Progress of MOF-Derived Functional Materials Toward Industrialization in Solar Cells and Metal-Air Batteries
Abstract
1. Introduction
2. MOF Based Functional Materials for Solar Cells
2.1. MOFs in Dye-Sensitized Solar Cells
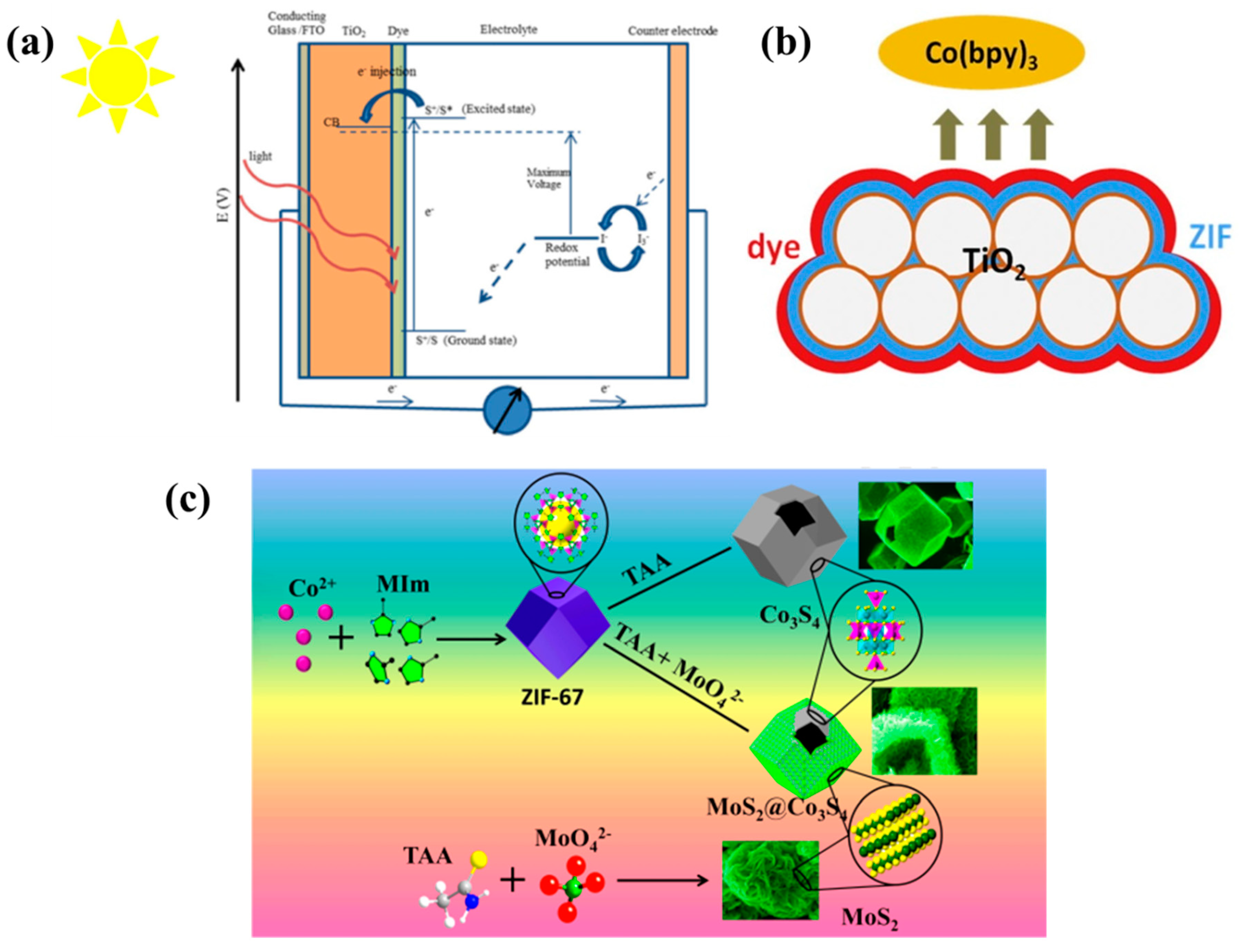
2.1.1. MOFs for DSSC Photoelectrode
2.1.2. MOFs for DSSC Counter Electrode
2.1.3. MOFs for DSSC Electrolyte
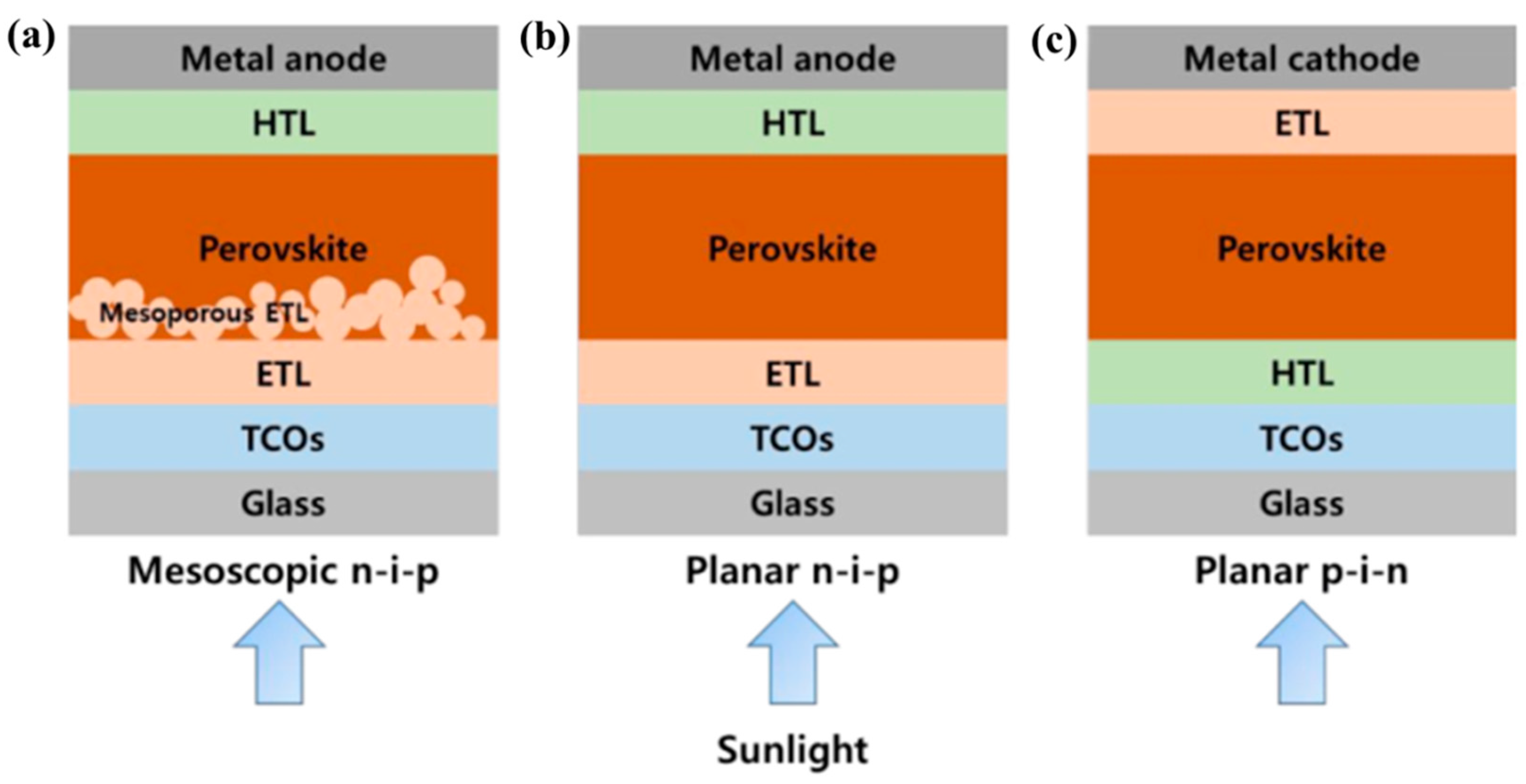
2.2. MOFs in Perovskite Solar Cells
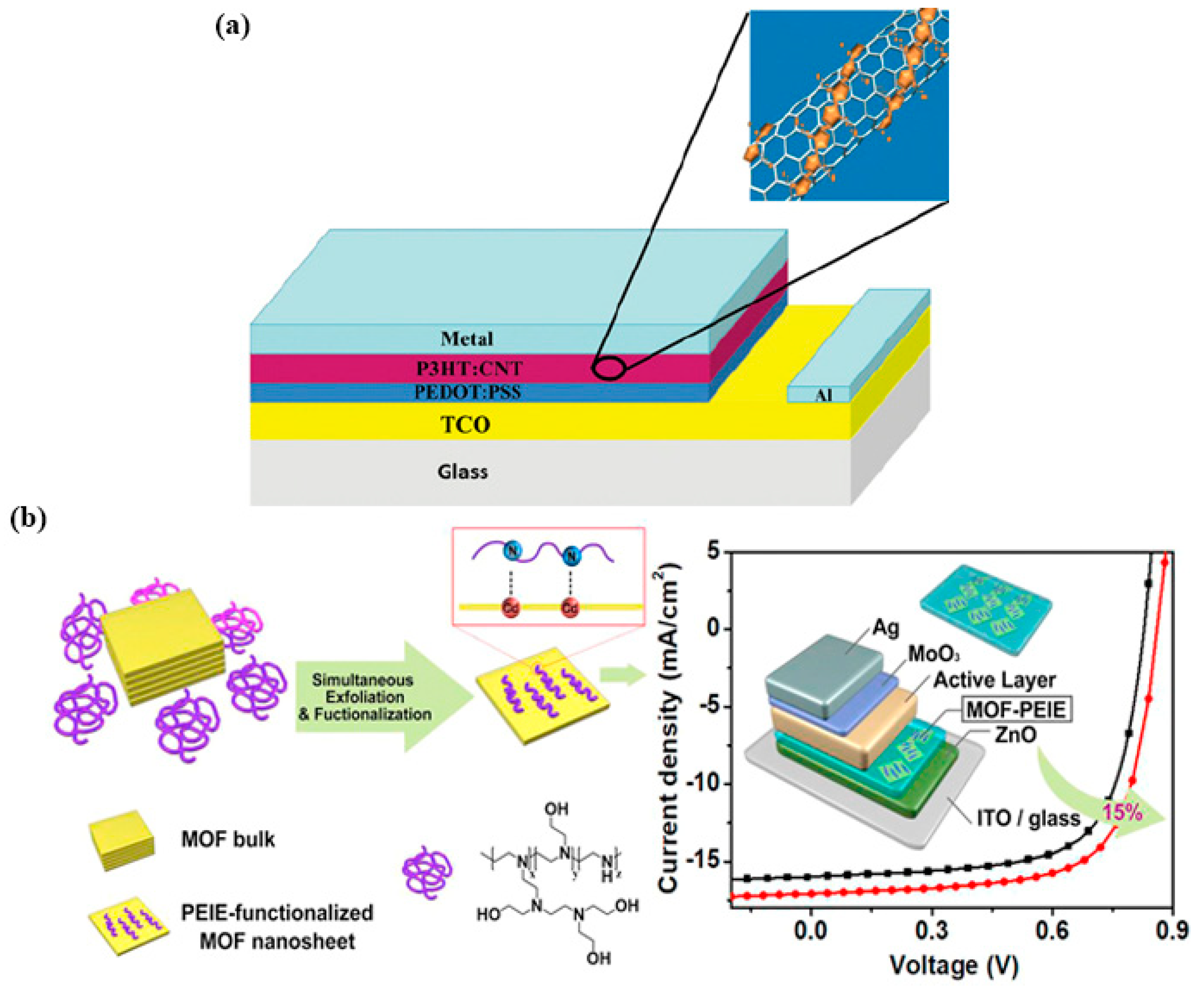
2.2.1. MOFs in the Perovskite/CTL Interface
2.2.2. MOFs for CTL or Incorporated with CTL
2.2.3. MOFs into Perovskite Thin Film
2.3. MOFs in Organic Solar Cells
3. MOF Based Functional Catalysts for Metal-Air Batteries
3.1. Zn-Air Batteries
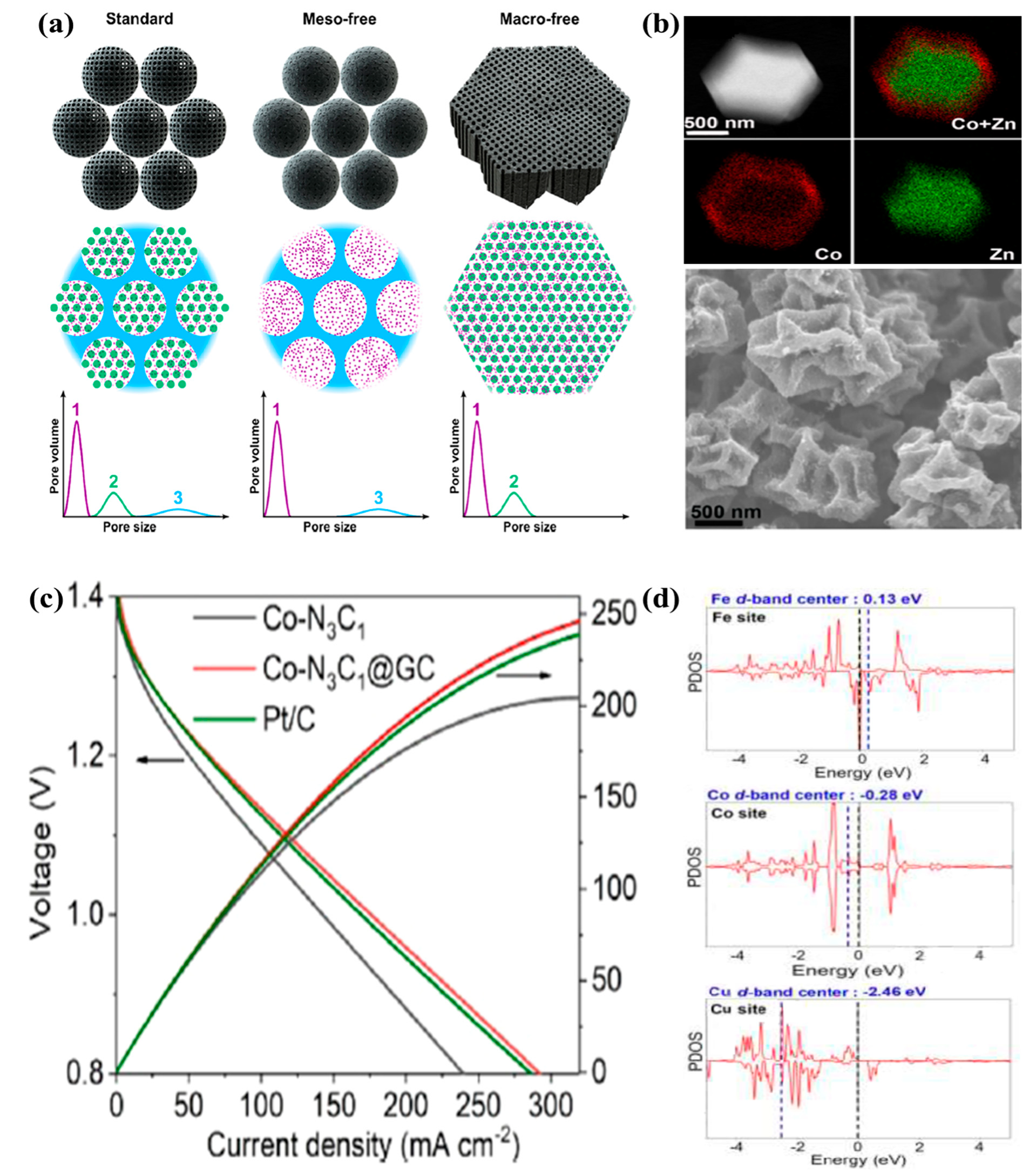
3.1.1. MOF-Derived Single-Atom Catalysts
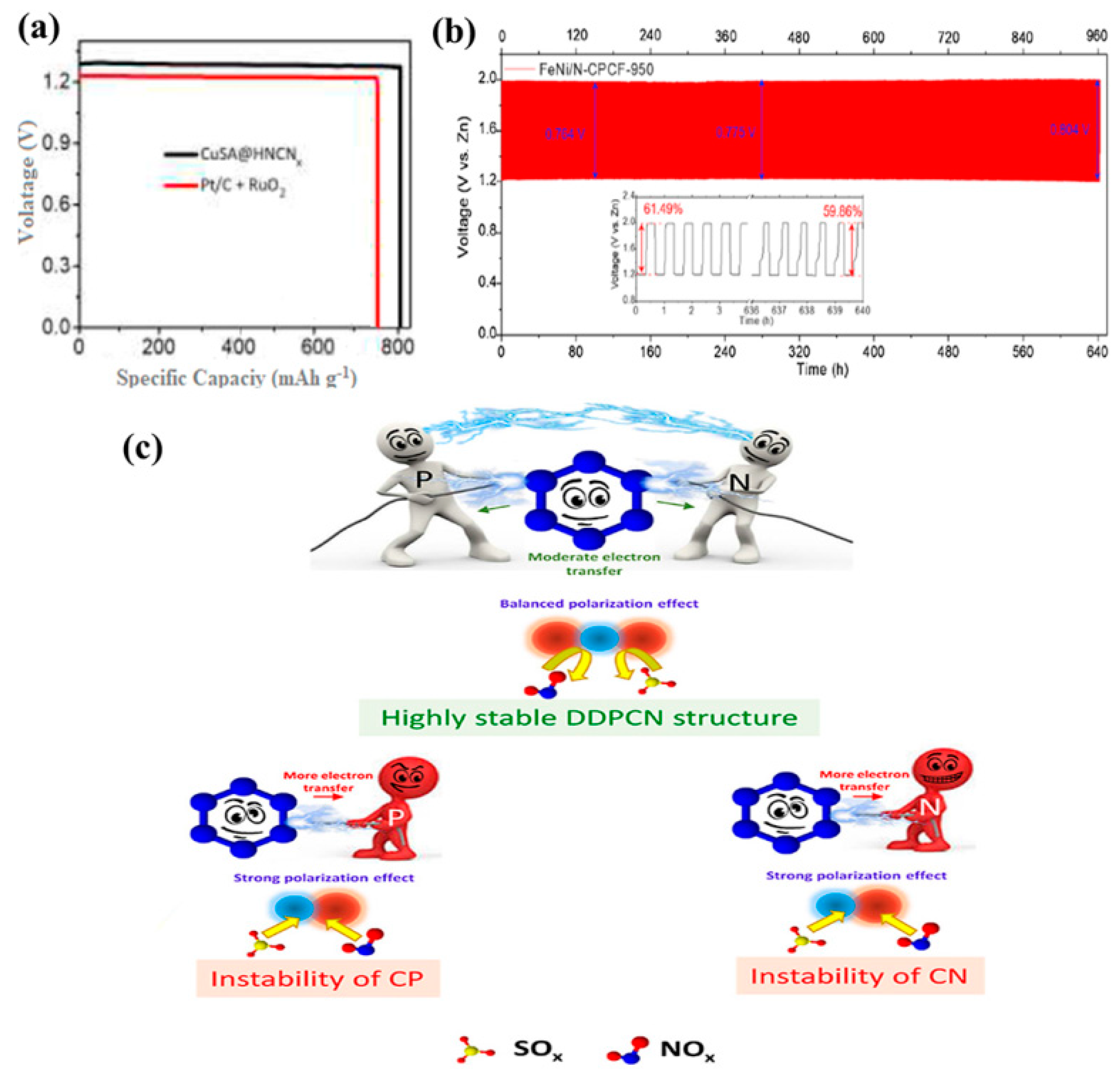
3.1.2. MOF-Derived Bimetallic Catalysts
3.1.3. Heteroatoms Doped MOF-Derived Carbon Catalysts
3.2. Li-Air Batteries
3.2.1. MOF-Derived Metals/Metal Oxides/Metal Carbides Based Catalysts
3.2.2. MOF-Derived Bimetallic and Hybrid Catalysts
3.2.3. Non-Metallic MOF-Derived Carbon Dopants
3.3. Al-Air Batteries
MOF-Based Electrocatalysts for Al-Air Battery
3.4. Na-Air Battery
MOF-Based Electrocatalysts for Na-Air Battery
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaker, M.T.; Khedr, A.E.; Kholeif, S. A Proposed Framework for Reducing Electricity Consumption in Smart Homes using Big Data Analytics. J. Comput. Sci. 2019, 15, 537. [Google Scholar] [CrossRef][Green Version]
- Kumari, A.; Tanwar, S.; Tyagi, S.; Kumar, N.; Obaidat, M.S.; Rodrigues, J.J.P.C. Fog Computing for Smart Grid Systems in the 5G Environment: Challenges and Solutions. IEEE Wirel. Commun. 2019, 26, 47. [Google Scholar] [CrossRef]
- Apostolaki-Iosifidou, E.; Woo, S.; Lipman, T. Challenges and Opportunities for Electric Vehicle Charging Detection Using Utility Energy Consumption Data. In Proceedings of the Transportation Research Board Annual Meeting, Washington, DC, USA, 13–17 January 2019. No. 19-05695. [Google Scholar]
- IEA. Electricity. In World Energy Outlook; IEA: Paris, France, 2019. [Google Scholar]
- Gurung, A.; Qiao, Q. Solar Charging Batteries: Advances, Challenges, and Opportunities. Joule 2018, 2, 1. [Google Scholar] [CrossRef]
- Tenfen, D.; Finardi, E.C.; Delinchant, B.; Wurtz, F. Lithium-ion Battery Modelling for the Energy Management Problem of Microgrids. Inst. Eng. Technol. 2016, 10, 576. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? Acs Energy Lett. 2017, 2, 1370. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Guo, S.; Zhou, H. Solar Energy Storage in the Rechargeable Batteries. Nano Today 2017, 16, 46. [Google Scholar] [CrossRef]
- Davari, E.; Ivey, D.G. Bifunctional Electrocatalysts for Zn–Air Batteries. Sustain. Energy Fuels 2017, 2, 39. [Google Scholar] [CrossRef]
- Fiakas, C.D. Metal-Air Battery Stocks. ALT Enegy Stocks, 4 April 2013. [Google Scholar]
- Corma, A.; Garcı, H.; Xamena, F.X.L.I. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606. [Google Scholar] [CrossRef]
- Hönicke, A.I.; Senkovska, I.; Bon, V.; Baburin, I.; Boenisch, N.; Raschke, S.; Evans, J.D. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450. [Google Scholar] [CrossRef]
- Schram, W.L.; Lampropoulos, I.; van Sark, W.G.J.H.M. Photovoltaic Systems Coupled with Batteries that are Optimally Sized for Household Self-Consumption: Assessment of peak shaving potential. Appl. Energy 2018, 223, 69. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Small, L.J.; Foster, M.E.; Wheeler, J.; Ullman, A.M.; Stavila, V.; Rodriguez, M.; Allendorf, M.D. MOF-Sensitized Solar Cells Enabled by a Pillared Porphyrin Framework. J. Phys. Chem. C 2017, 121, 4816. [Google Scholar] [CrossRef]
- Chen, T.; Huang, Y.; Li, C.; Kung, C.; Vittal, R.; Ho, K. Metal-Organic Framework/Sulfonated Polythiophene on Carbon Cloth as a Flexible Counter Electrode for Dye-Sensitized Solar Cells. Nano Energy 2016, 32, 19. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Xiang, W.; Amiinu, I.S.; Zhao, X. Zeolitic-Imidazolate-Framework (ZIF-8)/PEDOT: PSS Composite Counter Electrode for Low Cost and Efficient Dye-Sensitized Solar Cells. New J. Chem. 2018, 42, 17303. [Google Scholar] [CrossRef]
- Shen, D.; Pang, A.; Li, Y.; Dou, J.; Wei, M. Metal–Organic Frameworks at Interfaces of Hybrid Perovskite Solar Cells for Enhanced Photovoltaic. Chem. Commun. 2018, 54, 1253. [Google Scholar] [CrossRef]
- Xing, W.; Ye, P.; Lu, J.; Wu, X.; Chen, Y.; Zhu, T.; Peng, A. Tellurophene-Based Metal-Organic Framework Nanosheets for High- Performance Organic Solar Cells. J. Power Sources 2018, 401, 13. [Google Scholar] [CrossRef]
- O′Regan, B.; Gratzel, M. High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Lett. Nat. 1991, 353, 737. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cell. Chem. Rev. 2010, 110, 6595. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Yeoh, M.; Chan, K.-Y. Recent Advances in Photo-Anode for Dye-Sensitized Solar Cells: A review. Energy Res. 2017, 13, 381. [Google Scholar]
- Aghazada, S.; Nazeeruddin, M.K. Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 52. [Google Scholar] [CrossRef]
- Ammar, A.M.; Mohamed, H.S.H.; Yousef, M.M.K.; Abdel-hafez, G.M.; Hassanien, A.S.; Khalil, A.S.G. Dye-sensitized solar cells (DSSCs) based on extracted natural dyes. Hindawi 2019, 2019, 1867271. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Fl, A.; Lund, P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Z.; Wang, W.; Fu, L. Metal Organic Frameworks Derived High-Performance Photoanodes for DSSCs. J. Alloys Compd. 2020, 20, 154089. [Google Scholar] [CrossRef]
- Li, Y.; Pang, A.; Wang, C.; Wei, M. Metal–Organic Frameworks: Promising Materials for Improving the Open Circuit Voltage of Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 17259. [Google Scholar] [CrossRef]
- Gu, A.; Xiang, W.; Wang, T.; Gu, S.; Zhao, X. Enhance Photovoltaic Performance of Tris(2,20-bipyridine ) Cobalt (II)/(III) based Dye-sensitized Solar Cells via Modifying TiO2 Surface with Metal-Organic Frameworks. Sol. Energy 2017, 147, 126. [Google Scholar] [CrossRef]
- Alwin, S.; Ramasubbu, V.; Shajan, X.S. TiO2 Aerogel–Metal Organic Framework Nanocomposite: A New Class of Photoanode Material for Dye-Sensitized Solar Cell Applications. Bull. Mater. Sci. 2018, 41, 27. [Google Scholar] [CrossRef]
- Maza, W.A.; Haring, A.J.; Ahrenholtz, S.R.; Epleya, C.C.; Lina, S.Y.; Morris, A.J. Ruthenium(II)-Polypyridyl zirconium(IV) Metal-organic Frameworks as A New Class of Sensitized Solar Cells. Chem. Sci. 2015, 7, 719. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter Electrodes in Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2017, 46, 5975. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, M.; Wang, M.; Feng, C.; Wang, Z. Metal Selenides as Efficient Counter Electrodes for Dye-Sensitized Solar Cells. Acc. Chem. Res. 2017, 50, 895. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Kollu, P.; Jeong, S.K.; Grace, A.N. Low-cost and Efficient Counter Electrode with Carbon Wrapped Vo2(M) Nanofiber for Dye- Sensitized Solar Cells. Sci. Rep. 2019, 9, 5177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahajwalla, V.; Bhargava, P. Fabrication of A Counter Electrode for Dye-Sensitized Solar Cells (DSSCs) Using A Carbon Material Produced with the Organic Ligand 2-Methyl-8-Hydroxyquinolinol (Mq). Nanoscale Adv. 2019, 1, 3192. [Google Scholar] [CrossRef]
- Li, G.; Gao, X. Low-Cost Counter-Electrode Materials for Dye-Sensitized and Perovskite Solar Cells. Adv. Mater. 2019, 32, 1806478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Zhou, D.; Liao, P. Controlling Flexibility of Metal–Organic Frameworks. Natl. Sci. Rev. 2018, 5, 907. [Google Scholar] [CrossRef]
- He, A.H.; Sun, Q.; Gao, W.; Perman, J.A.; Sun, F.; Zhu, G.; Aguila, B.; Space, B.; Ma, S. A Stable Metal-Organic Framework Featuring Local Buffer Environment for Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2018, 57, 4657. [Google Scholar] [CrossRef]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461. [Google Scholar] [CrossRef]
- Chueh, C.; Chen, C.; Su, Y.; Konnerth, H.; Gu, Y.; Kung, C.; Wu, K.C. Harnessing MOF Materials in Photovoltaic Devices: Recent Advances, Challenges, and Perspectives. J. Mater. Chem. A 2019, 7, 17079. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Zhao, K.; Hao, M.; Zhang, Z.; Li, L.; Zhang, Y.; Zhang, W. A Facile Hydrothemal Synthesis of MoS2@Co3S4 Composites Based on Metal Organic Framework Compounds as a High-Efficiency Liquid-State Solar Cell Counter Electrode. J. Alloys Compd. 2020, 20, 154910. [Google Scholar] [CrossRef]
- Ou, J.; Xiang, J.; Liu, J.; Sun, L. Surface-Supported Metal-Organic Framework Thin-Film-Derived Transparent CoS1.097@N-Doped Carbon Film as an Efficient Counter Electrode for Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 14862. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Chen, S.; Gu, Z.; Zhang, J. Epitaxial Growth of Highly Transparent Metal-Porphyrin Framework Thin Films for Efficient Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 12, 1078. [Google Scholar] [CrossRef]
- Bella, F.; Bongiovanni, R.; Kumar, R.S.; Kulandainathan, M.A.; Stephan, A.M. Light Cured Networks Containing Metal Organic Frameworks as Efficient and Durable Polymer Electrolytes for Dye-Sensitized solar cells. J. Mater. Chem. A 2013, 1, 9033. [Google Scholar] [CrossRef]
- Sarwar, S.; Lee, M.; Park, S.; Dao, T.T.; Ullah, A.; Hong, S.; Han, C. Transformation of A Liquid Electrolyte to A Gel Inside Dye Sensitized Solar Cells for Better Stability and Performance. Thin Solid Film. 2020, 20, 30236. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. JACS 2009, 131, 6050. [Google Scholar] [CrossRef] [PubMed]
- NREL Transforming Energy. Best Research-Cell Efficiencies Chart. NREL 2019. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 2 August 2020).
- Seo, S.; Jeong, S.; Park, H.; Shin, H.; Park, N.-G. Atomic Layer Deposition for Efficient and Stable Perovskite Solar Cells. Chem. Commun. 2019, 55, 2403. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, J.; Liu, J.; Ouyang, L.; Hu, R.; Wang, H.; Yang, L.; Zhu, M. A General Metal-Organic Framework (MOF)-Derived Selenidation Strategy for In Situ Carbon-Encapsulated Metal Selenides as High-Rate Anodes for Na-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707573. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, H.U.; Shaheen, N.; Lin, R.; Qiu, M.; Xie, J.; Li, J.; Raza, R.; Mai, W.; Hu, C. High Energy Density Hybrid Supercapacitor Based on 3D Mesoporous Cuboidal Mn2O3 and MOF-Derived Porous Carbon Polyhedrons. Electrochim. Acta 2018, 18, 31296. [Google Scholar] [CrossRef]
- Hou, A.X.; Pan, L.; Huang, S.; Ou-yang, W.; Chen, X. Enhanced Efficiency and Stability of Perovskite Solar Cells using Porous Hierarchical TiO2 Nanostructures of Scattered Distribution as Scaffold. Electrochim. Acta 2017, 17, 30701. [Google Scholar]
- Aubrey, M.L.; Wiers, B.M.; Andrews, S.C.; Sakurai, T.; Reyes-lillo, S.E.; Hamed, S.M.; Yu, C.; Darago, L.E.; Mason, J.A.; Baeg, J.; et al. Electron delocalization and Charge Mobility as a Function of Reduction in a Metal–Organic Framework. Nat. Mater. 2018, 17, 625. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Zaake-hertling, H.; Hey-hawkins, E.; Agafonov, A.V.; Seisenbaeva, G.A.; Kesslerd, V.G.; Vinogradov, V.V. The first Depleted Heterojunction TiO2–MOF-Based Solar Cell. Chem. Commun. 2014, 1, 14. [Google Scholar] [CrossRef]
- Lee, C.; Chen, C.; Liao, Y.; Wu, K.C.; Chueh, C. Enhancing Efficiency and Stability of Photovoltaic Cells by Using Perovskite/Zr-MOF Heterojunction Including Bilayer and Hybrid Structures. Adv. Sci. 2018, 6, 1801715. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Park, J.; Han, I.K.; Lee, J.H.; Park, M.; Choi, K.M. Nanocrystalline Titanium Metal–Organic Frameworks for Highly Efficient and Flexible Perovskite Solar Cells. ACS Nano 2018, 12, 4968. [Google Scholar] [CrossRef] [PubMed]
- My, T.; Nguyen, H.; Bark, C.W. Synthesis of Cobalt-Doped TiO2 Based on Metal-Organic Frameworks as an Effective Electron Transport Material in Perovskite Solar Cells. ACS Omega 2020, 5, 2280. [Google Scholar]
- Zhang, Y.; Li, B.; Fu, L.; Li, Q.; Yin, L. MOF-Derived ZnO as Electron Transport Layer for Improving Light Harvesting and Electron Extraction Efficiency in Perovskite Solar Cells. Electrochim. Acta 2019, 330, 135280. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Jiang, A.; Xia, D.; Du, X.; Dong, Y.; Wang, P.; Fan, R.; Yang, Y. Metal Organic Framework Doped Spiro-OMeTAD with Increased Conductivity for Improving Perovskite Solar Cell Performance. Sol. Energy 2019, 188, 380. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, X.; Wu, R.; Shi, C.; Xue, R.; Zou, J.; Xu, C.; Zhao, J.; Zeng, W. Oriented Haloing Metal-organic Framework Providing High Efficiency and High Moisture-Resistance for Perovskite Solar Cells, Journal of Power Sources. J. Power Sources 2019, 433, 226699. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, L.; Fan, R.; Wang, A.; Ye, H.; Tian, C.; Hao, S.; Yang, Y. Metal–Organic Framework-Derived N-Rich Porous Carbon as an Auxiliary Additive of Hole Transport Layers for Highly Efficient and Long-Term Stable Perovskite Solar Cells. RRL Sol. 2019, 4, 1900380. [Google Scholar] [CrossRef]
- Chang, T.; Kung, C.; Chen, H.; Huang, T.; Kao, S.; Lu, H.; Lee, M.; Boopathi, K.M.; Chu, C. Planar Heterojunction Perovskite Solar Cells Incorporating Metal–Organic Framework Nanocrystals. Adv. Mater. 2015, 27, 7229. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, L.; Fan, R.; Zhang, J.; Hao, S.; Yang, Y. Heterojunction Incorporating Perovskite and Microporous Metal–Organic Framework Nanocrystals for Efficient and Stable Solar Cells. Nano Micro Lett. 2020, 12, 80. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.A.; Saini, V.; Bourdo, S.; Dervishi, E.; Biris, A.S. Organic Solar Cells: A Review of Materials, Limitations, and Possibilities for Improvement Omar. Part Sci. Technol. 2013, 6351, 427. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.; Zhang, X. Recent Progress on the Development of Metal-Air Batteries, Advanced Sustainable Systems. Adv. Sustain. Syst. 2017, 1, 1700036. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–Air batteries: Are They Ready for Prime Time? Chem. Sci. 2019, 10, 8924. [Google Scholar] [CrossRef] [PubMed]
- Nian, P.; Cao, Y.; Li, Y.; Zhang, X.; Wang, Y.; Liu, H.; Zhang, X. Reparation of A Pure ZIF-67 Membrane by Self-Conversion of Cobalt Carbonate Hydroxide Nanowires for H2 Separation. Cryst. Eng. Comm. 2018, 20, 2440. [Google Scholar] [CrossRef]
- Imaz, I.; Cano-sarabia, M.; Maspoch, D.; Carne, A. A Spray-Drying Strategy for Synthesis of Nanoscale Metal–Organic Frameworks and Their Assembly into Hollow Superstructures. Nat. Chem. 2013, 5, 203. [Google Scholar]
- Simon-Yarza, T.; Gimenez-Marqués, M.; Mrimi, R.; Mielcarek, A.; Gref, R.; Horcajada, P.; Serre, C.; Couvreur, P. A Smart Metal-Organic-Framework Nanomaterial for Lung Targeting. Angew. Chem. Int. Ed. 2017, 56, 15565. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef]
- Ji, H.; Wang, M.; Liu, S.; Sun, H.; Liu, J.; Qian, T.; Yan, C. Pyridinic and Graphitic Nitrogen-Enriched Carbon Paper as a Highly Active Bifunctional Catalyst for Zn-Air Batteries. Electrochim. Acta 2020, 19, 135562. [Google Scholar] [CrossRef]
- Tian, G.; Zhao, M.; Yu, D.; Kong, X.; Huang, J. Nitrogen-Doped Graphene/Carbon Nanotube Hybrids: In Situ Formation on Bifunctional Catalysts and Their Superior Electrocatalytic Activity for Oxygen Evolution/Reduction Reaction. Small 2014, 10, 2251. [Google Scholar] [CrossRef]
- Song, Z.; Han, X.; Deng, Y.; Zhao, N.; Hu, W.; Zhong, C. Clarifying the Controversial Catalytic Performance of Co(OH)2 for Oxygen Reduction/Evolution Reactions Toward Efficient Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 22694. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Xia, D.; Zou, R. Metal-Organic Frameworks and Their Derivatives for Metal-Air Batteries. Energy Storage Mater. 2019, 23, 2405. [Google Scholar] [CrossRef]
- Anand, G. Technological Breakthrough Achieved for Recharging Zinc-Air Battery. Trend Force, 23 August 2017. [Google Scholar]
- Tahil, W. The Zinc Air Battery and the Zinc Economy: A Virtuous Circle. Meridian Int. Res. 2007, 1–9. Available online: http://www.meridian-int-res.com/Projects/The_Zinc_Air_Solution.pdf (accessed on 2 August 2020).
- Cachet, C.; Saidani, B.; Wiart, R. The Behavior of Zinc Electrode in Alkaline Electrolytes: II. A Kinetic Analysis of Anodic Dissolution. Electrochem. Soc. 1992, 139, 644. [Google Scholar] [CrossRef]
- Han, X.; Ling, X.; Wang, Y.; Ma, T.; Zhong, C.; Hu, W.; Deng, Y. Spatial Isolation of Zeolitic Imidazole Frameworks-Derived Cobalt Catalysts: From Nanoparticle, Atomic Cluster to Single Atom. Angew. Chem. 2019, 131, 5413. [Google Scholar] [CrossRef]
- Liang, Z.; Guo, W.; Zhao, R.; Qiu, T.; Tabassum, H.; Zou, R. Engineering Atomically Dispersed Metal Sites for Electrocatalytic Energy Conversion. Nano Energy 2019, 64, 103917. [Google Scholar] [CrossRef]
- Han, G.; Zheng, Y.; Zhang, X.; Wang, Z.; Gong, Y.; Du, C.; Norouzi, M.; Yiu, Y.; Sham, T.; Gu, L.; et al. High loading single-atom Cu Dispersed on Graphene for Efficient Oxygen Reduction Reaction. Nano Energy 2019, 66, 104088. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Jiang, Z.; Xu, J.; Chen, L.; Xue, Y.; Nie, A.; Xie, Z.; Kuang, Q.; Zheng, L. Atomically Dispersed Hierarchically Ordered Porous Fe-N-C Electrocatalyst for High Performance Electrocatalytic Oxygen Reduction in Zn-Air Battery. Nano Energy 2020, 71, 104547. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.; Hyeon, T. Design Principle of Fe−N−C Electrocatalysts: How to Optimize Multimodal Porous Structures? J. Am. Chem. Soc. 2019, 141, 2035. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of Coordination Number over Single Co Sites Triggers the Efficient Electroreduction of CO2. Angew. Chem. Int. Ed. 2017, 57, 1944. [Google Scholar] [CrossRef]
- Hai, X.; Zhao, X.; Guo, N.; Yao, C.; Chen, C.; Liu, W.; Yan, H.; Li, J.; Chen, Z.; Li, X.; et al. Engineering Local and Global Structure of Single Co Atom for Superior Oxygen Reduction Reaction. ACS Catal. 2020, 10, 5862. [Google Scholar] [CrossRef]
- Wagh, N.K.; Shinde, S.S.; Lee, C.H.; Jung, J.; Kim, D.; Kim, S.; Lin, C.; Lee, S.U.; Lee, J. Densely Colonized Isolated Cu-N Single Sites for Efficient Bifunctional Electrocatalysts and Rechargeable Advanced Zn-Air Batteries. Appl. Catal. B Environ. 2020, 268, 118746. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Yun, X.; Ma, D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys Encapsulated in N-doped CNTs-Tangled Porous Carbon Fibers as Highly Efficient and Durable Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Battery. Appl. Catal. B Environ. 2019, 19, 118344. [Google Scholar]
- Agarwal, S.; Yu, X.; Manthiram, A. A Pair of Metal Organic Framework (MOF) -Derived Oxygen Reduction Reaction (ORR) and Oxygen Evolution Reaction (OER) Catalysts for Zinc-Air Batteries. Mater. Today Energy 2020, 16, 100405. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Qiao, J.; Chen, N.; Chen, W.; Sun, S.; National, I.; Recherche, D.; Matériaux, S.-E. Ultra-Long Life Rechargeable Zinc-Air Battery Based on High-Performance Trimetallic Nitride and NCNT Hybrid Bifunctional Electrocatalysts. Nano Energy 2019, 61, 86. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zeng, Y.; Chen, J.; Qiu, L.; Zhou, H.; Sun, C. Single Fe Atom on Hierarchically Porous S, N-Codoped Nanocarbon Derived from Porphyra Enable Boosted Oxygen Catalysis for Rechargeable Zn-Air Batteries. Small 2019, 15, 1900307. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active Sites of Nitrogen-Doped Carbon Materials for Oxygen Reduction Reaction Clarified using Model Catalysts. Science 2016, 351, 361. [Google Scholar] [CrossRef]
- Sarkar, S.; Biswas, A.; Purkait, T.; Das, M.; Kamboj, N.; Dey, R.S. Unravelling the Role of Fe−Mn Binary Active Sites Electrocatalyst for Efficient Oxygen Reduction Reaction and Rechargeable Zn-Air Batteries. Inorg. Chem. 2020, 59, 5194. [Google Scholar] [CrossRef]
- Najam, T.; Shoaib, S.; Shah, A.; Ali, H.; Song, Z.; Sun, H.; Peng, Z.; Cai, X. A Metal Free Electrocatalyst for High-Performance Zinc-Air Battery Application with Good Resistance towards Poisoning Species. Carbon 2020, 164, 12. [Google Scholar] [CrossRef]
- Wang, D.; Su, D. Heterogeneous Nanocarbon Materials for Oxygen Reduction Reaction. Energy Environ. Sci. 2014, 7, 576. [Google Scholar] [CrossRef]
- Maciel, I.O.; Pimenta, M.A.; Sumpter, B.G.; Meunier, V.; Lo, F. Synthesis, Electronic Structure, and Raman Scattering of Phosphorus-Doped Single-Wall Carbon Nanotubes. Nano Lett. 2008, 9, 2267. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L. Electrochemical Water Splitting Hot Paper Nitrogen, Phosphorus, and Fluorine Tri-doped Graphene as a Multifunctional Catalyst for Self-Powered Electrochemical Water Splitting. Angew. Chem. Int. Ed. 2016, 55, 1. [Google Scholar] [CrossRef]
- He, B.; Liu, F.; Yan, S. Temperature-Direct Growth of Graphitized, Ultra-hollow Carbon Framework of Highly Pyridinic Nitrogen Doped as an Efficient Electrocatalyst for Oxygen Reduction Reaction. J. Mater. Chem. A 2017, 5, 18064. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L. Heteroatom-Doped Graphitic Carbon Catalysts For Efficient Electrocatalysis Of Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7244. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, N.; Luo, M.; Sun, Y.; Yang, Y.; Qian, J.; Li, L. Long-life Lithium-O2 Battery Achieved by Integrating Quasi-Solid Electrolyte and Highly Active Pt3Co Nanowires Catalyst. Energy Storage Mater. 2019, 24, 707. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143, 1. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Y. Toward True Lithium-Air Batteries. Joule 2018, 2, 815. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H. A Lithium-Air Battery with A Potential to Continuously Reduce O2 from Air for Delivering Energy. J. Power Sources 2010, 195, 358. [Google Scholar] [CrossRef]
- Lai, N.; Cong, G.; Liang, Z.; Lu, Y.; Lai, N.; Cong, G.; Liang, Z.; Lu, Y. A Highly Active Oxygen Evolution Catalyst for Lithium-Oxygen Batteries Enabled by High- Surface-Energy Facets. Joule 2018, 2, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, N.; Lu, Y.; Zhou, Y.; Dong, C.; Lu, Y.; Wang, Y.; Lai, N.; Lu, Y.; Zhou, Y.; et al. A Solvent-Controlled Oxidation Mechanism of Li2O2 in Lithium-Oxygen Batteries. Joule 2017, 2, 1. [Google Scholar] [CrossRef]
- Jin, C.; Yang, Z.; Cao, X.; Lu, F.; Yang, R. A Novel Bifunctional Catalyst of Ba0.9Co0.5Fe0.4Nb0.1O3Ld Perovskite for Lithium-Air Battery. Int. J. Hydrogen Energy 2013, 39, 2526. [Google Scholar] [CrossRef]
- Geng, D.; Ding, N.; Hor, T.S.A.; Chien, S.W.; Liu, Z. Rom Lithium-Oxygen to Lithium-Air Batteries: Challenges and Opportunities. Adv. Energy Mater. 2016, 6, 1502164. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Guo, R.; Kou, Z.; Wang, T.; Guan, C.; Ding, J.; Chen, W.; Wang, J. 3D-Printed MOF-Derived Hierarchically Porous Frameworks for Practical High-Energy Density Li–O2 Batteries. Adv. Funct. Mater. 2018, 29, 1806658. [Google Scholar] [CrossRef]
- Meng, X.; Liao, K.; Dai, J.; Zou, X.; She, S.; Zhou, W.; Ye, F.; Shao, Z. Ultralong Cycle Life Li–O2 Battery Enabled by a MOF-Derived Ruthenium−Carbon Composite Catalyst with a Durable Regenerative Surface. ACS Appl. Mater. Interfaces 2019, 11, 20091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transitional Metal (Fe, Co, Ni) Encapsulated in Nitrogen-Doped Carbon Nanotubes as Bi-functional Catalysts for Oxygen Electrode Reactions. J. Mater. Chem. A 2016, 4, 1694. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Liu, Y.; Wang, Z. Nitrogen-Doped Carbon Nanotubes Encapsulated Cobalt Nanoparticles Hybrids for Highly Efficient Catalysis of Oxygen Reduction Reaction. J. Electrochem. Soc. 2018, 165, 3052. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Lin, H.; Gupta, S.; Wang, C.; Tao, Z.; Fu, H.; Wang, T.; Zheng, J.; Wu, G.; et al. Nano Energy Directly Converting Fe-doped Metal–Organic Frameworks into Highly Active and Stable Fe-N-C Catalysts for Oxygen Reduction in Acid. Nano Energy 2016, 25, 110. [Google Scholar] [CrossRef]
- Khalid, M.; Honorato, A.M.B.; Dai, L. Multifunctional Electrocatalysts Derived from Conducting Polymer and Metal Organic Framework Complexes. Nano Energy 2017, 17, 30819. [Google Scholar] [CrossRef]
- Hou, Y.; Hou, C.; Zhai, Y.; Li, H.; Chen, T.; Fan, Y.; Wang, H.; Wang, W. Enhancing the Electrocatalytic Activity of 2D Micro-Assembly Co3O4 Nanosheets for Li–O2 Batteries by Tuning Oxygen Vacancies and Co3þ/Co2þ ratio. Electrochim. Acta 2019, 324, 134884. [Google Scholar] [CrossRef]
- Ma, F.; Wu, H.B.; Xia, B.Y.; Xu, C.; Wen, X.; Lou, D. Hierarchical Beta-Mo2C Nanotubes Organized by Ultrathin Nanosheets as a Highly Efficient Electrocatalyst for Hydrogen Production. Angew. Chem. 2015, 54, 15395. [Google Scholar] [CrossRef]
- Jong, Y.; Hyun, J.; Park, S.; Park, J.; Lee, J.; Chan, Y. Highly Efficient Hierarchical Multiroom-Structured Molybdenum Carbide/ Carbon Composite Microspheres Grafted with Nickel-Nanoparticle-Embedded Nitrogen-Doped Carbon Nanotubes as Air Electrode for Lithium-Oxygen Batteries. Chem. Eng. J. 2018, 351, 886. [Google Scholar]
- Kelly, T.G.; Lee, K.X.; Chen, J.G. Pt-Modified Molybdenum Carbide for the Hydrogen Evolution Reaction: From Model Surfaces to Powder Electrocatalysts. J. Power Sources 2014, 271, 76. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, J.H.; Lee, J.Y.; Park, S.-K.; Kang, Y.C. Design of House Centipede-like MoC–Mo2C Nanorods Grafted with N-Doped Carbon Nanotubes as Bifunctional Catalysts for High-Performance Li–O2 Batteries. Chem. Eng. J. 2019, 19, 32757. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn–Air Batteries and Water Splitting. Adv. Mater. 2018, 1705431, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Zhou, J.; Liu, Y.; Wang, J.; Gao, X.; Han, Y.; Qi, P.; Wang, B. Carbon Dioxide in the Cage: Manganese Metal-Organic Frameworks for High Performance CO2 Electrodes in Li-CO2 Batteries. Energy Environ. Sci. 2018, 11, 1318. [Google Scholar] [CrossRef]
- Chao, F.; Wang, B.; Ren, J.; Lu, Y.; Zhang, W.; Wang, X.; Cheng, L.; Lou, Y.; Chen, J. Micro–Meso-Macroporous FeCo-N-C Derived from Hierarchical Bimetallic FeCo-ZIFs as Cathode Catalysts for Enhanced Li–O2 Batteries Performance. J. Energy Chem. 2019, 35, 212. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Leng, L.; Liu, M.; Huang, L.; Tian, X.; Song, H.; Lu, X. MOF-Templated Sword-Like Co3O4@NiCo2O4 Sheet Arrays on Carbon Cloth as Highly Efficient Li–O2 Battery Cathode. J. Power Sources 2020, 450, 227725. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, F.; Zhang, N.; Han, X.; Chen, J. Nanocomposite of Fe2O3@C@MnO2 as an Efficient Cathode Catalyst for Rechargeable Lithium-Oxygen Batteries. Small 2015, 2, 5545. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, P.; Lee, J.; Jake, T.; Cha, Y.; Ha, S.; Song, M. Enhanced Cycling Performance of Rechargeable Li–O2 Batteries via LiOH Formation and Decomposition Using High-Performance MOF-74@CNTs Hybrid Catalysts. Energy Storage Mater. 2018, 18, 30801. [Google Scholar] [CrossRef]
- Wang, H.; Yin, F.; Liu, N.; Kou, R.; He, X.; Sun, C.; Chen, B.; Liu, D.; Yin, H. Engineering Fe–Fe3C@Fe–N–C Active Sites and Hybrid Structures from Dual Metal–Organic Frameworks for Oxygen Reduction Reaction in H2–O2 Fuel Cell and Li–O2 Battery. Adv. Funct. Mater. 2019, 29, 1901531. [Google Scholar] [CrossRef]
- Chatterjee, A.; Or, S.W. Metal–Organic Framework-Derived MnO/CoMn2O4@N–C nanorods with Nanoparticle Interstitial Decoration in Core@Shell Structure as Improved Bifunctional Electrocatalytic Cathodes for Li–O2 Batteries. Electrochim. Acta 2020, 20, 135809. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Li, M.; Ran, Z.; Shu, C.; Long, J. Cobalt Encapsulated within Porous MOF-Derived Nitrogen-Doped Carbon as an Efficient Bifunctional Electrocatalyst for Aprotic Lithium-Oxygen Battery. J. Alloys Compd. 2019, 810, 151877. [Google Scholar] [CrossRef]
- Yu, S.; Luo, S.; Zhan, Y.; Huang, H.; Wang, Q. Metal-Organic Framework-Derived Cobalt Nanoparticle Space Confined in Nitrogen-Doped Carbon Polyhedra Networks as High-Performance Bifunctional Electrocatalyst for Rechargeable Li–O2 Batteries. J. Power Sources 2020, 453, 227899. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal Organic Frameworks for Energy Storage and Conversion. Energy Storage Mater. 2015, 15, 30056. [Google Scholar] [CrossRef]
- Egan, D.R.; de León, C.P.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in Electrode Materials and Electrolytes for Aluminum-Air Batteries. J. Power Sources 2013, 236, 293. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as Anode for Energy Storage and Conversion: A Review. J. Power Sources 2002, 110, 1. [Google Scholar] [CrossRef]
- Hang, C.; Zhang, J.; Zhu, J.; Li, W.; Kou, Z.; Huang, Y. In Situ Exfoliating and Generating Active Sites on Graphene Nanosheets Strongly Coupled with Carbon Fiber toward Self-Standing Bifunctional Cathode for Rechargeable Zn–Air Batteries. Adv. Energy Mater. 2018, 8, 1703539. [Google Scholar] [CrossRef]
- Xu, J.; Chang, Z.; Wang, Y.; Liu, D.; Zhang, Y.; Zhang, X. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Mater. 2016, 28, 9620. [Google Scholar] [CrossRef]
- Mori, R. Electrochemical Properties of A Rechargeable Aluminum–Air Battery with A Metal–Organic Framework as Air Cathode Material. RSC Adv. 2017, 7, 6389. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Khamis, E.; Adeel, S. Novel Package for Inhibition of Aluminium Corrosion in Alkaline Solutions. Mater. Chem. Phys. 2010, 124, 773. [Google Scholar] [CrossRef]
- Flamini, D.O.; Saidman, S.B.; Bessone, J.B. Aluminum Activation Produced by Gallium. Corros. Sci. 2006, 48, 1413. [Google Scholar] [CrossRef]
- Bessone, J.B.; Flamini, D.O.; Saidman, S.B. Comprehensive Model for the Activation Mechanism of Al–Zn Alloys Produced by Indium. Corros. Sci. 2005, 47, 95. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-Solid-State Al e Air Batteries with Polymer Alkaline Gel Electrolyte. J. Power Sources 2014, 251, 470. [Google Scholar] [CrossRef]
- Nestoridi, M.; Pletcher, D.; Wood, R.J.K.; Wang, S.; Jones, R.L.; Stokes, K.R.; Wilcock, I. The Study of Aluminium Anodes for High Power Density Al/Air Batteries with Brine Electrolytes. J. Power Sources 2008, 178, 445. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Ren, J.; Zhang, Y.; Peng, H. An All-Solid-State Fiber-Shaped Aluminum–Air Battery with Flexibility, Stretchability, and High Electrochemical Performance. Angew. Chem. 2016, 128, 1. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Zhang, Z.; Wang, L.; Sun, P.; Wang, S. Design of Three Dimensional Interconnected Hierarchical Micro- Mesoporous Structure of Graphene as Support Material for Spinel NiCoO Electrocatalyst toward Oxygen Reduction Reaction. J. Phys. Chem. C 2018, 48, 27469. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wang, L.; Lu, X.; Wang, S.; Niu, X. Vertical-Space-Limit Synthesis of Bi-Functional Fe, N codoped Two- Dimensional Multilayers Graphene Electrocatalysts for Zn-air Battery. Energy Technol. 2019, 7, 1900123. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Ju, J.; Zhang, W.; He, L.; Zhang, J.; Fu, C.; Sun, B. Pace-Confined Synthesis of CoNi nanoalloy in N-doped Porous Carbon Frameworks as Efficient Oxygen Reduction Catalyst for Neutral and Alkaline Aluminum-air Batteries. Energy Storage Mater. 2020, 20, 30022. [Google Scholar]
- Liu, Y.; Jiang, H.; Hao, J.; Liu, Y.; Shen, H.; Li, W.; Li, J. Metal-Organic Framework Derived Reduced Graphene Oxide Supported ZnO/ZnCo2O4/C Hollow Nanocages as Cathode Catalysts for Aluminum-O2 Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31841. [Google Scholar] [CrossRef]
- Li, J.; Zhou, N.; Song, J.; Fu, L.; Yan, J.; Tang, Y.; Wang, H. Cu-MOF-Derived Cu/Cu2O Nanoparticles and CuNxCy Species to Boost Oxygen Reduction Activity of Ketjenblack Carbon in Al-Air Battery. ACS Sustain. Chem. Eng. 2018, 6, 413. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Hong, Q.; Cao, Y.; Li, X.; Bai, J. Porous N,S-Codoped Carbon Architectures with Bimetallic Sulphide Nanoparticles Encapsulated in Graphitic Layers: Highly Active and Robust Electrocatalysts for the Oxygen Reduction Reaction in Al-Air Batteries. Chem. Eng. J. 2017, 17, 31415. [Google Scholar] [CrossRef]
- Lou, P.; Li, C.; Cui, Z.; Guo, X. Job-Sharing Cathode Design for Li–O2 Batteries with High Energy Efficiency Enabled by In-Situ Ionic Liquid Bonding to Cover Carbon Surface Defects. J. Mater. Chem. A 2015, 8, 1703539. [Google Scholar]
- Zhang, S.S.; Foster, D.; Read, J. Discharge Characteristic of A Non-Aqueous Electrolyte Li/O2 Battery. J. Power Sources 2010, 195, 1235. [Google Scholar] [CrossRef]
- Wei, S.; Xu, S.; Agrawral, A.; Choudhury, S.; Lu, Y.; Tu, Z.; Ma, L.; Archer, L.A. A Stable Room-Temperature Sodium–Sulfur Battery. Nat. Commun. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Wei, S.; Ozhabes, Y.; Gunceler, D.; Zachman, M.J.; Tu, Z.; Shin, J.H.; Nath, P.; Agrawal, A.; Kourkoutis, L.F.; et al. Designing Solid-Liquid Interphases for Sodium Batteries. Nat. Commun. 2017, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xia, G.; Lemmon, J.P.; Yang, Z. Advanced Materials for Sodium-Beta Alumina Batteries: Status, Challenges and Perspectives. J. Power Sources 2010, 195, 2431. [Google Scholar] [CrossRef]
- Kim, S.; Seo, D.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710. [Google Scholar] [CrossRef]
- Kim, M.; Ju, H.; Kim, J. Highly Efficient Bifunctional Catalytic Activity of Bismuth Rhodium Oxide Pyrochlore Through Tuning the Covalent Character for Rechargeable Aqueous Na-Air Batteries. J. Mater. Chem. A 2018, 6, 8523. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Mazor, H.; Goor, M.; Avshalomov, S. Parameter Analysis of A Practical Lithium- and Sodium-Air Electric Vehicle Battery. J. Power Sources 2011, 196, 6835. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can Hybrid Na–Air Batteries Outperform Nonaqueous Na–O2 Batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H.; Gwon, H.; Kang, K. Sodium–Oxygen Batteries with Alkyl-Carbonate and Ether Based Electrolytes. Phys. Chem. Chem. Phys. 2013, 15, 3623. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal–Air Batteries. Nat. Chem. 2011, 3, 546. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2012, 1383, 1383–1385. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A Metal-Free Bifunctional Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions. Nat. Nanotechnol. 2015, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hou, C.; Wang, C.; Yang, X.; Chen, Y. Ir4+-Doped NiFe LDH to Expedite Hydrogen Evolution Kinetics as A Pt-Like Electrocatalyst for Water Splitting. Chem. Commun. 2018, 54, 6400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries. Electrochem. Energy Rev. 2018, 1, 1. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, T.; Su, F.; Wu, Y.; Kang, Y.; Chen, K.; Yao, Y.; Ma, W.; Yang, B.; Dai, Y.; et al. Highly dispersed Co Nanoparticles Decorated on N-doped Defective Carbon Nano-Framework for Hybrid Na-Air battery. Dalton Trans. 2020, 49, 1811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiaowei, L.; Qi, C.; Yuexiu, H.; Jiaqi, D.; Xinghao, H.; Yu, L. Metal-Organic Framework-Derived Hierarchical Co3O4@MnCo2O4.5 Nanocubes with Enhanced Electrocatalytic Activity for Na-O2 Batteries. Nanoscale 2019, 11, 5285. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilal, M.E.; Aboulouard, A.; Akbar, A.R.; Younus, H.A.; Horzum, N.; Verpoort, F. Progress of MOF-Derived Functional Materials Toward Industrialization in Solar Cells and Metal-Air Batteries. Catalysts 2020, 10, 897. https://doi.org/10.3390/catal10080897
Hilal ME, Aboulouard A, Akbar AR, Younus HA, Horzum N, Verpoort F. Progress of MOF-Derived Functional Materials Toward Industrialization in Solar Cells and Metal-Air Batteries. Catalysts. 2020; 10(8):897. https://doi.org/10.3390/catal10080897
Chicago/Turabian StyleHilal, Mohamed Elhousseini, Abdelkhalk Aboulouard, Abdul Rehman Akbar, Hussein A. Younus, Nesrin Horzum, and Francis Verpoort. 2020. "Progress of MOF-Derived Functional Materials Toward Industrialization in Solar Cells and Metal-Air Batteries" Catalysts 10, no. 8: 897. https://doi.org/10.3390/catal10080897
APA StyleHilal, M. E., Aboulouard, A., Akbar, A. R., Younus, H. A., Horzum, N., & Verpoort, F. (2020). Progress of MOF-Derived Functional Materials Toward Industrialization in Solar Cells and Metal-Air Batteries. Catalysts, 10(8), 897. https://doi.org/10.3390/catal10080897







