, 671–675. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, T.H.; Ko, C.H.; Park, H.C.; Song, I.K. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/MxOy–Al2O3 (M=Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrog. Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Jun, K.-W.; Park, S.-E.; Oh, Y.-S. Methane reforming over Ni/Ce-ZrO2 catalysts: Effect of nickel content. Appl. Catal. A Gen. 2002, 226, 63–72. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Park, S.-E. Methane-reforming reactions over Ni/Ce-ZrO2/θ-Al2O3 catalysts. Appl. Catal. A Gen. 2003, 251, 275–283. [Google Scholar] [CrossRef]
- Mierczynski, P.; Stępińska, N.; Mosinska, M.; Chalupka, K.; Albinska, J.; Maniukiewicz, W.; Rogowski, J.; Nowosielska, M.; Szynkowska, M.I. Hydrogen Production via the Oxy-Steam Reforming of LNG or Methane on Ni Catalysts. Catalysts 2020, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Mierczynski, P.; Mosinska, M.; Stepinska, N.; Chalupka, K.; Nowosielska, M.; Maniukiewicz, W.; Rogowski, J.; Goswami, N.; Vasilev, K.; Szynkowska, M.I. Effect of the support composition on catalytic and physicochemical properties of Ni catalysts in oxy-steam reforming of methane. Catal. Today 2020. [Google Scholar] [CrossRef]
- Mosinska, M.; Stepinska, N.; Chalupka, K.; Maniukiewicz, W.; Szynkowska, M.I.; Mierczynski, P. Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes. Catalysts 2020, 10, 855. [Google Scholar] [CrossRef]
- Stawowska, J.; Bartczak, W.M. Computer modeling of metal catalyst deactivation caused by carbon deposit. Przemysł Chem. 2003, 82, 778–782. [Google Scholar]
- Dantas, S.C.; Escritori, J.C.; Soares, R.R.; Hori, C.E. Effect of different promoters on Ni/CeZrO2 catalyst for autothermal reforming and partial oxidation of methane. Chem. Eng. J. 2010, 156, 380–387. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Pino, L.; Specchia, S. Syngas production by methane oxy-steam reforming on Me/CeO2 (Me=Rh, Pt, Ni) catalyst lined on cordierite monoliths. Appl. Catal. B Environ. 2015, 162, 551–563. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Specchia, S.; Cipitì, F.; Specchia, V. Methane oxy-steam reforming reaction: Performances of Ru/γ-Al2O3 catalysts loaded on structured cordierite monoliths. Int. J. Hydrog. Energy 2014, 39, 18592–18603. [Google Scholar] [CrossRef]
- Yoshida, K.; Begum, N.; Ito, S.-i.; Tomishige, K. Oxidative steam reforming of methane over Ni/α-Al2O3 modified with trace noble metals. Appl. Catal. A Gen. 2009, 358, 186–192. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Hashemipour, H.; Talebizadeh, A. Syngas production through methane oxy-steam reforming over a Ni/SiO2 nanocatalyst prepared by a modified impregnation method. Micro Nano Lett. 2016, 11, 890–895. [Google Scholar] [CrossRef]
- Roh, H.S.; Jun, K.W.; Dong, W.S.; Back, S.C.; Park, S.E. Methane reforming reactions over stable Ni/ θ -Al2O3 catalysts. J. Ind. Eng. Chem. 2002, 8, 464–471. [Google Scholar]
- Jiménez-González, C.; Gil-Calvo, M.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidative Steam Reforming and Steam Reforming of Methane, Isooctane, and N-Tetradecane over an Alumina Supported Spinel-Derived Nickel Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3920–3929. [Google Scholar] [CrossRef]
- Miletić, N.; Izquierdo, U.; Obregón, I.; Bizkarra, K.; Agirrezabal-Telleria, I.; Bario, L.V.; Arias, P.L. Oxidative steam reforming of methane over nickel catalysts supported on Al2O3–CeO2–La2O3. Catal. Sci. Technol. 2015, 5, 1704–1715. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Seo, J.G.; Youn, M.H.; Song, J.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over ordered mesoporous nickel–alumina catalyst. Int. J. Hydrog. Energy 2012, 37, 17967–17977. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Park, S.; Chung, J.S.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni/Al2O3–ZrO2 xerogel catalysts: Effect of calcination temperature of Al2O3–ZrO2 xerogel supports. Int. J. Hydrog. Energy 2009, 34, 3755–3763. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Jung, J.C.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous nickel–alumina aerogel catalyst. Int. J. Hydrog. Energy 2010, 35, 6738–6746. [Google Scholar] [CrossRef]
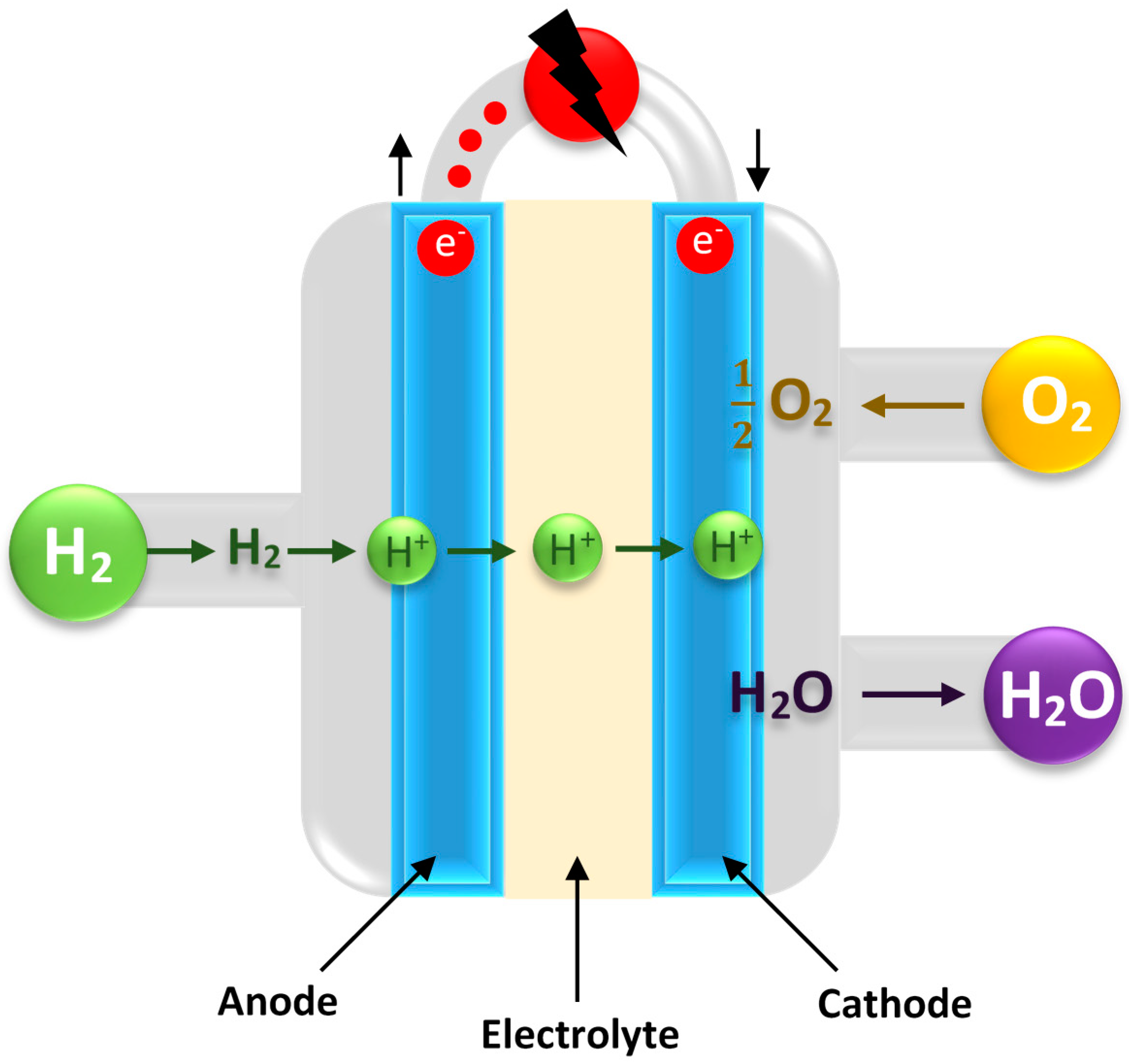
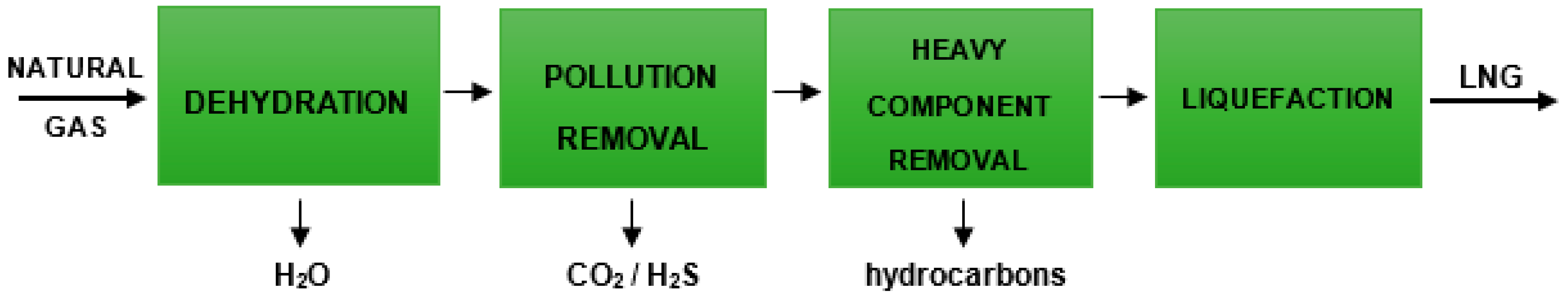

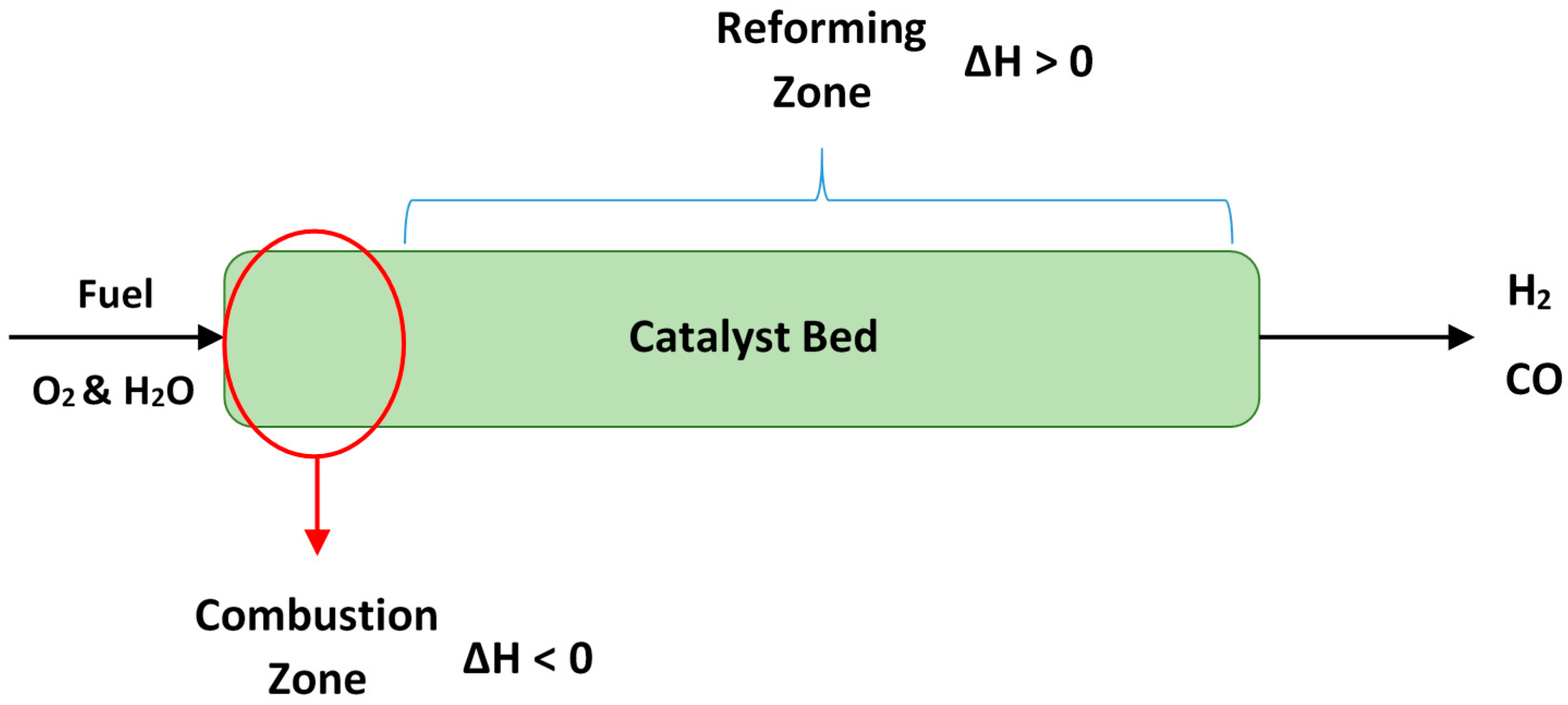
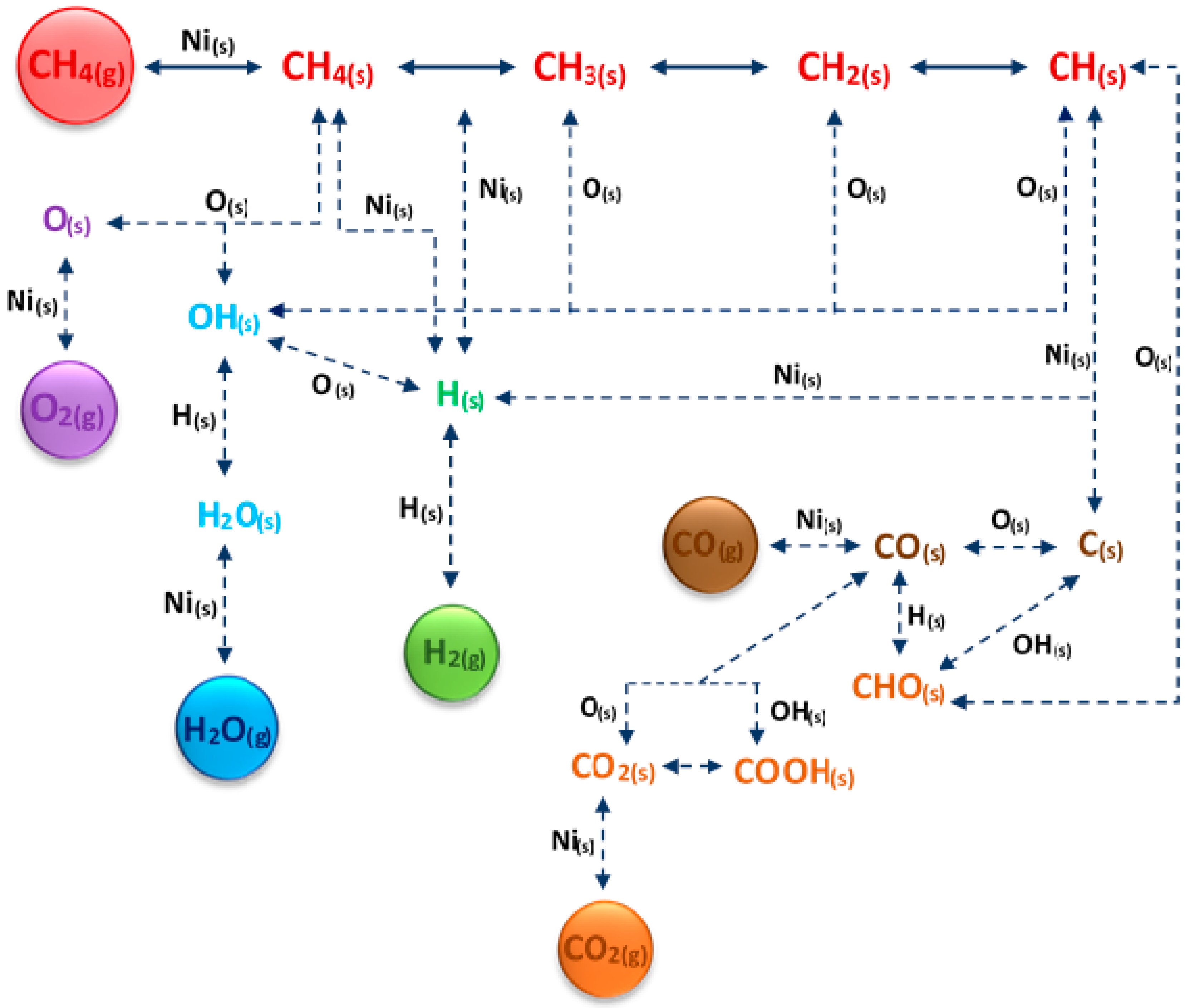
| Process | Reaction Equation | H2/CO Ratio | ∆H (kJ/mol) |
|---|---|---|---|
| Steam reforming (SR) | ≥3 | 206 −41 | |
| Partial oxidation (POM) | 2 | −37 | |
| Dry reforming (DR) | 1 | 247 | |
| Oxy-steam reforming (OSR) | It depends on the reaction mixture composition | 206 −41 −37 −880 |
| Process | Reaction Equation | ∆H (kJ/mol) |
|---|---|---|
| Water gas shift (WGS) Reverse water gas shift (RWGS) | −41 | |
| 41 | ||
| Carbon deposit formation | +75 | |
| −172 | ||
| −131 | ||
| Coal gasification | 131 | |
| −395 | ||
| −110 | ||
| 172 | ||
| Methanation | −206 | |
| −247 |
| Catalyst | Preparation Method | SBET [m2/g] | Reduction of NiO [%] * | Active Metal Dispersion [%] | Metal Particle Size [nm] | TOSR [°C] | Catalyst Weight [g] | CH4 Conv. [%] | H2 | CO | CO2 | H2 /CO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni(5)/La2O3 [136] | IP | 9 | - | - | - | 700 | 0.2 | 39 | 0 s | 0 s | 54 s | 0 |
| Ni(5)/CeO2 [136] | IP | 17 | - | - | - | 700 | 0.2 | 100 | 61 s | 34 s | 5 s | 1.8 |
| Ni(5)/ZrO2 [136] | IP | 94 | 99 | - | - | 700 | 0.2 | 88 | 63 s | 31 s | 4 s | 2.0 |
| Ni(5)/CeO2·La2O3 (2:1) [136] | IP | 23 | - | - | - | 700 | 0.2 | 99 | 64 s | 31 s | 5 s | 2.1 |
| Ni(5)/CeO2·La2O3 (1:1) [136] | IP | 25 | - | - | - | 700 | 0.2 | 98 | 64 s | 30 s | 6 s | 2.1 |
| Ni(5)/CeO2·La2O3 (1:2) [136] | IP | 20 | - | - | - | 700 | 0.2 | 43 | 0 s | 1 s | 46 s | 0 |
| Ni(5)/CeO2·ZrO2 (2:1) [136] | IP | 42 | - | - | - | 700 | 0.2 | 97 | 65 s | 31 s | 4 s | 2.1 |
| Ni(5)/La2O3·ZrO2 (2:1) [136] | IP | 4 | - | - | - | 700 | 0.2 | 28 | 0 s | 0 s | 66 s | 0 |
| Ni(20)/ZrO2 [135] | IP | 77 | 80 | - | 32 *** | 700 | 0.2 | 97 | 91 y | 89 s | 11 s | 1.7 |
| Ni(20)/CeO2(5)-ZrO2 [135] | IP | 79 | 60 | - | 24 *** | 700 | 0.2 | 87 | 101 y | 75 s | 24 s | 4.6 |
| Ni(20)/La2O3(5)-ZrO2 [135] | IP | 74 | 99 | - | 20 *** | 700 | 0.2 | 97 | 90 y | 88 s | 12 s | 1.7 |
| Ag(1)-Ni(20)/ZrO2 [137] | SIP | 69 | - | - | - | 700 | 0.2 | 96 | 83 y | 89 s | 11 s | 1.9 |
| Pt(0.1)/γ-Al2O3 [39] | IP | - | - | - | - | 850 | 0.08 | 87 | - | 75 s | - | 2.8 |
| Ni(2.6)/γ-Al2O3 [39] | IP | - | 100 | - | 4.8 **** | 850 | 0.08 | 97 | - | 79 s | - | 2.9 |
| Pt(0.1)/Ni(2.6)/γ-Al2O3 [39] | SIP | - | 100 | - | 5.0 **** | 850 | 0.08 | >99 | - | 82 s | - | 2.7 |
| Pt(0.1)-Ni(2.6)/γ-Al2O3 [39] | CIP | - | 100 | - | 4.5 **** | 850 | 0.08 | >99 | - | 81 s | - | 2.7 |
| Ni(7.5)/CeO2 [140] | IP | 5 | - | 2.9 ** | - | 750 | - | 98 | 68 c | 19 c | 13 c | 3.2 |
| Rh(1.5)/CeO2 [140] | IP | 14 | - | 22.2 ** | - | 750 | - | 99 | 68 c | 19 c | 13 c | 3.2 |
| Pt(1.5)/CeO2 [140] | IP | 16 | - | 23.1 ** | - | 750 | - | 99 | 68 c | 19 c | 13 c | 3.2 |
| Ru(1.5)/γ-Al2O3 [141] | IP | 192 | - | 0.9 ** | 22 *** | 750 | - | >99 | 70 c | 20 c | 10 c | 3.3 |
| Ni(15)/MgO [89] | MS | 16 | - | 1.7 ** | 58 ** | 750 | 0.05 | 92 | - | 55 s | - | 3.3 |
| Ni(15)/Ce-ZrO2 [133] | MS | 40 | 85 | 0.9 ** | 112 ** | 750 | - | 99 | - | 69 y | - | 3.4 |
| Ni(15)/Ce-ZrO2/θ-Al2O3 [134] | IP | 128 | 92 | 5.0 ** | 11.3 *** | 750 | 0.05 | 98 | - | 72 s | - | 3.0 |
| Ni(10.6)/α-Al2O3 [142] | IP | - | - | 3.4 ** | 28 a | 850 | 0.045 | >99 | - | 81 s | - | 2.8 |
| Pd(0.07)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | 64 | - | 91 s | - | 1.9 |
| Pd(0.07)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 6.6 ** | 15 a | 850 | 0.045 | >99 | - | 81 s | - | 2.8 |
| Pt(0.14)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | 96 | - | 83 s | - | 2.3 |
| Pt(0.14)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 4.6 ** | 21 a | 850 | 0.045 | >99 | - | 81 s | - | 2.9 |
| Au(0.14)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | <1 | - | - | - | - |
| Au(0.14)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 2.6 ** | 23 *** | 850 | 0.045 | >99 | - | 80 s | - | 2.8 |
| Ir(0.14)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | 93 | - | 82 s | - | 2.4 |
| Ir(0.14)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 3.8 ** | 26 a | 850 | 0.045 | >99 | - | 80 s | - | 2.9 |
| Rh(0.07)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | >99 | - | 81 s | - | 2.8 |
| Rh(0.07)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 5.2 ** | 19 a | 850 | 0.045 | >99 | - | 79 s | - | 2.9 |
| Ru(0.07)/α-Al2O3 [142] | IP | - | - | - | - | 850 | 0.045 | >99 | - | 81 s | - | 2.7 |
| Ru(0.07)-Ni(10.6)/α-Al2O3 [142] | CIP | - | - | 4.3 ** | 23 a | 850 | 0.045 | >99 | - | 81 s | - | 2.9 |
| Ni(10)/γ-Al2O3 [38] | MI | 186 | 85 | 4.6 *** | - | 750 | 0.06 | 99 | 2.4 n | 0.8 n | - | 3.2 |
| Ni(10)/γ-Al2O3 [38] | IP | - | - | - | - | 750 | 0.06 | 58 | 1.3 n | 0.3 n | - | 1.6 |
| Ni(5)/SiO2 [143] | OAmIP | 304 | - | - | 5.4 *** | 750 | 0.1 | 92 | 2.3 n | 0.7 n | 0.2 n | 3.4 |
| Ni(5)/SiO2 [143] | IP | 270 | - | - | 10.8 *** | 750 | 0.1 | 47 | 1.0 n | 0.2 n | 0.3 n | 5.2 |
| Ni(12)/θ-Al2O3 [144] | IP | 145 | 66 | 30.1 ** | 21.2 *** | 750 | 0.05 | 99 | - | 71 s | - | 3.0 |
| Ni(17)/γ-Al2O3 [145] | CP | 94 | - | - | - | 600 | 0.125 | 75 | 31 y | 19 y | 59 y | - |
| Rh(1)/γ-Al2O3 [145] | - | 132 | - | 33 | 9 *** | 600 | 0.125 | 90 | 50 y | 19 y | 70 y | - |
| Ni(9)/γ-Al2O3 [146] | SG | 126 | - | 4.4 b | 4 *** | 550 | - | 54 | 2.4 s | - | - | 4.7 |
| Ni(9)/γ-Al2O3-CeO2 [146] | SG | 132 | - | 5.5 b | 5 *** | 550 | - | 45 | 2.2 s | - | - | 4.0 |
| Ni(9)/γ-Al2O3-La2O3 [146] | SG | 131 | - | 6.0 b | 7 *** | 550 | - | 52 | 1.4 s | - | - | 3.8 |
| Ni(9)/γ-Al2O3-CeO2-La2O3 [146] | SG | 127 | - | 4.7 b | 4 *** | 550 | - | 52 | 2.1 s | - | - | 5.8 |
| Catalyst | Preparation Method | SBET [m2/g] | Reduction of NiO [%] * | Active Metal dispersion [%] | Metal Particle Size [nm] | TOSR [°C] | Catalyst Weight [g] | CH4 Conv. [%] | C2H6 Conv. [%] | C3H8 Conv. [%] | C4H10 Conv. [%] | H2 | CO | CO2 | H2 /CO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni(20)/ZrO2 [135] | IP | 77 | 80 | - | 35 *** | 700 | 0.2 | 98 | 100 | 100 | 100 | 57 y | 87 s | 13 s | 2.0 |
| Ni(20)/CeO2(5)-ZrO2 [135] | IP | 79 | 60 | - | 22 *** | 700 | 0.2 | 96 | 100 | 100 | 100 | 60 y | 85 s | 15 s | 2.1 |
| Ni(20)/La2O3(5)-ZrO2 [135] | IP | 74 | 99 | - | 41 *** | 700 | 0.2 | 94 | 100 | 100 | 100 | 61 y | 86 s | 14 s | 2.2 |
| Ag(1)-Ni(20)/ZrO2 [137] | SIP | 69 | 88 | - | - | 700 | 0.2 | 72 | 100 | 100 | 100 | 59 y | 64 s | 36 s | 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosinska, M.; Szynkowska, M.I.; Mierczynski, P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts 2020, 10, 896. https://doi.org/10.3390/catal10080896
Mosinska M, Szynkowska MI, Mierczynski P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts. 2020; 10(8):896. https://doi.org/10.3390/catal10080896
Chicago/Turabian StyleMosinska, Magdalena, Malgorzata I. Szynkowska, and Pawel Mierczynski. 2020. "Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview" Catalysts 10, no. 8: 896. https://doi.org/10.3390/catal10080896
APA StyleMosinska, M., Szynkowska, M. I., & Mierczynski, P. (2020). Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts, 10(8), 896. https://doi.org/10.3390/catal10080896







