Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses
Abstract
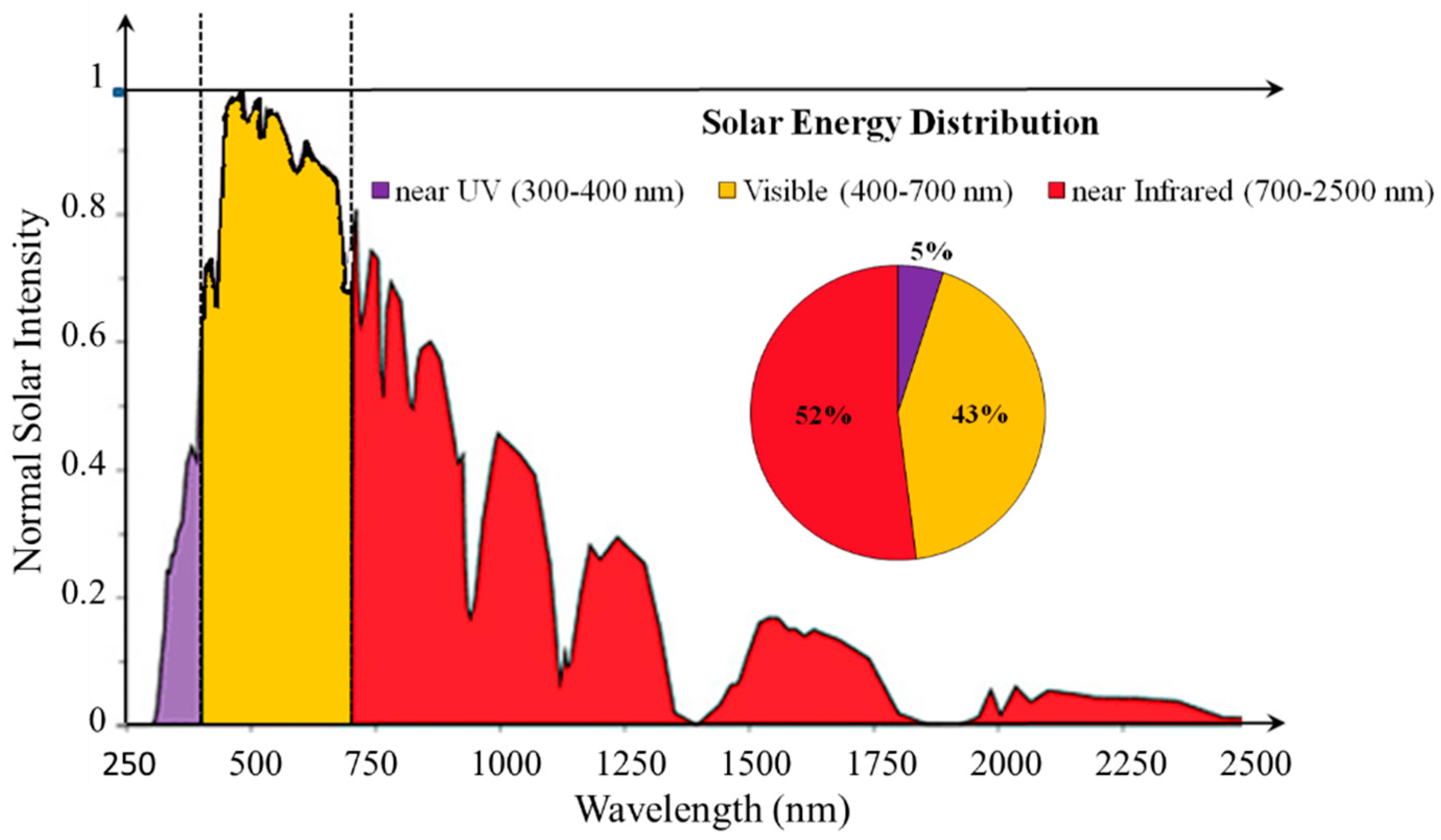
1. Introduction
2. Photocatalysts for Air Treatment
2.1. Commercial TiO2-Based Photocatalysts
2.2. Synthesis of TiO2-Based Photocatalysts
3. Photocatalytic Reactors for Air Treatment
3.1. Type of Irradiation
3.2. Position of Light Source
3.3. Photocatalysts Deposition Methods in Photo-Air Reactors
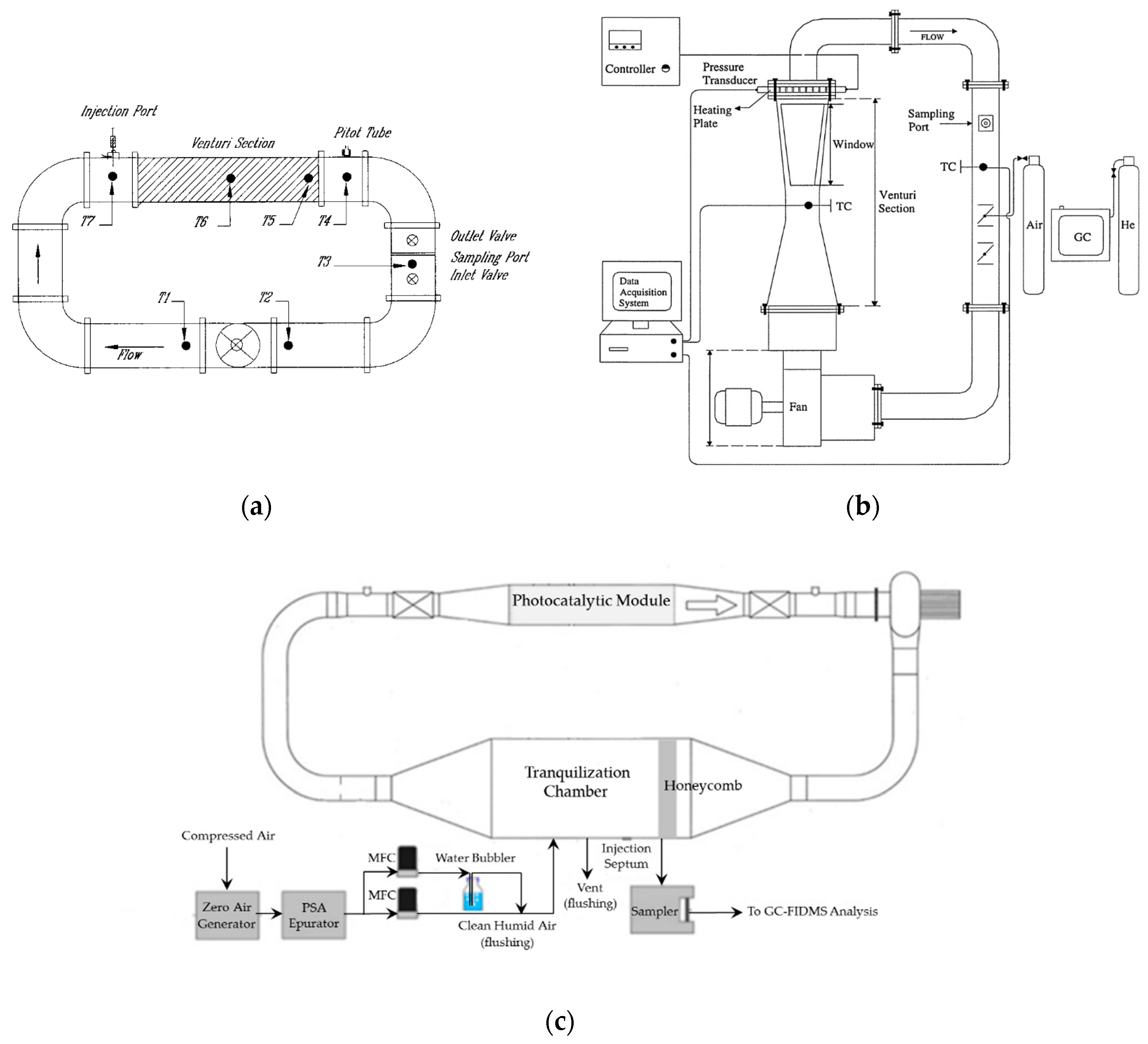
3.4. Experimental Photocatalytic Air Reactors for the Photodegradation of Organic Pollutants
4. Photodegradation Modeling Studies for Air Treatment Using TiO2-Based Photocatalysts
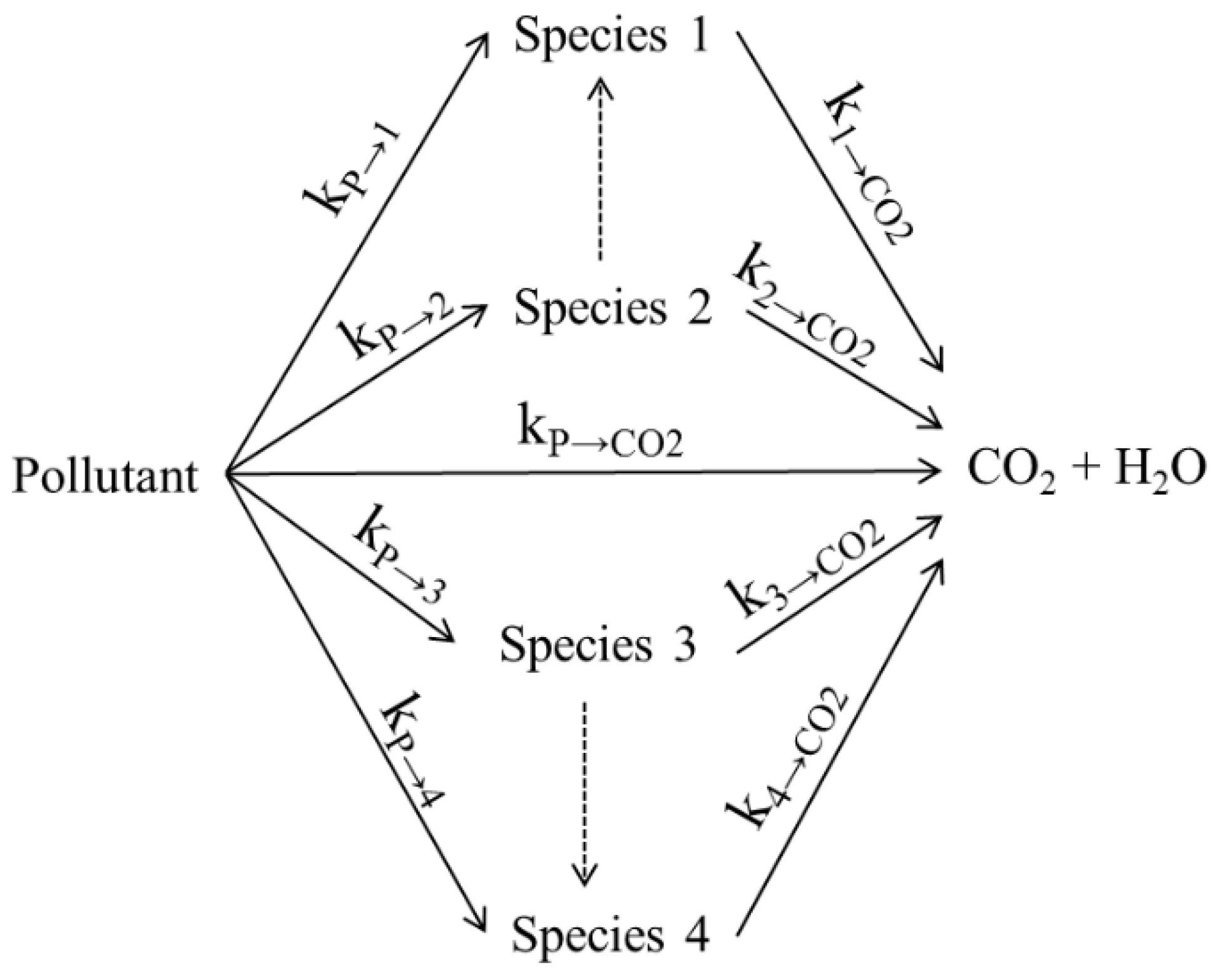
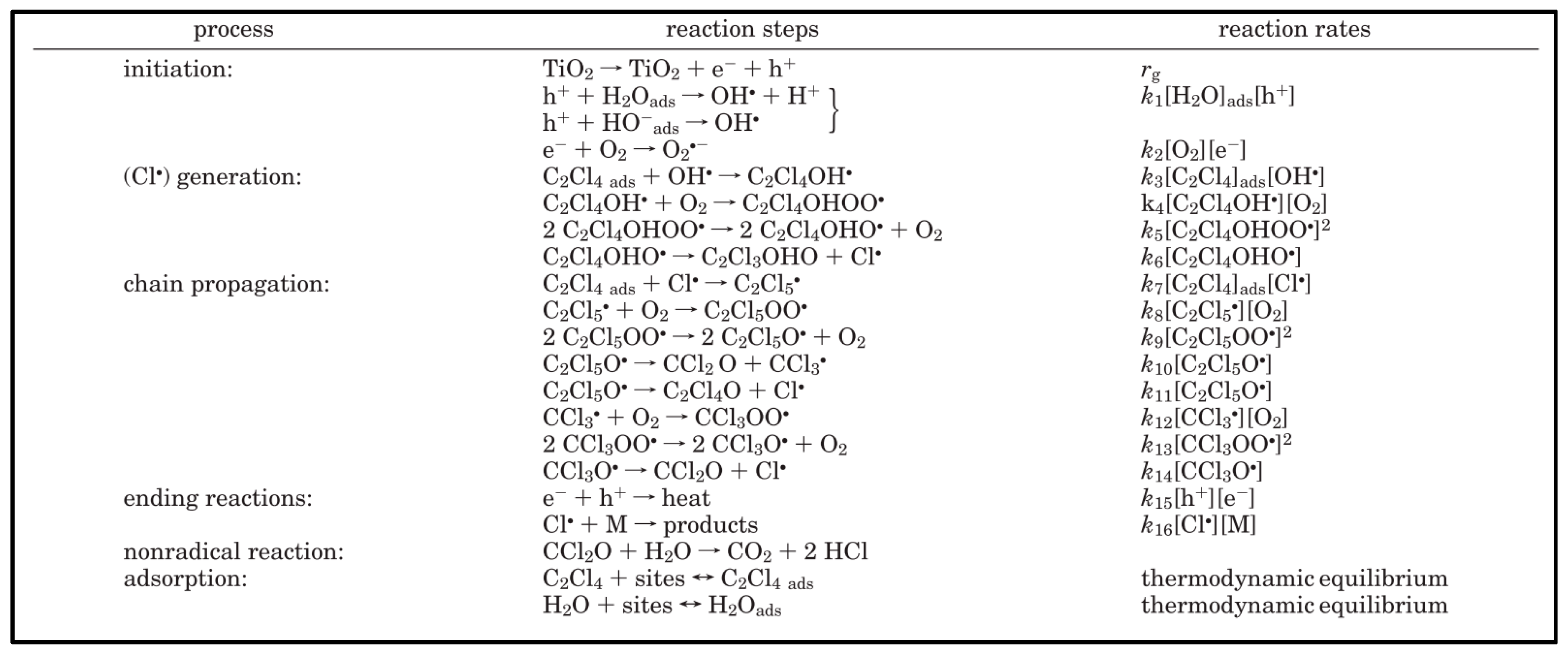
4.1. Reaction Mechanism Development
4.2. Adsorption and Kinetic Modeling Development
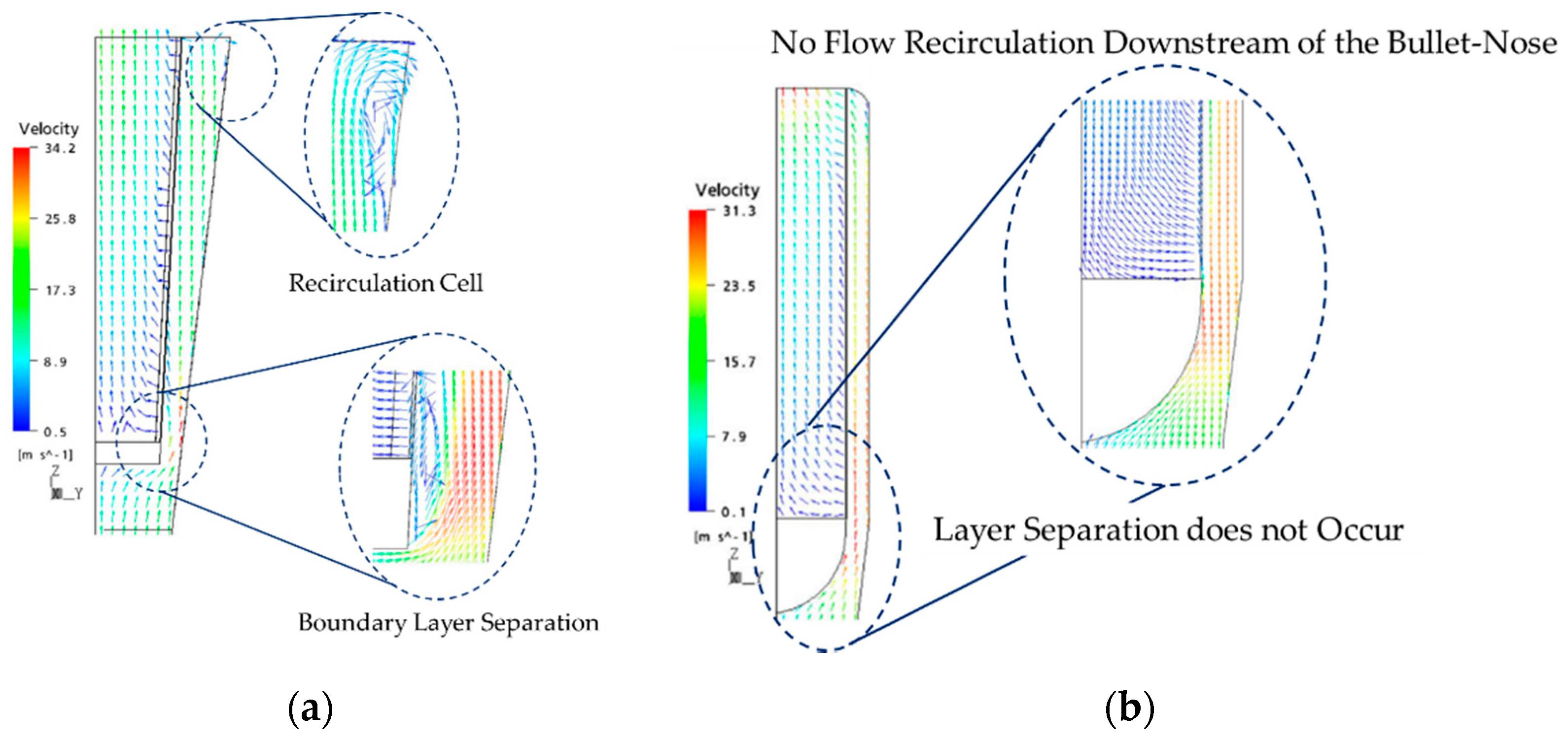
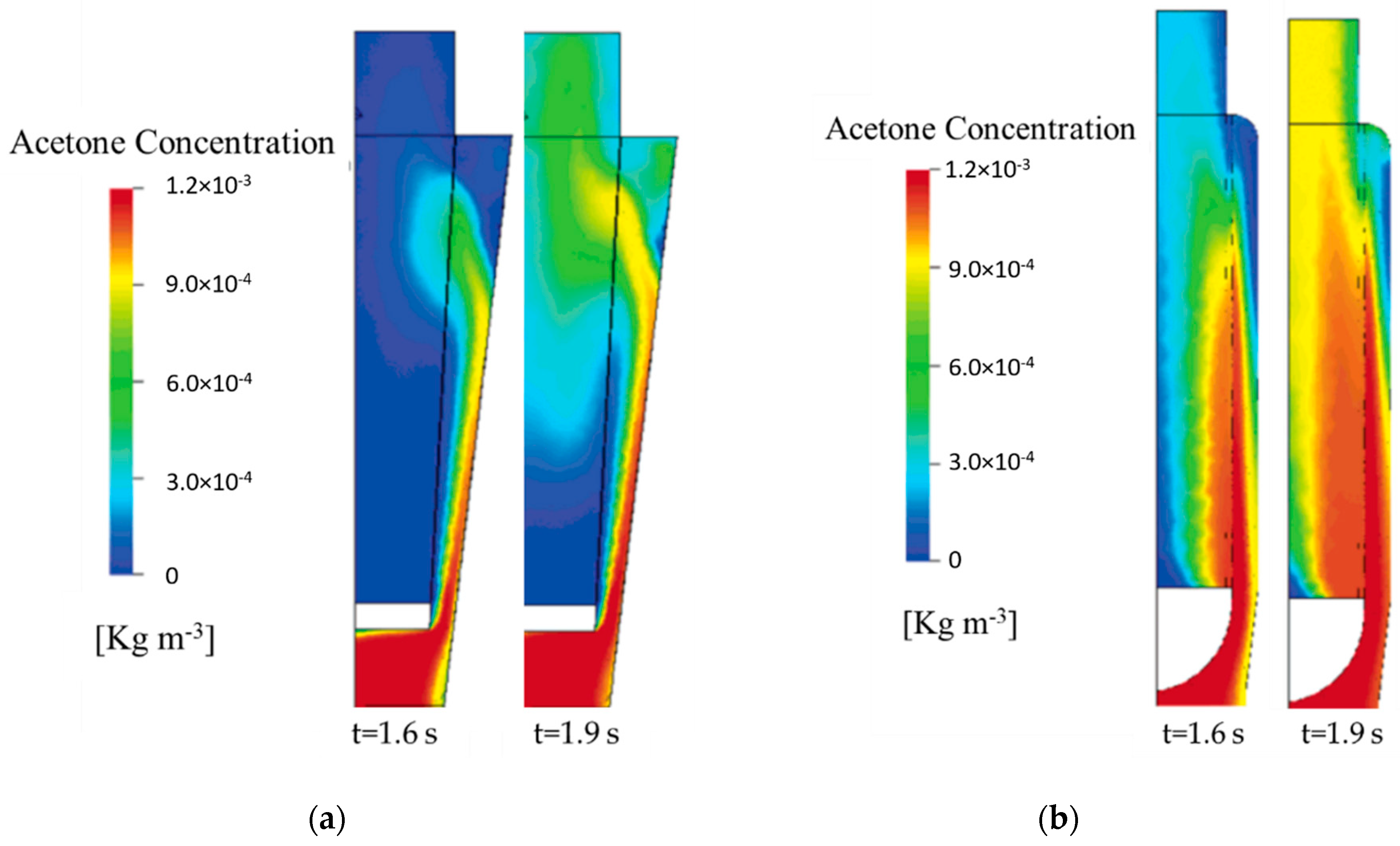
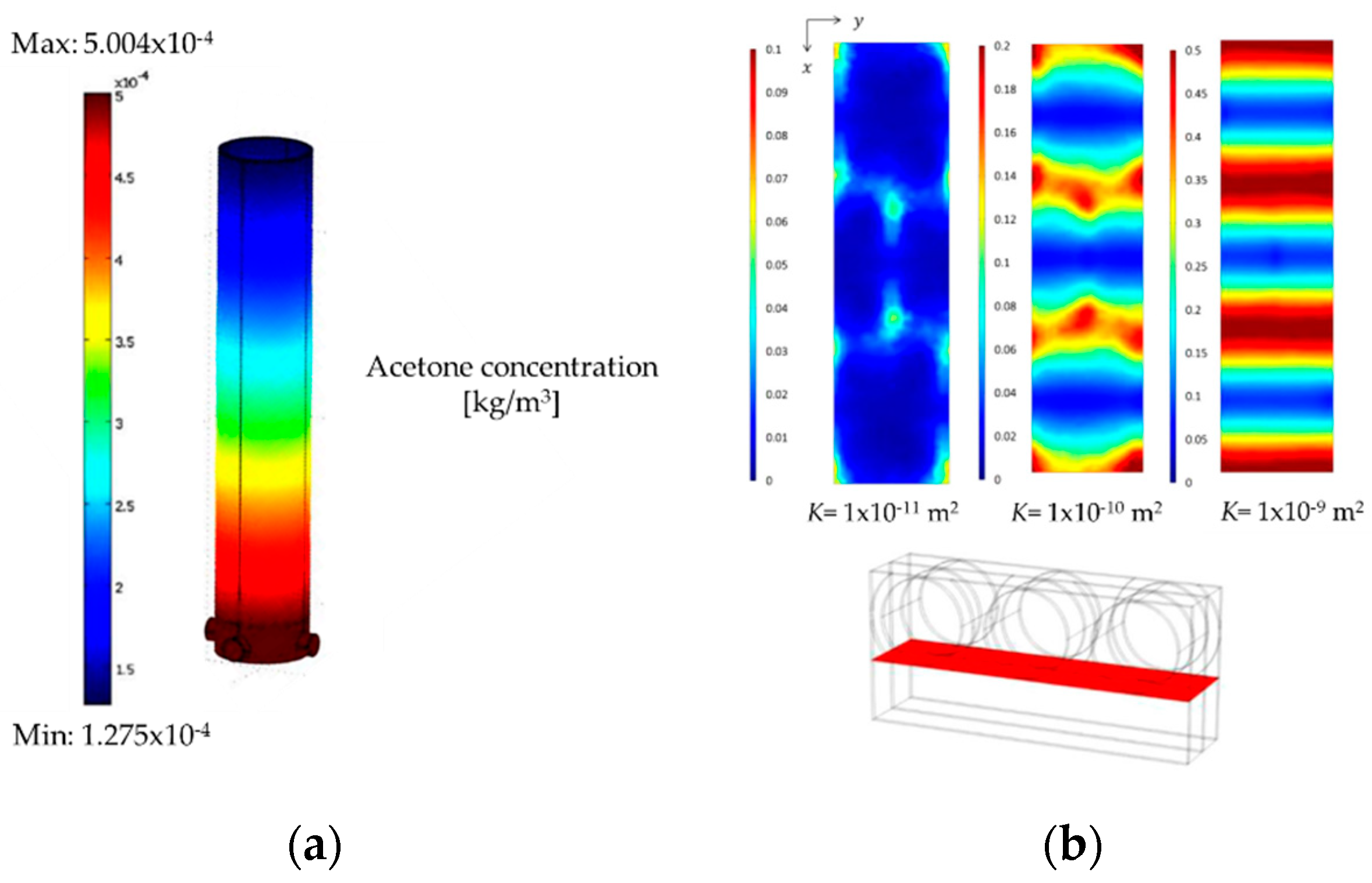
4.3. Computation Fluid Dynamic (CFD) Development
- (a)
- Reynolds-averaged mass,
- (b)
- Momentum,
- (c)
- Acetone transport,
5. Energy Efficiency Studies for Air Treatment Photoreactors
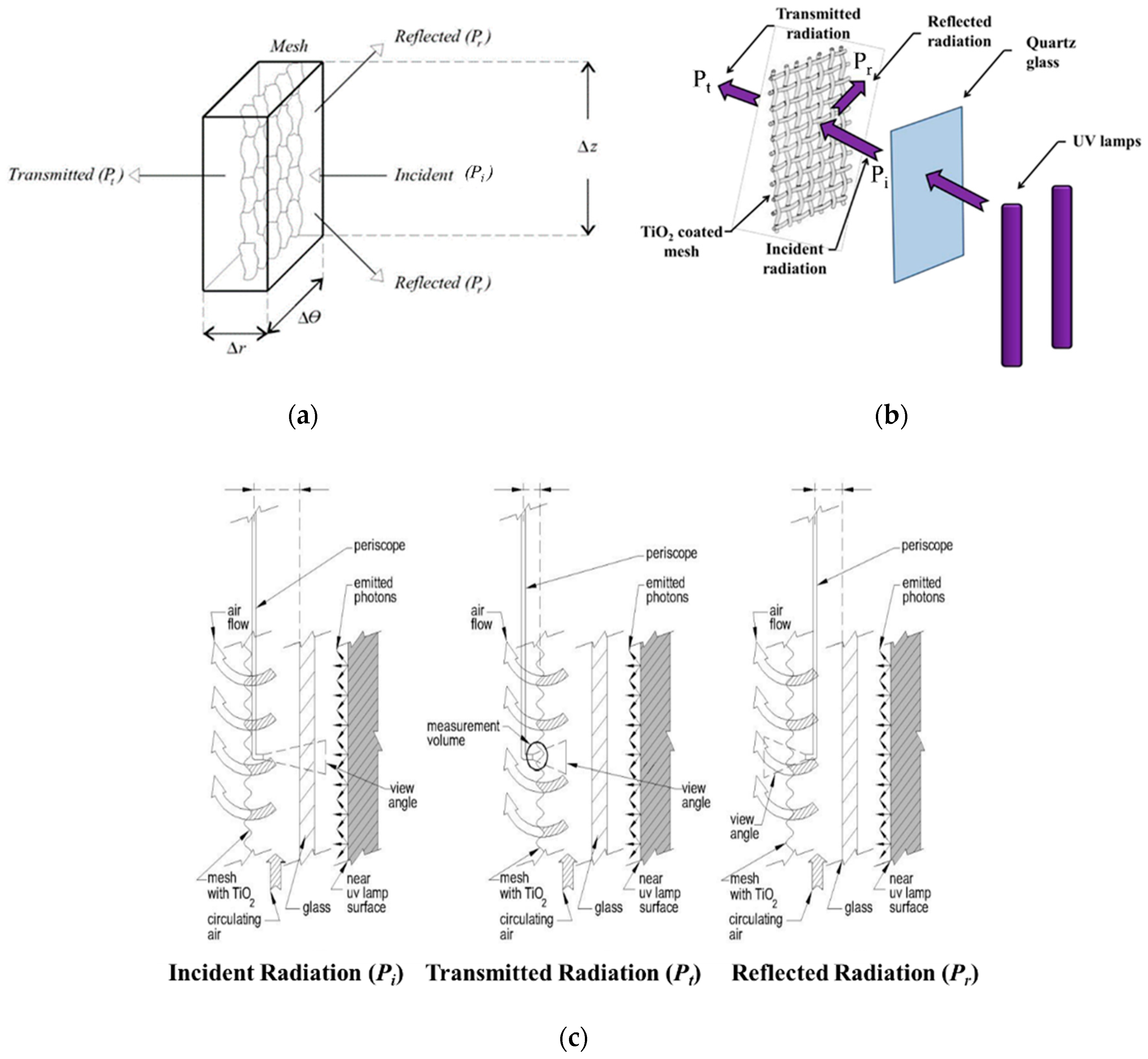
5.1. Quantum Yield (QY or )
- (a)
- elemental carbon balance:
- (b)
- elemental hydrogen balance:
- (c)
- elemental oxygen balance:
5.2. Photochemical Thermodynamic Efficiency Factors (PTEFs)
6. Challenges and Opportunities for Photocatalytic Conversion of Air Pollutants
7. Conclusions
- (a)
- TiO2-based photocatalysts irradiated with near-UV light offer an important application for the photocatalytic oxidation of organic pollutants (i.e., VOCs), both in air and water.
- (b)
- TiO2 photocatalysts are exceptional materials for the photoconversion of organic pollutants. These semiconductor materials can be modified by a diversity of methods such as deposition, impregnation, or doping.
- (c)
- A diversity of immobilization techniques can be employed for PCO. The immobilization selection method is dependent on the mesh material type (i.e., quartz, glass, stainless steel, polymers, or fabric).
- (d)
- CFD models allow the effective pretesting of various operational conditions and flow patterns in photocatalytic reactors.
- (e)
- Different reaction mechanisms can be considered for the complete mineralization of VOCs into CO2 and H2O, with the “parallel-in series” method proving to be a most relevant approach for the photoconversion of organic pollutants in air and water.
- (f)
- Macroscopic irradiation energy balances are required to calculate the photons absorbed by the semiconductor material during PCO.
- (g)
- Tracking all chemical species, including model pollutants and intermediates, is required to assess OH• consumption rates.
- (h)
- Quantum Yields provide energy efficiencies in photocatalytic reactors, with an adequate definition based on the ratio of OH• radicals consumed over the rate of photons absorbed by the photocatalyst.
- (i)
- The PTEF is a valuable parameter to determine photocatalytic reactor efficiencies on the basis of thermodynamics principles.
- (j)
- Photocatalytic reactors have the potential to be re-engineered for SARS-CoV2 (COVID-19) virus inactivation in hospitals and other indoor enclosed spaces. It is anticipated that this will be achieved with high IQYs (Inactivation Quantum Yields).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Uniformly irradiated mesh area holding an optimum loading of TiO2 (m2) |
| Ag | Silver |
| Au | Gold |
| c | Speed of light (3 × 108 m/s) |
| C | Carbon |
| Co(CH3CO2)2 | Cobalt (II) acetate |
| C2H4O | Acetaldehyde |
| C3H6O | Acetone |
| Mn(CH3CO2)2 | Manganese (II) acetate |
| CO2 | Carbon dioxide |
| Co | Cobalt |
| Cr | Chrome |
| Acetone concentration (kg/m3) | |
| Ceq,i | Equilibrium of species “i” in the gas phase concentration (mol/m3 or ppm) |
| Ceq,Inf | Inflection point observed on the adsorption isotherm (mol/m3) |
| D | Diffusivity of acetone in air (m2/s) |
| Dp-BJH | Pore diameter (nm) |
| Dt | Eddy diffusivity (m2/s) |
| e- | Electron |
| Eav | Average photon energy (kJ/mol photon). |
| EBG | Energy band gap (eV) |
| eV | Electron volts |
| Fe | Iron |
| Fe+3 | Iron-III |
| h | Planck’s constant (6.63 × 10−34 J/s)) |
| h+ | Hole |
| H2O | Water |
| HNO3 | Nitric acid |
| K | Permeability (m2) |
| ki | Equilibrium kinetic constant of specie “i” (mol/s m3) |
| Adsorption equilibrium constant (m3/mol or ppm−1) | |
| Langmuir adsorption equilibrium constant (m3/mol or ppm−1) | |
| Freundlich adsorption equilibrium constant (m3/mol or ppm−1) | |
| L | Liters |
| Mn | Manganese |
| MS2 | Non-pathogenicity to humans norovirus structure |
| n | Number of layers |
| N | Nitrogen (solid) |
| N2 | Nitrogen (gas) |
| NH3 | Ammonia |
| NH4F | Ammonium fluoride |
| NH4VO3 | Ammonium metavanadate |
| NO | Nitrogen oxide |
| NO2 | Nitrogen dioxide |
| Carbon atoms at initial conditions (atoms of carbon inactivated/s) | |
| O− | Oxidized |
| O2 | Oxygen |
| O2- | Superoxide radicals |
| OH• | Hydroxyl radicals |
| Pa | Rate of absorbed photons (mol of photons/s). |
| Incident radiation | |
| Reflected radiation | |
| Transmitted radiation | |
| Pd | Palladium |
| Pt | Platinum |
| q (θ,z,λ) | Radiation measured (W/cm2 nm) |
| Qeq, ads | Existing equilibrium adsorption surface concentration (mol/gcat) |
| Qeq, max | Maximum equilibrium adsorption surface concentration (mol/gcat) |
| Qeq, max-1 | Langmuir maximum equilibrium adsorption surface concentrations (mol/gcat) |
| Qeq, max-2 | Freundlich maximum equilibrium adsorption surface concentrations (mol/gcat) |
| R+ | Reduced |
| S | Sulphur |
| SBET | Surface Area (m2/g) |
| Acetone source/sink term (kg/m3 s) | |
| Momentum source/sink term (kg/m2 s2) | |
| t | Time (s) |
| Ti | Titanium |
| TiCl4 | Titanium tetrachloride |
| TiO2 | Titanium dioxide |
| Ti(OCH(CH3)2)4 | Titanium tetraisopropoxide |
| V | Total volume in PCAR reactors (m3) |
| V | Vanadium |
| V2O5 | Vanadium (V) oxide |
| Vp-BJH | Pore volume (cm3/g) |
| W(CO6) | Tungsten hexacarbonyle |
| wt.% | Weight percent (%) |
| Acronyms | |
| A | Anatase |
| AAS-ASC | Air Assisted Spray-Automatized Spinning Coating |
| BET | Brunauer-Emmett-Teller |
| BJH | Barrett-Joyner-Halenda |
| BTX | Benzene-Toluene-Xylene |
| CB | Conduction Band |
| CFD | Computational Fluid Dynamics |
| COVID-19 | Corona Virus -19 (SARS-CoV2) |
| CREC | Chemical Reactor Engineer Centre |
| CVD | Chemical Vapor Deposition |
| DB | Debye Sherrer |
| DP25 | Degussa P25 (Commercial TiO2) |
| FQE | Formal Quantum Efficiency |
| HEPA | High-Efficiency Particulate Air Filter |
| IQYs | Inactivation Quantum Yields |
| LED | Light-Emitting Diode |
| LH | Langmuir-Hinshelwood |
| MIEB | Macroscopic Irradiation Energy Balances |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCAR | Photocatalytic Oxidation |
| PFU | Particle Forming Units |
| PTEFs | Photocatalytic Thermodynamic Efficiency Factors |
| PU | Porous Polyurethane |
| QYs | Quantum Yields |
| QYapp | Apparent Quantum Yield |
| R | Rutile |
| RVE | Representative Volume Element |
| SARS | Severe Acute Respiratory Syndrome |
| SS | Stainless Steel |
| SST | Shear Stress Transport |
| UV | Ultraviolet |
| VB | Valence Band |
| VOCs | Volatile Organic Compounds |
| VUV | Vacuum Ultraviolet |
| Symbols | |
| θ | Angular position (°) |
| Dimensionless surface species concentration | |
| Rate of photoconversion of the model pollutant “i” (mol/min*m2) | |
| Enthalpy invested in the formation of the OH• radicals (J/mol) | |
| λ | Radiation wavelength (nm) |
| Fluid density (kg/m3) | |
| Fluid velocity vector (m/s) | |
| Dimensionless ratio between the interface slip velocity | |
| Stress tensor (N/m2) | |
| Reynolds stress tensor (N/m2) | |
| Stoichiometric coefficient | |
| r | Radial position (cm) |
| Fraction of photon energy used to form OH• radicals | |
| Gradient vector of x, y and z coordinates | |
| Tensor product |
References
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Ibrahim, H.; Lasa, H. de Photo-catalytic conversion of air borne pollutants Effect of catalyst type and catalyst loading in a novel photo-CREC-air unit. Appl. Catal. B Environ. 2002, 38, 201–213. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S. Evaluation of VOC Degradation in Photo- Catalytic Air Reactors: TiO2 Immobilization, Energy Efficiency and Kinetic Modeling. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2016. [Google Scholar]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Dales, R.; Liu, L.; Wheeler, A.J.; Gilbert, N.L. Quality of indoor residential air and health. Can. Med. Assoc. J. 2008, 179, 147–152. [Google Scholar] [CrossRef]
- Indoor Air Quality | EPA’s Report on the Environment (ROE) | US EPA. Available online: https://www.epa.gov/report-environment/indoor-air-quality (accessed on 20 April 2020).
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, J.M.; Serrano-Rosales, B.; De Lasa, H. Energy efficiencies in a photo-CREC-air reactor: Conversion of model organic pollutants in air. Ind. Eng. Chem. Res. 2012, 51, 5715–5727. [Google Scholar] [CrossRef]
- Castrillón, S.R.V.; De Lasa, H.I. Performance evaluation of photocatalytic reactors for air purification using Computational Fluid Dynamics (CFD). Ind. Eng. Chem. Res. 2007, 46, 5867–5880. [Google Scholar] [CrossRef]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reaction Engineering, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 978-0-387-27591-8. [Google Scholar]
- Sirivallop, A.; Areerob, T.; Chiarakorn, S. Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Sity Solvothermal Method for Gas Phase Ammonia Removal. Catalysts 2020, 10, 251. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S.; Serrano-Rosales, B.; de Lasa, H. Immobilized particle coating for optimum photon and TiO2 utilization in scaled air treatment photo reactors. Appl. Catal. B Environ. 2016, 198, 211–223. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Ibrahim, H.; De Lasa, H. Flow field investigation in a photocatalytic reactor for air treatment (Photo-CREC-air). Chem. Eng. Sci. 2006, 61, 3343–3361. [Google Scholar] [CrossRef]
- Hernandez, J.M.G.; Rosales, B.S.; de Lasa, H. The photochemical thermodynamic efficiency factor (PTEF) in photocatalytic reactors for air treatment. Chem. Eng. J. 2010, 165, 891–901. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S.; Serrano-Rosales, B.; de Lasa, H. Energy efficiency limits in Photo-CREC-Air photocatalytic reactors. Chem. Eng. Sci. 2016, 156, 77–88. [Google Scholar] [CrossRef]
- Ibrahim, H.; de Lasa, H. Photo-catalytic degradation of air borne pollutants apparent quantum efficiencies in a novel photo-CREC-air reactor. Chem. Eng. Sci. 2003, 58, 943–949. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photocatalytic Hydrogen Production Under Near-UV Using Pd-Doped Mesoporous TiO2 and Ethanol as Organic Scavenger. Catalysts 2019, 9, 33. [Google Scholar] [CrossRef]
- Escobedo, S.; Serrano, B.; de Lasa, H. Quantum yield with platinum modified TiO2 photocatalyst for hydrogen production. Appl. Catal. B Environ. 2013, 523–536. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts 2020, 10, 74. [Google Scholar] [CrossRef]
- Deng, X.; Yue, Y.; Gao, Z. Gas-phase photo-oxidation of organic compounds over nanosized TiO2 photocatalysts by various preparations. Appl. Catal. B Environ. 2002, 39, 135–147. [Google Scholar] [CrossRef]
- Benmami, M.; Chhor, K.; Kanaev, A.V. High photocatalytic activity of monolayer nanocoatings prepared from non-crystalline titanium oxide sol nanoparticles. Chem. Phys. Lett. 2006, 422, 552–557. [Google Scholar] [CrossRef]
- Esterkin, C.R.; Negro, A.C.; Alfano, O.M.; Cassano, A.E. Air pollution remediation in a fixed bed photocatalytic reactor coated with TiO2. AIChE J. 2003, 51, 2298–2310. [Google Scholar] [CrossRef]
- Peill, N.J.; Hoffmann, M.R. Development and Optimization of a TiO2-Coated Fiber-Optic Cable Reactor: Photocatalytic Degradation of 4-Chlorophenol. Environ. Sci. Technol. 1995, 29, 2974–2981. [Google Scholar] [CrossRef]
- Zhu, R.; Che, S.; Liu, X.; Lin, S.; Xu, G.; Ouyang, F. A novel fluidized-bed-optical-fibers photocatalytic reactor (FBOFPR) and its performance. Appl. Catal. A Gen. 2014, 471, 136–141. [Google Scholar] [CrossRef]
- Wu, Y.T.; Yu, Y.H.; Nguyen, V.H.; Lu, K.T.; Wu, J.C.S.; Chang, L.M.; Kuo, C.W. Enhanced xylene removal by photocatalytic oxidation using fiber-illuminated honeycomb reactor at ppb level. J. Hazard. Mater. 2013, 262, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Carneiro, J.T.; Moulijn, J.A.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. A Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Miranda, S.M.; Rodrigues-Silva, C.; Faria, J.L.; Silva, A.M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Gas phase oxidation of n-decane and PCE by photocatalysis using an annular photoreactor packed with a monolithic catalytic bed coated with P25 and PC500. Appl. Catal. B Environ. 2015, 165, 306–315. [Google Scholar] [CrossRef]
- Han, Z.; Chang, V.W.C.; Zhang, L.; Tse, M.S.; Tan, O.K.; Hildemann, L.M. Preparation of TiO2-coated polyester fiber filter by spray-coating and its photocatalytic degradation of gaseous formaldehyde. Aerosol Air Qual. Res. 2012, 12, 1327–1335. [Google Scholar] [CrossRef]
- Birnie, M.; Riffat, S.; Gillott, M. Photocatalytic reactors: Design for effective air purification. Int. J. Low-Carbon Technol. 2006, 1, 47–58. [Google Scholar] [CrossRef]
- Hou, W.M.; Ku, Y. Photocatalytic decomposition of gaseous isopropanol in a tubular optical fiber reactor under periodic UV-LED illumination. J. Mol. Catal. A Chem. 2013, 374–375, 7–11. [Google Scholar] [CrossRef]
- Ibrahim, H.; De Lasa, H. Kinetic Modeling of the Photocatalytic Degradation of Air-Borne Pollutants. AIChE J. 2004, 50, 1017–1027. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Kozák, O.; Lacný, Z.; Plachá, D.; Praus, P.; Obalová, L. Influence of reactor geometry on the yield of CO2 photocatalytic reduction. Catal. Today 2011, 176, 212–214. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S.; Moreira, J.; Serrano-Rosales, B.; de Lasa, H. Kinetics of the pollutant photocatalytic conversion in a Photo-CREC-Air Reactor. Chem. Eng. J. 2017, 317, 1069–1082. [Google Scholar] [CrossRef]
- Escobedo, S.; Serrano, B.; Calzada, A.; Moreira, J.; de Lasa, H. Hydrogen production using a platinum modified TiO2 photocatalyst and an organic scavenger. Kinetic modeling. Fuel 2016, 181, 438–449. [Google Scholar] [CrossRef]
- Vincent, G.; Schaer, E.; Marquaire, P.M.; Zahraa, O. CFD modelling of an annular reactor, application to the photocatalytic degradation of acetone. Process Saf. Environ. Prot. 2011, 89, 35–40. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Ang, M.; Pareek, V. Some aspects of photocatalytic reactor modeling using computational fluid dynamics. Chem. Eng. Sci. 2013, 101, 764–784. [Google Scholar] [CrossRef]
- Queffeulou, A.; Geron, L.; Schaer, E. Prediction of photocatalytic air purifier apparatus performances with a CFD approach using experimentally determined kinetic parameters. Chem. Eng. Sci. 2010, 65, 5067–5074. [Google Scholar] [CrossRef]
- Ibrahim, H.; De Lasa, H. Novel photocatalytic reactor for the destruction of airborne pollutants reaction kinetics and quantum yields. Ind. Eng. Chem. Res. 1999, 38, 3211–3217. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Dosta, S.; Guilemany, J.M.; Brillas, E. Solar photoelectrocatalytic degradation of Acid Orange 7 azo dye using a highly stable TiO2 photoanode synthesized by atmospheric plasma spray. Appl. Catal. B Environ. 2013, 132–133, 142–150. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.; Ma, W. Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Top. Catal. 2005, 35, 269–278. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Serrano, B.; Ortíz, A.; Moreira, J.; De Lasa, H.I. Energy efficiency in photocatalytic reactors for the full span of reaction times. Ind. Eng. Chem. Res. 2009, 48, 9864–9876. [Google Scholar] [CrossRef]
- Escobedo, S.; Rusinque, B.; de Lasa, H. Photochemical Thermodynamic Efficiency Factors (PTEFs) for Hydrogen Production Using Different TiO2 Photocatalysts. Ind. Eng. Chem. Res. 2019, 58, 22225–22235. [Google Scholar] [CrossRef]
- Serrano, B.; Ortíz, A.; Moreira, J.; De Lasa, H.I. Photocatalytic thermodynamic efficiency factors. Practical limits in photocatalytic reactors. Ind. Eng. Chem. Res. 2010, 49, 6824–6833. [Google Scholar] [CrossRef]
- Keller, V.; Keller, N.; Ledoux, M.J.; Lett, M.C. Biological agent inactivation in a flowing air stream by photocatalysis. Chem. Commun. 2005, 2918–2920. [Google Scholar] [CrossRef]
- Gerrity, D.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J. Environ. Sci. Heal.—Part A Toxic/Hazardous Subst. Environ. Eng. 2008, 43, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci. Technol. 2018, 52, 557–566. [Google Scholar] [CrossRef]
- Yao, Y.; Ochiai, T.; Ishiguro, H.; Nakano, R.; Kubota, Y. Antibacterial performance of a novel photocatalytic-coated cordierite foam for use in air cleaners. Appl. Catal. B Environ. 2011, 106, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A review on the synthesis and characterization of metal organic frameworks for photocatalytic water purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Salaices, M.; Serrano, B.; De Lasa, H.I. Photocatalytic conversion of organic pollutants extinction coefficients and quantum efficiencies. Ind. Eng. Chem. Res. 2001, 40, 5455–5464. [Google Scholar] [CrossRef]
- Rosales, B.S.; Moreira Del Rio, J.; Guayaquil, J.F.; De Lasa, H. Photodegradation Efficiencies in a Photo-CREC Water-II Reactor Using Several TiO2 Based Catalysts. Int. J. Chem. React. Eng. 2016, 14, 685–701. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Haghighatmamaghani, A.; Haghighat, F.; Lee, C.S. Performance of various commercial TiO2 in photocatalytic degradation of a mixture of indoor air pollutants: Effect of photocatalyst and operating parameters. Sci. Technol. Built Environ. 2019, 25, 600–614. [Google Scholar] [CrossRef]
- Nuño, M.; Ball, R.J.; Bowen, C.R. Photocatalytic Properties of Commercially Available TiO2 Powders for Pollution Control. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; InTech: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Sun, H.; Ullah, R.; Chong, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Room-light-induced indoor air purification using an efficient Pt/N-TiO2 photocatalyst. Appl. Catal. B Environ. 2011, 108–109, 127–133. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Figueroa, S.J.A.; Fernández-García, M. Iron-sulfur codoped TiO2 anatase nano-materials: UV and sunlight activity for toluene degradation. Appl. Catal. B Environ. 2012, 117–118, 310–316. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Novel adsorption and photocatalytic oxidation for removal of gaseous toluene by V-doped TiO2/PU under visible light. J. Hazard. Mater. 2015, 300, 493–503. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Selvaraj, M.; Selvamani, A.; Vijayan, P.; Sasi Rekha, N.R.; Shanthi, K. Enhanced visible-light photocatalytic activity of V2O5/S-TiO2 nanocomposites. Appl. Catal. A Gen. 2012, 449, 31–46. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Shawaqfah, M. Solar oxidation of toluene over Co doped nano-catalyst. Chemosphere 2020, 255, 126878. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Zhang, L.; Hu, P.; Ye, X.; Leung, D.Y.C. Enhanced degradation of gaseous benzene under vacuum ultraviolet (VUV) irradiation over TiO2 modified by transition metals. Chem. Eng. J. 2015, 259, 534–541. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Hirakawa, T.; Koga, C.; Negishi, N.; Takeuchi, K.; Matsuzawa, S. An approach to elucidating photocatalytic reaction mechanisms by monitoring dissolved oxygen: Effect of H2O2 on photocatalysis. Appl. Catal. B Environ. 2009, 87, 46–55. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, H.; Tian, C. Photocatalytic mechanisms of modified titania under visible light. Res. Chem. Intermed. 2011, 37, 91–102. [Google Scholar] [CrossRef]
- Wang, K.; Tsai, H.; Hsieh, Y. A study of photocatalytic degradation of trichloroethylene in vapor phase on TiO2 photocatalyst. Chemosphere 1998, 36, 2763–2773. [Google Scholar] [CrossRef]
- Barakat, C.; Gravejat, P.; Guaitella, O.; Thevenet, F.; Rousseau, A. Oxidation of isopropanol and acetone adsorbed on TiO2 under plasma generated ozone flow: Gas phase and adsorbed species monitoring. Appl. Catal. B Environ. 2014, 147, 302–313. [Google Scholar] [CrossRef]
- Kim, M.S.; Liu, G.; Nam, W.K.; Kim, B.W. Preparation of porous carbon-doped TiO2 film by sol-gel method and its application for the removal of gaseous toluene in the optical fiber reactor. J. Ind. Eng. Chem. 2011, 17, 223–228. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven N-F-codoped TiO2 photocatalysts. 1. Synthesis by spray pyrolysis and surface characterization. Chem. Mater. 2005, 17, 2588–2595. [Google Scholar] [CrossRef]
- Salas, S.E.; Hugo De Lasa, S. Photocatalytic Water Splitting using a Modified Pt- TiO2. Kinetic Modeling and Hydrogen Production Efficiency. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2013. [Google Scholar]
- Imoberdorf, G.E.; Irazoqui, H.A.; Alfano, O.M.; Cassano, A.E. Scaling-up from first principles of a photocatalytic reactor for air pollution remediation. Chem. Eng. Sci. 2007, 62, 793–804. [Google Scholar] [CrossRef]
- Arsac, F.; Bianchi, D.; Chovelon, J.M.; Conchon, P.; Ferronato, C.; Lair, A.; Sleiman, M. Photocatalytic degradation of organic pollutants in water and in air. An analytical approach. Mater. Sci. Eng. C 2008, 28, 722–725. [Google Scholar] [CrossRef]
- Debono, O.; Thevenet, F.; Gravejat, P.; Hequet, V.; Raillard, C.; Lecoq, L.; Locoge, N. Toluene photocatalytic oxidation at ppbv levels: Kinetic investigation and carbon balance determination. Appl. Catal. B Environ. 2011, 106, 600–608. [Google Scholar] [CrossRef]
- Debono, O.; Thévenet, F.; Gravejat, P.; Héquet, V.; Raillard, C.; Le Coq, L.; Locoge, N. Gas phase photocatalytic oxidation of decane at ppb levels: Removal kinetics, reaction intermediates and carbon mass balance. J. Photochem. Photobiol. A Chem. 2013, 258, 17–29. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Lee, C.S.; Lakdawala, N. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation. J. Hazard. Mater. 2013, 261, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.K.; Lee, S.C. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Nuño, M.; Ball, R.J.; Bowen, C.R. Study of solid/gas phase photocatalytic reactions by electron ionization mass spectrometry. J. Mass Spectrom. 2014, 49, 716–726. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Cassano, A.E.; Irazoqui, H.A.; Alfano, O.M. Optimal design and modeling of annular photocatalytic wall reactors. Catal. Today 2007, 129, 118–126. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Irazoqui, H.A.; Cassano, A.E.; Alfano, O.M. Photocatalytic Degradation of Tetrachloroethylene in Gas Phase on TiO2 Films: A Kinetic Study. Ind. Eng. Chem. Res. 2005, 44, 6075–6085. [Google Scholar] [CrossRef]
- Héquet, V.; Batault, F.; Raillard, C.; Thévenet, F.; Le Coq, L.; Dumont, É. Determination of the clean air delivery rate (CADR) of photocatalytic oxidation (PCO) purifiers for indoor air pollutants using a closed-loop reactor. Part II: Experimental results. Molecules 2017, 22, 408. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, H.; Zhang, P. Performance of an innovative VUV-PCO purifier with nanoporous TiO2 film for simultaneous elimination of VOCs and by-product ozone in indoor air. Build. Environ. 2018, 142, 379–387. [Google Scholar] [CrossRef]
- Onoda, K.; Yoshikawa, S. Applications of anodized TiO2 films for environmental purifications. Appl. Catal. B Environ. 2008, 80, 277–285. [Google Scholar] [CrossRef]
- Taranto, J.; Frochot, D.; Pichat, P. Photocatalytic treatment of air: Comparison of various TiO2, coating methods, and supports using methanol or n-Octane as tst pollutant. Ind. Eng. Chem. Res. 2009, 48, 6229–6236. [Google Scholar] [CrossRef]
- El-Kalliny, A.S.; Ahmed, S.F.; Rietveld, L.C.; Appel, P.W. Immobilized photocatalyst on stainless steel woven meshes assuring efficient light distribution in a solar reactor. Drink. Water Eng. Sci. 2014, 7, 41–52. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Silva, A.M.T.; Ângelo, J.R.M.; Silva, G.V.; Mendes, A.M.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic oxidation of gaseous perchloroethylene over TiO2 based paint. J. Photochem. Photobiol. A Chem. 2015, 311, 41–52. [Google Scholar] [CrossRef]
- Pulišová, P.; Boháček, J.; Šubrt, J.; Szatmáry, L.; Bezdička, P.; Večerníková, E.; Balek, V. Thermal behaviour of titanium dioxide nanoparticles prepared by precipitation from aqueous solutions. J. Therm. Anal. Calorim. 2010, 101, 607–613. [Google Scholar] [CrossRef]
- Motola, M.; Satrapinskyy, L.; Roch, T.; Šubrt, J.; Kupčík, J.; Klementová, M.; Jakubičková, M.; Peterka, F.; Plesch, G. Anatase TiO2 nanotube arrays and titania films on titanium mesh for photocatalytic NOX removal and water cleaning. Catal. Today 2017, 287, 59–64. [Google Scholar] [CrossRef]
- Ma, C.M.; Ku, Y.; Kuo, Y.L.; Chou, Y.C.; Jeng, F.T. Effects of silver on the photocatalytic degradation of gaseous Isopropanol. Water. Air. Soil Pollut. 2009, 197, 313–321. [Google Scholar] [CrossRef]
- Xu, W.; Raftery, D. Photocatalytic oxidation of 2-propanol on TiO2 powder and TiO2 monolayer catalysts studied by solid-state NMR. J. Phys. Chem. B 2001, 105, 4343–4349. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Mak, C.L.; Chan, L.Y. Photodegradation of volatile organic compounds (VOCs) and NO for indoor air purification using TiO2: Promotion versus inhibition effect of NO. Appl. Catal. B Environ. 2003, 42, 119–129. [Google Scholar] [CrossRef]
- Marinangeli, R.E.; Ollis, D.F. Photo-assisted heterogeneous catalysis with optical fibers II. Nonisothermal single fiber and fiber bundle. AIChE J. 1980, 26, 1000–1008. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Ribbens, S.; Tytgat, T.; Hauchecorne, B.; Smits, M.; Meynen, V.; Cool, P.; Martens, J.A.; Lenaerts, S. The benefit of glass bead supports for efficient gas phase photocatalysis: Case study of a commercial and a synthesised photocatalyst. Chem. Eng. J. 2011, 174, 318–325. [Google Scholar] [CrossRef]
- Hachem, C.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Decolourization of textile industry wastewater by the photocatalytic degradation process. Dye. Pigment. 2001, 49, 117–125. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Development of easy and reproducible immobilization techniques using TiO2 for photocatalytic degradation of aquatic pollutants. Acta Hydrochim. Hydrobiol. 2004, 32, 201–213. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; González-Elipe, A.R.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Fernández, A.; Lassaletta, G.; Jiménez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Rodgher, V.; Moreira, J.; de Lasa, H.; Serrano, B. Photocatalytic degradation of malic acid using a thin coated TiO2 -film: Insights on the mechanism of photocatalysis. AIChE J. 2014, 60, 3286–3299. [Google Scholar] [CrossRef]
- Harizanov, O.; Harizanova, A. Development and investigation of sol-gel solutions for the formation of TiO2 coatings. Sol. Energy Mater. Sol. Cells 2000, 63, 185–195. [Google Scholar] [CrossRef]
- Yamazaki-Nishida, S.; Nagano, K.; Phillips, L.A.; Cervera-March, S.; Anderson, M.A. Photocatalytic degradation of trichloroethylene in the gas phase using titanium dioxide pelle. J. Photochem. Photobiol. A Chem. 1993, 70, 95–99. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.K. Fabrication of durable superhydrophobic coatings on cotton fabrics with photocatalytic activity by fluorine-free chemical modification for dual-functional water purification. New J. Chem. 2017, 41, 4618–4628. [Google Scholar] [CrossRef]
- Langlet, M.; Kim, A.; Audier, M.; Herrmann, J.M. Sol-gel preparation of photocatalytic TiO2 films on polymer substrates. J. Sol-Gel Sci. Technol. 2002, 25, 223–234. [Google Scholar] [CrossRef]
- Yamazaki, S.; Tanimura, T.; Yoshida, A.; Hori, K. Reaction mechanism of photocatalytic degradation of chlorinated ethylenes on porous TiO2 pellets: Cl radical-initiated mechanism. J. Phys. Chem. A 2004, 108, 5183–5188. [Google Scholar] [CrossRef]
- Harizanov, O.A.; Gesheva, K.A.; Stefchev, P.L. Sol-gel and CVD-metal oxide coatings for solar energy utilization. Ceram. Int. 1996, 22, 91–94. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Farhanian, D.; Haghighat, F.; Lee, C.S.; Lakdawala, N. Impact of design parameters on the performance of ultraviolet photocatalytic oxidation air cleaner. Build. Environ. 2013, 66, 148–157. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies—Abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Salaices, M.; Serrano, B.; de Lasa, H.I. Photocatalytic conversion of phenolic compounds in slurry reactors. Chem. Eng. Sci. 2004, 59, 3–15. [Google Scholar] [CrossRef]
- Moreira, J.; Serrano, B.; Ortiz, A.; de Lasa, H. A unified kinetic model for phenol photocatalytic degradation over TiO2 photocatalysts. Chem. Eng. Sci. 2012, 78, 186–203. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical Processes for Water Treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Ortiz-Gomez, A.; Serrano-Rosales, B.; Salaices, M.; De Lasa, H. Photocatalytic oxidation of phenol: Reaction network, kinetic modeling, and parameter estimation. Ind. Eng. Chem. Res. 2007, 46, 7394–7409. [Google Scholar] [CrossRef]
- Guayaquil, J.F.; Escobedo, S.; Calzada, A.; Serrano, B.; de Lasa, H. Hydrogen Production via Water Dissociation Using Pt–TiO2 Photocatalysts: An Oxidation–Reduction Network. Catalysts 2017, 7, 324. [Google Scholar] [CrossRef]
- Yu, Q.L.; Ballari, M.M.; Brouwers, H.J.H. Indoor air purification using heterogeneous photocatalytic oxidation. Part II: Kinetic study. Appl. Catal. B Environ. 2010, 99, 58–65. [Google Scholar] [CrossRef]
- Besov, A.S.; Vorontsov, A.V.; Parmon, V.N. Fast adsorptive and photocatalytic purification of air from acetone and dimethyl methylphosphonate by TiO2 aerosol. Appl. Catal. B Environ. 2009, 89, 602–612. [Google Scholar] [CrossRef]
- Boulamanti, A.K.; Philippopoulos, C.J. Photocatalytic degradation of C5-C7 alkanes in the gas-phase. Atmos. Environ. 2009, 43, 3168–3174. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Knauth, P. Electrical properties and defect chemistry of anatase (TiO2). Solid State Ionics 2006, 177, 229–236. [Google Scholar] [CrossRef][Green Version]
- Sauer, L.M.; Ollis, F.D. Acetone Oxidation in Photocatalytic Monolith Reactor. J. Catal. 1994, 149, 81–94. [Google Scholar] [CrossRef]
- Trong On, D.; Desplantier-Giscard, D.; Danumah, C.; Kaliaguine, S. ChemInform Abstract: Perspectives in Catalytic Applications of Mesostructured Materials. ChemInform 2010, 33, 299–357. [Google Scholar] [CrossRef]
- Maudhuit, A.; Raillard, C.; Héquet, V.; Le Coq, L.; Sablayrolles, J.; Molins, L. Adsorption phenomena in photocatalytic reactions: The case of toluene, acetone and heptane. Chem. Eng. J. 2011, 170, 464–470. [Google Scholar] [CrossRef]
- Obee, T.N. Photooxidation of sub-parts-per-million toluene and formaldehyde levels on titania using a glass-plate reactor. Environ. Sci. Technol. 1996, 30, 3578–3584. [Google Scholar] [CrossRef]
- Ding, X.; An, T.; Li, G.; Zhang, S.; Chen, J.; Yuan, J.; Zhao, H.; Chen, H.; Sheng, G.; Fu, J. Preparation and characterization of hydrophobic TiO2 pillared clay: The effect of acid hydrolysis catalyst and doped Pt amount on photocatalytic activity. J. Colloid Interface Sci. 2008, 320, 501–507. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yang, R.; Xu, Q.J.; Mo, J.H. Characteristics of photocatalytic oxidation of toluene, benzene, and their mixture. J. Air Waste Manag. Assoc. 2007, 57, 94–101. [Google Scholar] [CrossRef]
- Chin, P.; Yang, L.P.; Ollis, D.F. Formaldehyde removal from air via a rotating adsorbent combined with a photocatalyst reactor: Kinetic modeling. J. Catal. 2006, 237, 29–37. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.S. UV-PCO device for indoor VOCs removal: Investigation on multiple compounds effect. Build. Environ. 2008, 43, 246–252. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Blondeau, P.; Kozinski, J. Modeling and physical interpretation of photocatalytic oxidation efficiency in indoor air applications. Build. Environ. 2010, 45, 2689–2697. [Google Scholar] [CrossRef]
- Salvadores, F.; Alfano, O.M.; Ballari, M.M. Kinetic study of air treatment by photocatalytic paints under indoor radiation source: Influence of ambient conditions and photocatalyst content. Appl. Catal. B Environ. 2020, 268, 118694. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, J.; Zhang, X.; Li, Z.; Yu, T.; Ye, J.; Zou, Z. BaCeO3 as a novel photocatalyst with 4f electronic configuration for water splitting. Solid State Ionics 2008, 178, 1711–1713. [Google Scholar] [CrossRef]
- Jacoby, W.A.; Blake, D.M.; Noble, R.D.; Koval, C.A. Kinetics of theoxidation of trichloroethylene in air via heterogeneous photocatalysis. J. Catal. 1995, 157, 87–96. [Google Scholar] [CrossRef]
- Baxter, R.J.; Hu, P. Insight into why the Langmuir-Hinshelwood mechanism is generally preferred. J. Chem. Phys. 2002, 116, 4379–4381. [Google Scholar] [CrossRef]
- Vincent, G.; Marquaire, P.M.; Zahraa, O. Photocatalytic degradation of gaseous 1-propanol using an annular reactor: Kinetic modelling and pathways. J. Hazard. Mater. 2009, 161, 1173–1181. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Modeling and validation of a photocatalytic oxidation reactor for indoor environment applications. Chem. Eng. Sci. 2011, 66, 5945–5954. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Monteiro, R.A.R.; Silva, A.M.T.; Silva, G.V.; Faria, J.L.; Mendes, A.M.; Vilar, V.J.P.; Boaventura, R.A.R. Insights into UV-TiO2 photocatalytic degradation of PCE for air decontamination systems. Chem. Eng. J. 2012, 204–205, 244–257. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Stoyanova, I.V.; Kozlov, D.V.; Simagina, V.I.; Savinov, E.N. Kinetics of the photocatalytic oxidation of gaseous acetone over platinized titanium dioxide. J. Catal. 2000, 189, 360–369. [Google Scholar] [CrossRef]
- Valadés-Pelayo, P.J.; Guayaquil Sosa, F.; Serrano, B.; de Lasa, H. Eight-lamp externally irradiated bench-scale photocatalytic reactor: Scale-up and performance prediction. Chem. Eng. J. 2015, 282, 142–151. [Google Scholar] [CrossRef]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. A new method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 1966, 58, 18–27. [Google Scholar] [CrossRef]
- Degrave, R.; Moreau, J.; Cockx, A.; Schmitz, P. Multiscale analysis and modelling of fluid flow within a photocatalytic textile. Chem. Eng. Sci. 2015, 130, 264–274. [Google Scholar] [CrossRef]
- Cassano, A.E.; Martín, C.A.; Brandi, R.J.; Alfano, O.M. Photoreactor Analysis and Design: Fundamentals and Applications. Ind. Eng. Chem. Res. 1995, 34, 2155–2201. [Google Scholar] [CrossRef]
- Davydov, L.; Smirniotis, P.G.; Pratsinis, S.E. Novel differential reactor for the measurement of overall quantum yields. Ind. Eng. Chem. Res. 1999, 38, 1376–1383. [Google Scholar] [CrossRef]
- Valadés-Pelayo, P.J.; Moreira del Rio, J.; Solano-Flores, P.; Serrano, B.; de Lasa, H. Establishing photon absorption fields in a Photo-CREC Water II Reactor using a CREC-spectroradiometric probe. Chem. Eng. Sci. 2014, 116, 406–417. [Google Scholar] [CrossRef]
- Serrano, B.; De Lasa, H. Photocatalytic Degradation of Water Organic Pollutants. Kinetic Modeling and Energy Efficiency. Ind. Eng. Chem. Res. 1997, 36, 4705–4711. [Google Scholar] [CrossRef]
- Tran Thi Thuong, H.; Tran Thi Kim, C.; Nguyen Quang, L.; Kosslick, H. Highly active brookite TiO2-assisted photocatalytic degradation of dyes under the simulated solar−UVA radiation. Prog. Nat. Sci. Mater. Int. 2019, 29, 641–647. [Google Scholar] [CrossRef]
- Di Paola, A.; Addamo, M.; Bellardita, M.; Cazzanelli, E.; Palmisano, L. Preparation of photocatalytic brookite thin films. Thin Solid Films 2007, 515, 3527–3529. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Wang, J.; Chen, C.; Peng, T. SrCO3 -modified brookite/anatase TiO2 heterophase junctions with enhanced activity and selectivity of CO2 photoreduction to CH4. Appl. Surf. Sci. 2019, 476, 937–947. [Google Scholar] [CrossRef]
- Cao, S.; Chan, T.S.; Lu, Y.R.; Shi, X.; Fu, B.; Wu, Z.; Li, H.; Liu, K.; Alzuabi, S.; Cheng, P.; et al. Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes. Nano Energy 2020, 67, 104287. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Duan, G.; Yang, X.; Liu, X. Facile preparation of anatase-brookite-rutile mixed-phase N-doped TiO2 with high visible-light photocatalytic activity. J. Environ. Chem. Eng. 2015, 3, 603–608. [Google Scholar] [CrossRef]
- Ma, Y.; Kobayashi, K.; Cao, Y.; Ohno, T. Development of visible-light-responsive morphology-controlled brookite TiO2 nanorods by site-selective loading of AuAg bimetallic nanoparticles. Appl. Catal. B Environ. 2019, 245, 681–690. [Google Scholar] [CrossRef]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, E.R. Role of UV Light in Photodamage, Skin Aging, and Skin Cancer. Am. J. Clin. Dermatol. 2009, 10, 19–24. [Google Scholar] [CrossRef]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Goswami, D.Y. Decontamination of ventilation systems using photocatalytic air cleaning technology. J. Sol. Energy Eng. Trans. ASME 2003, 125, 359–365. [Google Scholar] [CrossRef]
- Chotigawin, R.; Sribenjalux, P.; Supothina, S.; Johns, J.; Charerntanyarak, L.; Chuaybamroong, P. Airborne Microorganism Disinfection by Photocatalytic HEPA Filter. EnvironmentAsia 2010, 3, 1–7. [Google Scholar]
- Pigeot-Remy, S.; Lazzaroni, J.C.; Simonet, F.; Petinga, P.; Vallet, C.; Petit, P.; Vialle, P.J.; Guillard, C. Survival of bioaerosols in HVAC system photocatalytic filters. Appl. Catal. B Environ. 2014, 144, 654–664. [Google Scholar] [CrossRef]
- Stuart, J.I.; Salaices, M.; Valvano, M.A.; De Lasa, H.I. Photocatalytic Inactivation of MS2 Bacteriophage and E. coli. Kinetics Modeling and Quantum Efficiency. In Proceedings of the 18th North American Catalysis Society Conference, Cancun, Mexico, 1–6 June 2003; pp. 1–2. Available online: http://nacatsoc.org/18nam/Orals/257-Photocatalytic Inactivation of MS2 Bacteriophage.pdf (accessed on 20 April 2020).
- Armon, R.; Narkis, N.; Neeman, I. Photocatalytic Inactivation of Different Bacteria and Bacteriophages in Drinking Water at Different TiO2 Concentration With or Without Exposure to O2. J. Adv. Oxid. Technol. 1998, 3. [Google Scholar] [CrossRef]


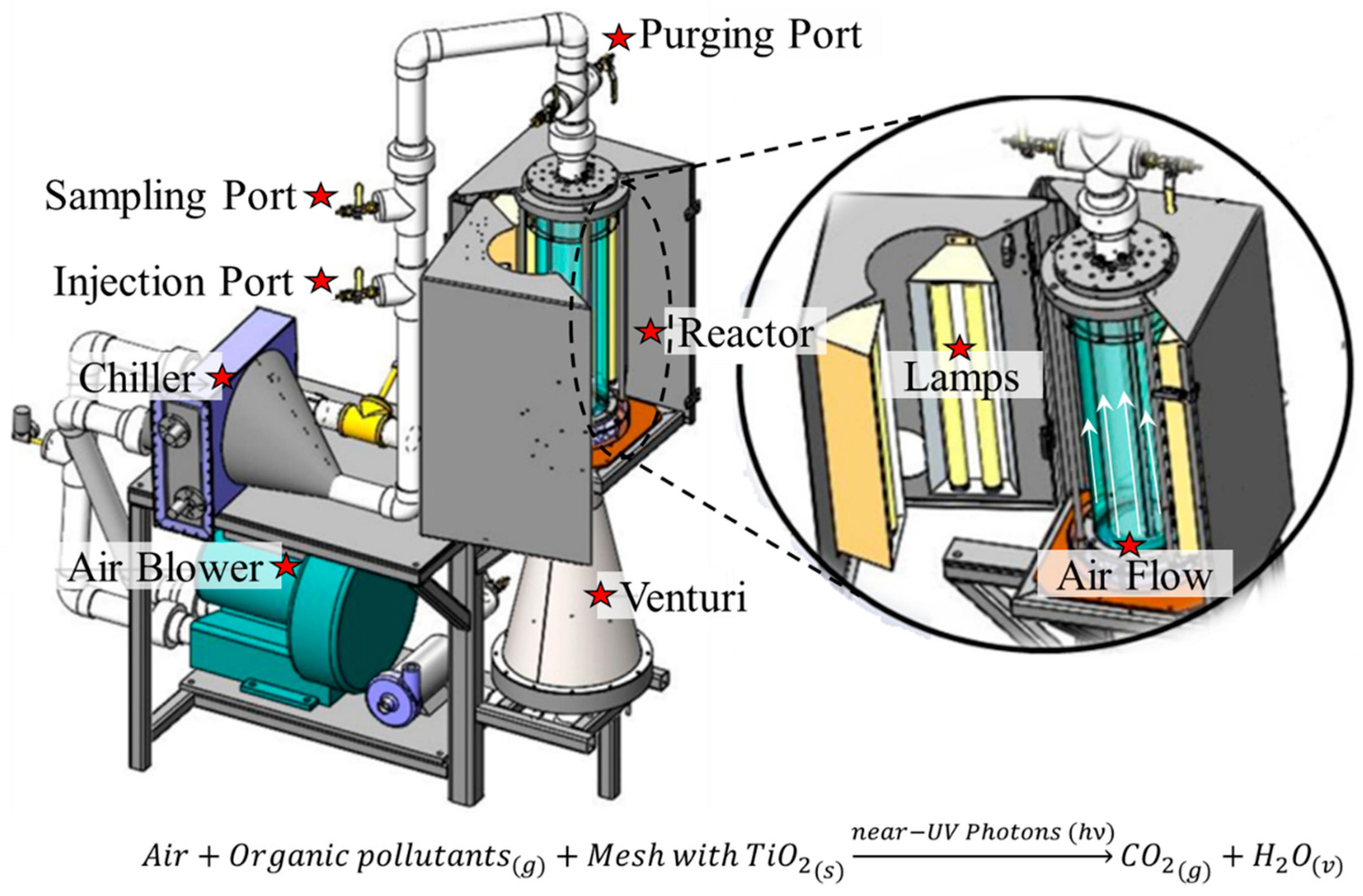
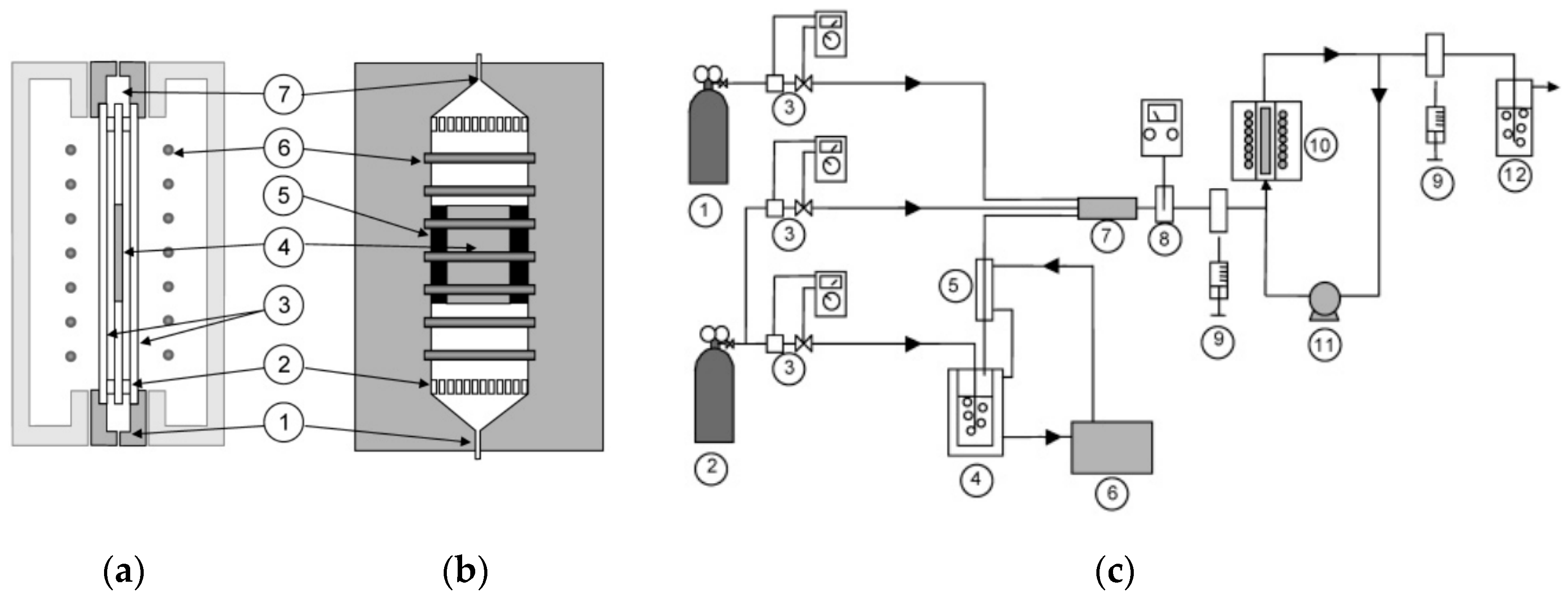

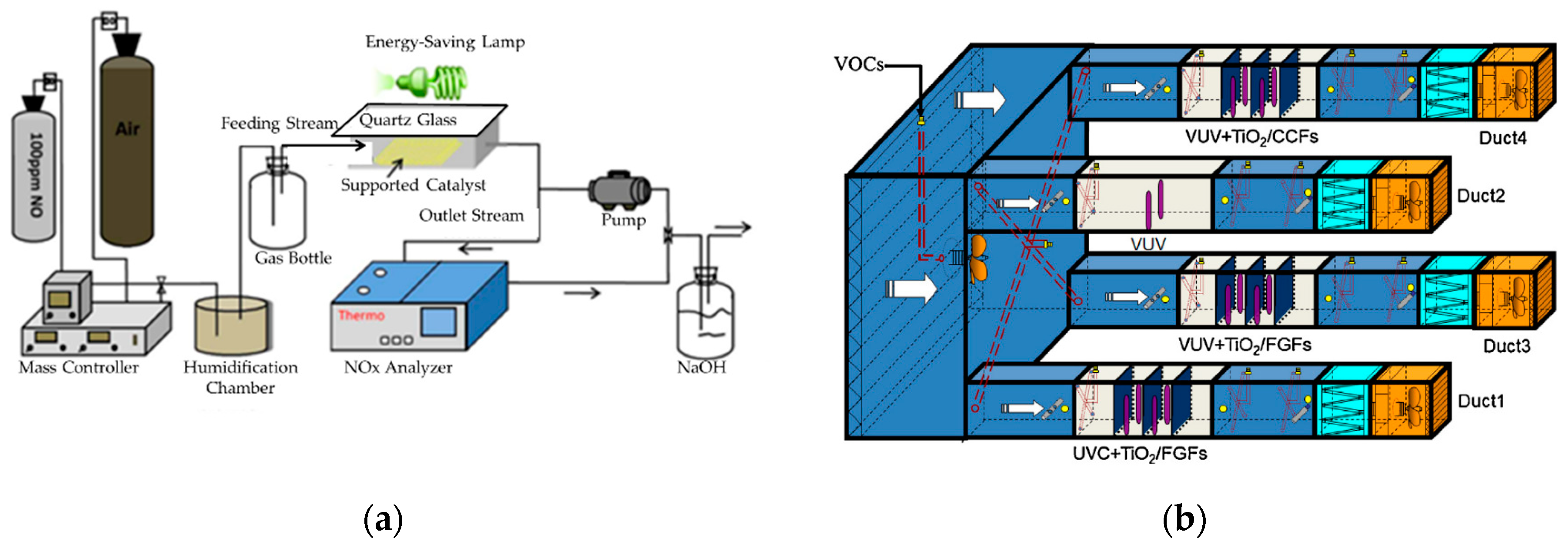

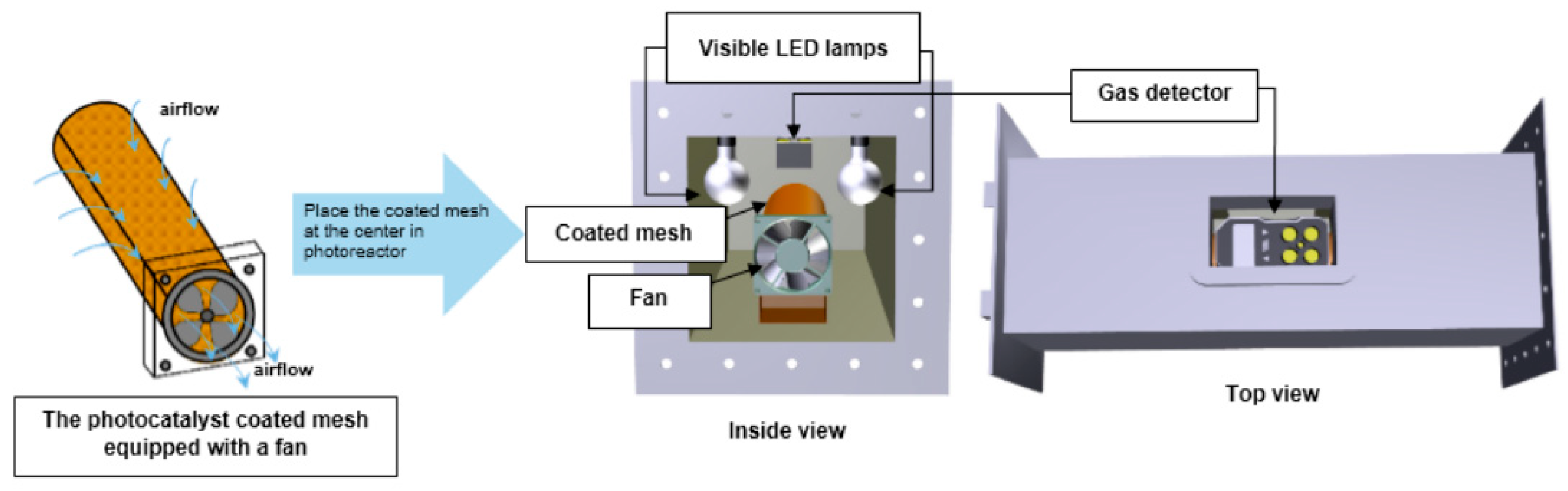


| Reference | Year | TiO2-Based Photocatalysts | Crystalline Phase | Crystallite Size-DB (nm) | SBET (m2/g) | Vp-BJH (cm3/g) | Dp-BJH (nm) | EBG (eV) |
|---|---|---|---|---|---|---|---|---|
| [44] | 2019 | Evonik-Degussa P25 (DP25) | 80% A 20% R | 21 | 54 | 0.1 | 7.5 | 3.2 |
| [10,53,54] | 2001 2005 2016 | Hombikat UV-100 | 99% A 1% R | <10 | >250 | - | ≈10 | 3.2 |
| [55] | 2014 | Cristal PC105 | 100% A | 23 | 80 | 0.35 | 15 | 3.19 |
| Cristal AT-1 | 12 | 192 | - | 120 | 3.15 | |||
| Kronos Titan 1077 | 130 | - | - | |||||
| [56,57] | 2019 | PC 500 | 100% A | 9.5 | 345 | - | - | 3.35 |
| S5-300A | 7.9 | 330 | - | - | - | |||
| [58] | 2011 | 1% Pt/N-TiO2 | - | 5 | 94.5 | - | 6 | 2.69 |
| [59] | 2012 | 1.5% Fe-doped TiO2 | - | ≈18 | 41.6 | 0.062 | 4 | ≈2.95 |
| ≈1% S-doped TiO2 | ≈14 | 42.7 | 0.186 | ≈15 | ≈3.18 | |||
| 1.5% Fe/ ≈ 1% S co-doped TiO2 | ≈1 | 77.8 | 0.191 | 7.7 | ≈3.01 | |||
| [11] | 2020 | TiO2 | 100% A | 61.48 | 11.64 | 0.02 | 7.99 | 3.2 |
| 5% N-TiO2 | 11.45 | 122.80 | 0.19 | 5.87 | 3.02 | |||
| 10% Ag-TiO2 | 15.81 | 23.6 | 0.16 | 26.54 | 1.7 | |||
| 5% N/Ag-TiO2 | 11.47 | 81.16 | 0.26 | 15.97 | 1.5 | |||
| [60] | 2015 | 6 wt.% V-TiO2/PU | - | - | 192.5 | - | - | 2.83 |
| [61] | 2012 | TiO2 | 100% A | 21.6 | 81.6 | 0.2 | 5.8 | 3.11 |
| S-TiO2 (4) | 15.6 | 51.7 | 0.13 | 4.8 | 2.7 | |||
| V2O5/TiO2 | 35.2 | 66.3 | 0.15 | 4.6 | 2.8 | |||
| V2O5/S-TiO2 (4) | 32.7 | 16.5 | - | - | 2.5 | |||
| [62] | 2020 | 5 wt.% Co/TiO2 | 100% A | ≈11.1 | ≈80.4 | - | 6.1 | ≈2.76 |
| [63] | 2015 | 1 wt.% Mn/TiO2 | 97% A 3% R | 24.5 | 49.7 | - | - |
| Reference | Year | Lamp Type | λ = Wavelength (nm) | Nominal Output Power (W) |
|---|---|---|---|---|
| [2,38] | 1999 & 2002 | Peng-Ray® Mercury UV | 300 to 410 | 16 |
| [75] | 2007 | Philips TL 4W/08 F4T5/BLB | 300 to 410 | 4 |
| [76] | 2008 | Philips HPK Mercury UV | 200 to 600 | 125 |
| [58] | 2011 | Philips MSR 575/2 10H metal halide | ≈240 to 800 | 575 |
| [77,78] | 2011 & 2013 | Philips UV PL—L-40 | 300 to 400 | 40 |
| [61] | 2012 | Tungsten | 410 to 800 | 500 |
| [79] | 2013 | VUV & UVC Low-pressure mercury (Ster-L-Ray®, Atlantic Ultraviolet Corp.) | 150 to 280 | 18.4 |
| [55] | 2014 | Jelosil HG 500 halogen | 315 to 400 | 500 |
| [80] | 2014 | Fluorescent domestic energy-saving light | - | 13 |
| [81] | 2016 | GaN UV-LEDs | 376 to 387 | ≈20 |
| [12] | 2016 | Polychromatic EIKO Global | 325 to 410 | 15 |
| [17,18] | 2013 & 2019 | USHIO Blacklight Blue (BLB) | 340 to 410 | 15 |
| [11] | 2020 | Visible LED | - | 16 |
| [19] | 2020 | Philips mercury visible-light | 300 to 700 | 15 |
| Reference | Year | Immobilization Deposition Method | Material Type | Total Area (cm2) | Mesh Open Area (%) | Photocatalyst Loading (g/cm2) |
|---|---|---|---|---|---|---|
| [2] | 2002 | Spread Coating | Fibrous Glass Mesh | 510 | - | - |
| [28] | 2006 | N2 Spray Coating | Non- Woven Dry Polyester Fabric Mesh | 50 | HVAC Filter | 0.24 ± 0.01 |
| [86] | 2008 | Galvanostatic Anodization of thin TiO2 Films | Metallic Titanium Plate | 100 | - | - |
| [87] | 2009 | Dip-Coating | Nonwoven Cellulose-Fiber Tissue | 25 | - | ≈0.093 |
| Quartz Plate | ≈0.025 | |||||
| [88] | 2014 | 304 SS Woven Wire Mesh | 214 | 52 | ≈0.027 | |
| [89] | 2015 | Cellulose Acetate Monolithic Structure | 806.4 | - | ≈0.0015 | |
| [12] | 2016 | Air Assisted Spray with Automatized Spinning (TiO2-ASS-ASC) | 304 SS Woven Wire Mesh | 1042 | 36 | 2.2 |
| [90,91] | 2010 & 2017 | TiO2 Liquid Deposition | U-VIX TiO2 Mesh | 50 | - | ≈5.41 |
| [11] | 2020 | Brush Painting | 304 SS Woven Wire Mesh | 700 | 52 | 1.5 ± 0.5 |
| Ref. | Year | Eq. * | Cpollutant (μmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [120] | 1994 | 1 | Blend Acetone + Water: (7.11) + (≈1206) | 4.57 × 107 μmol/m3 min | |||||
| (m3/mg) | |||||||||
| 0.208 | 0.0102 | ||||||||
| [125] | 2007 | Blend Toluene (0.173) + Benzene (0.037) | kTol | kBen | |||||
| (m3/mg) | 10−7 (mol/m2 s) | ||||||||
| 0.24 ± 0.04 | 0.77 ± 0.44 | 6.77 ± 0.65 | 1.56 ± 0.51 | ||||||
| [122] | 2011 | 2 | Toluene (5 × 10−4) | Qeq,m1 | Qeq,m2 | - | |||
| (mmol/m2) | (m3/mmol) | ||||||||
| 0.59 ± 0.06 | 0.41 ± 0.02 | 0.42 ± 0.05 | 1.28 ± 0.5 | ||||||
| [31] | 2004 | 4 | Acetone (60) | 10−5 (m3/μmol) | 10−4 (μmol/m3 min) | ||||
| 4.5 ± 0.18 | 2.04 ± 0.001 | ||||||||
| Acetaldehyde (50) | 0.71 ± 0.03 | 0.27 ± 0.02 | |||||||
| [8] | 2012 | Acetone (49) | 4.8 ± 0.09 | 13.15 ± 0.03 | |||||
| Acetaldehyde (320) | 0.31 ± 0.04 | 1.97 ± 1.18 | |||||||
| [118] | 2009 | 104 (m3/mol) | 10−7 (mol/m2 s) | ||||||
| Pentane (1386) | 1.14 | 1.81 | |||||||
| i-Pentane (1386) | 1.51 | 1.97 | |||||||
| Hexane (1160) | 1.25 | 2.16 | |||||||
| i-Hexane (1160) | 1.54 | 2.48 | |||||||
| Heptane (998) | 2.83 | 3.03 | |||||||
| Water (1 × 10−3) | 0.00112 | - | |||||||
| [16] | 2017 | 5 | Acetone (50) | 10−5 (m3/μmol) | 10−4 (μmol/m3 min) | ||||
| 4 ± 1 | 2.7 | ||||||||
| Acetaldehyde (50) | 9 ± 2 | 2.4 | |||||||
| [136] | 2000 | 6 | Acetone (8608) | ||||||
| 10−3 (ppm−1) | 10−10 (mol/s) | ||||||||
| 88 ± 0.7 | 0.97 ± 0.02 | 23.8 ± 0.4 | 22.5 ± 0.8 | ||||||
| 100 ± 7 | 0.35 ± 0.2 | 29.1 ± 2 | 7.86 ± 2.2 | ||||||
| Ref. | Year | Pollutant (μmol/L) | Temperature (°C) | PTEFs % | |
|---|---|---|---|---|---|
| [8,14] | 2012 | Acetone (25–49) | ≈44 | 215–140 | 3.3–2.2 |
| Acetaldehyde (160–240) | 400–250 | 5.3–3.3 | |||
| [12,15] | 2016 | Acetone (50–250) | 50–133 | ≈10 | |
| Acetaldehyde (65–325) | 80–125 | ≈24 |
| Organism | First Order Inactivation Constant (min−1) | IQYs% (Carbons Inactivated/Photon) |
|---|---|---|
| MS2 Bacteriophage | 0.8175 | 2200 |
| E. coli | 0.2377 | 5.19 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo, S.; de Lasa, H. Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses. Catalysts 2020, 10, 966. https://doi.org/10.3390/catal10090966
Escobedo S, de Lasa H. Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses. Catalysts. 2020; 10(9):966. https://doi.org/10.3390/catal10090966
Chicago/Turabian StyleEscobedo, Salvador, and Hugo de Lasa. 2020. "Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses" Catalysts 10, no. 9: 966. https://doi.org/10.3390/catal10090966
APA StyleEscobedo, S., & de Lasa, H. (2020). Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses. Catalysts, 10(9), 966. https://doi.org/10.3390/catal10090966