Abstract
The search for economic and sustainable sources of polyunsaturated fatty acids (PUFAs) within the framework of the circular economy is encouraged by their proven beneficial effects on health. The extraction of monkfish liver oil (MLO) for the synthesis of omega-3 ethyl esters was performed to evaluate two blending systems and four green solvents in this work. Moreover, the potential solubility of the MLO in green solvents was studied using the predictive simulation software COnductor-like Screening MOdel for Realistic Solvents (COSMO-RS). The production of ethyl esters was performed by one or two-step reactions. Novozym 435, two resting cells (Aspergillus flavus and Rhizopus oryzae) obtained in our laboratory and a mix of them were used as biocatalysts in a solvent-free system. The yields for Novozym 435, R. oryzae and A. flavus in the one-step esterification were 63, 61 and 46%, respectively. The hydrolysis step in the two-step reaction led to 83, 88 and 93% of free fatty acids (FFA) for Novozym 435, R. oryzae and A. flavus, respectively. However, Novozym 435 showed the highest yield in the esterification step (85%), followed by R. oryzae (65%) and A. flavus (41%). Moreover, selectivity of polyunsaturated fatty acids of R. oryzae lipase was evidenced as it slightly esterified docosahexaenoic acid (DHA) in all the esterification reactions tested.
1. Introduction
Numerous scientific studies have demonstrated the health benefits of PUFAs, in particular those known as omega-3 [1]. The main omega-3 fatty acids are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The main health effects of omega-3 fatty acids isolated and combined as ethyl esters have been described for cardiovascular diseases (CVD) [2,3]. Beneficial effects have also been described in diseases such as diabetes [4], immune system problems [5] and cancer [6,7]. Likewise, positive interactions have even been found in diseases such as autism spectrum disorder (ASD) [8] and Alzheimer’s [9]. Consequently, omega-3 fatty acid and its ethyl esters have become important as nutraceutical ingredients in functional food products and in pharmaceuticals [10,11], among other uses.
The fatty acid content of marine fish, whether oily or white, is high in omega-3 polyunsaturated fatty acids. These acids are synthesized by microalgae, and reach the fish through the food chain [12]. Fish oil, apart from being used in the food and pharmaceutical industry, is also used in agriculture and mainly in aquaculture as a feed additive [13]. They have also been used as a pesticide carrier, in paints and in leather manufacturing [14]. In addition, the fatty acid esters of simple alcohols are used in a wide variety of applications such as biodiesel fuel or biodiesel additives, lubricants, metalworking coolants, drilling and printing fluids, inks and solvents in alkyd resins [15].
According to the APROMAR report on aquaculture and fisheries in Spain 2019 [16], the EU consumed 13 million tons of aquatic products in 2018. There are no specific data on the consumption of monkfish in Spain; however, according to the European fisheries market report for 2019 [17], more than 50.746 tonnes of monkfish were landed in 2017. The waste and coproducts that can be obtained from the fishing industry are variable. The data presented by Erasmus et al. [18] showed that the greatest amount of monkfish waste was produced on fishing vessels (more than 60%). This species of fish has a large head that ends up being discarded with the gonads and livers [18,19]. Therefore, these byproducts or residues can be used as starting materials to prepare new commercial products within the circular economy concept [20]. Fish viscera accounts for 12 to 18% [19] of a fish and are considered animal byproducts (ABPs) not intended for human consumption. Although monkfish liver has an important culinary value in some restaurants as a gourmet dish, it is usually considered as an ABP and is discarded with the rest of the viscera in the vast majority of cases [20,21,22]. The monkfish, a white fish, contains ca. 30% oil, with a fatty acid profile showing the presence of DHA, EPA, gadoleic acid and oleic acid, among other fatty acids characteristic of fish oils [23].
Currently, there are various methods to determine the lipid content of biological samples. Among them, the Folch method (FM) is commonly used to determine the lipid content in fatty samples in the laboratory [24]. This method takes advantage of the solvent system consisting of chloroform/methanol in a 2:1 ratio to extract the sample. n-Hexane is another useful solvent for the extraction of natural products. Its low boiling point, low polarity and chemical stability make it one of the most used solvents to extract nonpolar compounds in the food industry. Nevertheless, these solvents are volatile organic compounds (VOCs) mainly sourced from nonrenewable resources. They are flammable, volatile and toxic, and are responsible for environmental pollution and the greenhouse effect [25,26]. Furthermore, these solvents are now strictly regulated by the European Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). Therefore, industries have been forced to use more environmentally friendly alternatives as green solvents.
Waste fish oils are traditionally extracted by hydraulic pressing or by the use of heat or solvents [27]. The main disadvantage of these methods, apart from those already mentioned, is that the quality of the product can be affected. High temperatures used degrade the thermolabile compounds of the oils, and the use of solvents can have a negative impact on the final product [27]. Consequently, solvents must be chosen with care as they are strictly regulated for food activity by various regulations such as the Codex Alimentarius [28]. In recent years, green extraction methods have been recognized as a promising alternative to traditional organic solvents. Green extraction methods include supercritical fluid extraction (SCF-CO2), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE) and enzymatic hydrolysis. They can improve yields, product quality and omega-3 fatty acid content [27]. Therefore, the search for more environmentally friendly solvent extraction processes is a priority [29,30,31].
Tanzi et al. [29] extracted oil from microalgae (rich in mono and polyunsaturated fatty acids) using terpenes as green solvents (d-limonene, α-pinene and p-cymene) in order to replace n-hexane. The extracted mass of crude oil was higher using terpenes than n-hexane. In addition, de Jesus et al. [32] used 2-methyltetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME) to extract lipids from microalgae Chlorella pyrenoidosa, yielding up to 89 mg/g of microalgae biomass. CPME, 2-MeTHF, dimethyl carbonate (DMC) and limonene (LMN) are considered green solvents and have been used in sustainable and environmentally friendly extraction processes [33]. Moreover, the extraction capacity of these solvents can be studied with computer tools, which saves time and resources in experimentation and maximizes the chances of success. COnductor-like Screening MOdel for Realistic Solvents (COSMO-RS) is a software that is used worldwide to predict the most suitable solvents for the extraction of natural products [31,33,34].
Sustainable oil extraction processes are the first step in the exploitation of fish coproducts. While the application and use of fish oils rich in omega-3 is widespread, it is usually more common to use them in the form of esters for their stability [2,10]. Free fatty acids, mainly PUFAs, are considered more susceptible to oxidation than ethyl esters [35]. As high degree unsaturated acids, EPA and DHA oxidize very easily, causing undesirable flavors, which diminish the nutritional quality and safety of food containing them [36]. Ester synthesis can be performed in one-step (transesterification) or two-step (hydrolysis followed by esterification) reactions. These reactions can be catalyzed by enzymes, which allow the development of efficient and fast processes. Lipases are widely used in industry because they have the capacity to catalyze different reactions. Moreover, several scientific articles have demonstrated the selectivity of some lipases for different fatty acids in both hydrolysis and esterification reactions. Moreno-Pérez et al. [37] studied the selectivity of two lipases (Thermomyces lanuginosus (TLL) and Lecitase Ultra, a phospholipase with lipolytic activity) immobilized in different supports (hydrophobic Sepabeads C18 and a Duolite anion exchanger) in the synthesis of ethyl esters of omega-3 fatty acids by the ethanolysis of sardine oil in solvent-free systems. They achieved an increase in the activity of TLL and Lecitase. Moreover, the Sepabeads support showed high selectivity for EPA ethyl ester (EPA-EE) synthesis. Castejón et al. [38] studied the enzymatic production of enriched structured triacylglycerols of EPA and DHA (STAG) from Camelina sativa oil by two-stage selective hydrolysis-esterification. A noteworthy selectivity of the different lipases tested towards EPA-EE compared to DHA-EE was found. Zangh et al. [35] also reported selectivity between DHA and EPA in the production of DHA-rich triacylglycerides (TAGs) using the commercial enzyme Novozym 435. Ranjan-Moharana et al. [39] described the use of phospholipase A1 for omega-3 enrichment in anchovy oil.
COSMO-RS software was used in this work to predict the extractive potential of monkfish liver oil (MLO) of four alternative green solvents (2-MeTHF, CPME, DMC and LMN). Afterward, the experimental extraction of MLO was carried out to develop a comprehensive, sustainable and environmentally friendly process. The Folch method (a mixture of chloroform and methanol) was used as a reference. Moreover, two systems of agitation were tested (roller mixer and ULTRA-TURRAX®). The recovered MLO was used to prepare the fatty acid ethyl esters (FAEEs) using three biocatalysts, a commercial enzyme (Novozym 435) and two resting cells (Rhizopus oryzae and Aspergillus flavus) for 24, 48 and 72 h. In addition, the selectivity of these biocatalysts for the different fatty acids present in the MLO was studied. In this sense, we developed a process that potentiates the use of fish coproducts (participating in the circular economy) to synthesize products with industrial potential, such as the ethyl esters of PUFAs, which are significant for various applications, such as cosmetics, food pharmacy and others.
2. Results and Discussion
2.1. COSMO-RS Prediction
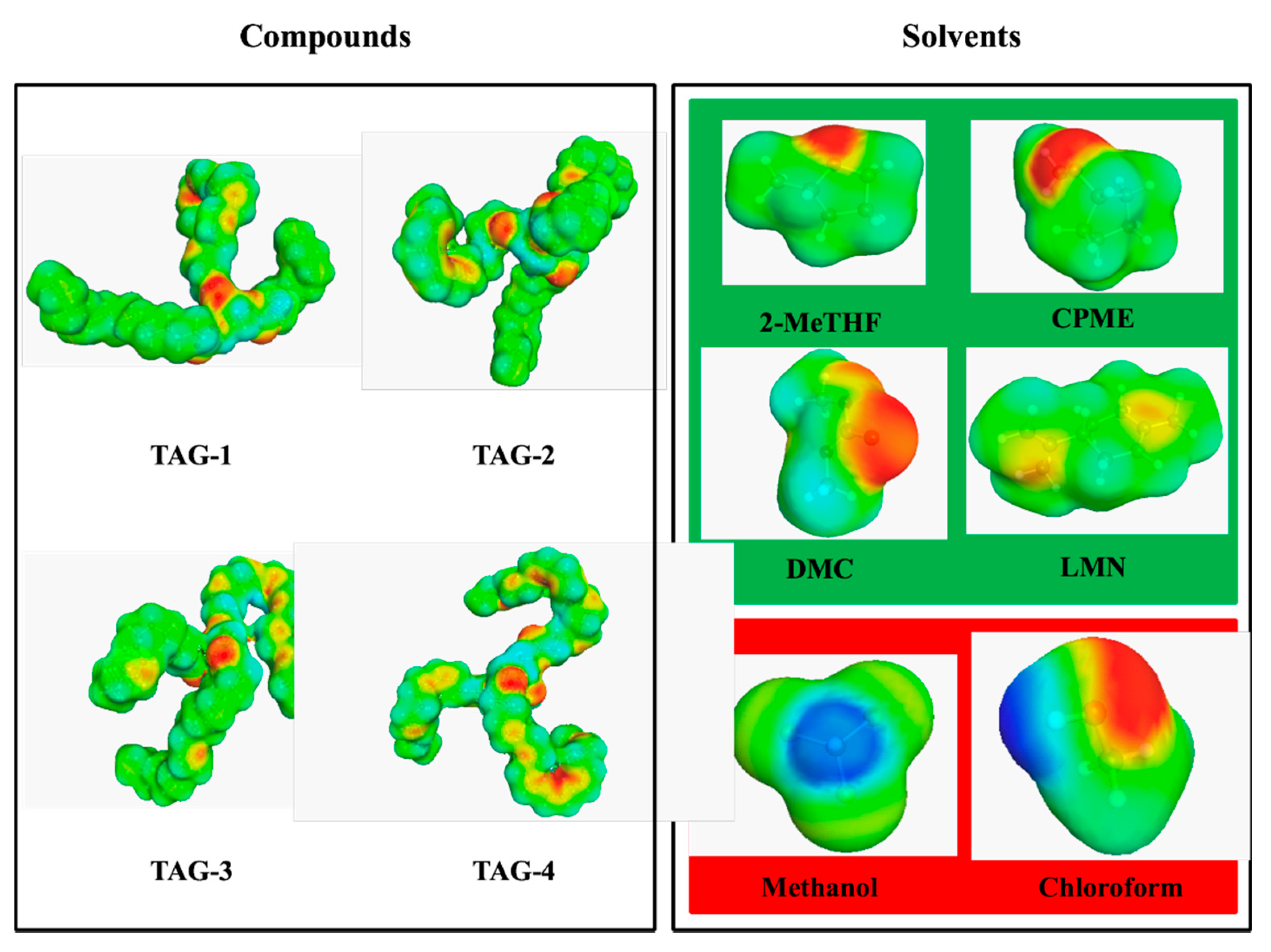
A COSMO-RS simulation was conducted to determine the relative solubility of the four main TAGs of MLO in the targeted solvents. 1H-NMR oil analysis was used to determine that TAGs were the main lipids present in the fish oil and to determine the main fatty acids present in the oil. GC-FID analysis confirmed that these TAGs were mainly composed of long carbon chains such as palmitic acid (C16:0), oleic acid (C18:1n9), EPA (C20:5n3) and DHA (C22:6n3). Therefore, we decided to use the four main fatty acids in the oil to define four TAG structures: TAG-1(R1 (C16:0); R2 (C22:6n3); R3 (C16:0)); TAG-2 (R1 (C18:1n9); R2 (C20:5n3); R3 (C16:0)); TAG-3 (R1 (C18:1n9); R2 (C22:6n3); R3 (C18:1n9)); TAG-4 (R1 (C22:6n3); R2 (C22:6n3); R3 (C22:6n3)) as models for COSMO-RS analysis (Figure 1). These main components were modeled with ChemSketch software and used for the predictive study. COSMO-RS integrates a quantum chemistry approach that allows the calculation of several properties, such as the relative solubility of a compound in several solvents. This means that the analysis of the σ profile and the σ potential of the components of the mixture (TAGs and solvents) provides important information about the molecules that can be used to predict possible interactions in the fluid phase.
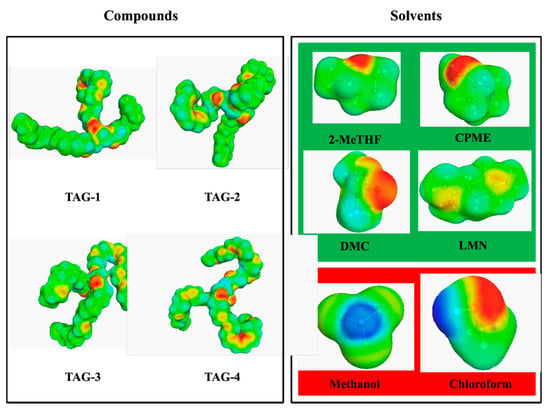
Figure 1.
Model of α-surfaces by COSMO-RS of compounds and solvents used in the theoretical study. Compounds (TGAs): TAG-1 (R1 (C16:0); R2 (C22:6n3); R3 (C16:0)); TAG-2 (R1 (C18:1n9); R2 (C20:5n3); R3 (C16:0)); TAG-3 (R1 (C18:1n9); R2 (C22:6n3); R3 (C18:1n9)); TAG-4 (R1 (C22:6n3); R2 (C22:6n3); R3 (C22:6n3)). Green solvents (green color): 2-methyltetrahydrofuran (2-MeTHF), cyclopentyl methyl ether (CPME), dimethyl carbonate (DMC) and limonene (LMN). Reference solvent (red color): Folch reagent (FR), chloroform/methanol (2:1, v/v).
Table 1 shows the solubility of the model TAGs from MLO in the solvents used in this study. The solubility is expressed in log10(x_solub) (best solubility is set to 0, and all solvents are given relative to the best solvent) and percentage of probability of solubility for a better understanding of the results. The solvent used as a reference was the Folch reagent (FR), a mixture of chloroform and methanol (2:1, v/v), considered to be the most reliable method for full recovery of total lipids [40]. Three of the green solvents tested, 2-MeTHF, CPME and LMN, showed a higher probability of solubility (60–100%) than the reference solvent for TAG-1 to TAG-3 and TAG-4 was similar. Even though DMC presented a low probability of solubility (0–20%) for three of the four model TAGs, this green solvent showed better solubility percentage than the FR. Finally, taking into consideration the theoretical results obtained by the COSMO-RS computational predictive method, we decided to perform the experimental study using the four green solvents with the potential to replace the FR solvent in the extraction of lipids (2-MeTHF, CPME, DMC and LMN).

Table 1.
COSMO-RS relative solubility (log10(x_solub)) and probability of solubility of triacylglycerides (TAGs) from monkfish liver oil using four different green solvents and Folch reagent (FR) as a reference solvent.
2.2. Monkfish Liver Oil (MLO) Extraction
The solid-liquid extraction of oil from fresh monkfish liver using five different solvents was performed by maceration using a roller mixer and an ULTRA-TURRAX® system. Folch reagent [24] was used until exhaustion (until no color was observed in the solvent) to determine the maximum content of oil in the liver. A percentage of 39.0% w/w was the maximum content of oil in the fresh material, with 49.8% moisture content. This was the first determination of the oil content in monkfish liver as far as we know. This value was higher than the percentage reported for tuna liver, with an oil yield of 17.5% [41], or from salmon byproducts (head, frame and viscera), with an oil content ranging from 13.09 to 19.2% [42]. Ciriminna et al. [43] reported 1.5% of oil content from anchovy heads.
2.2.1. Extraction in the Two Systems: Roller Mixer (RM) and ULTRA-TURRAX® (UT)
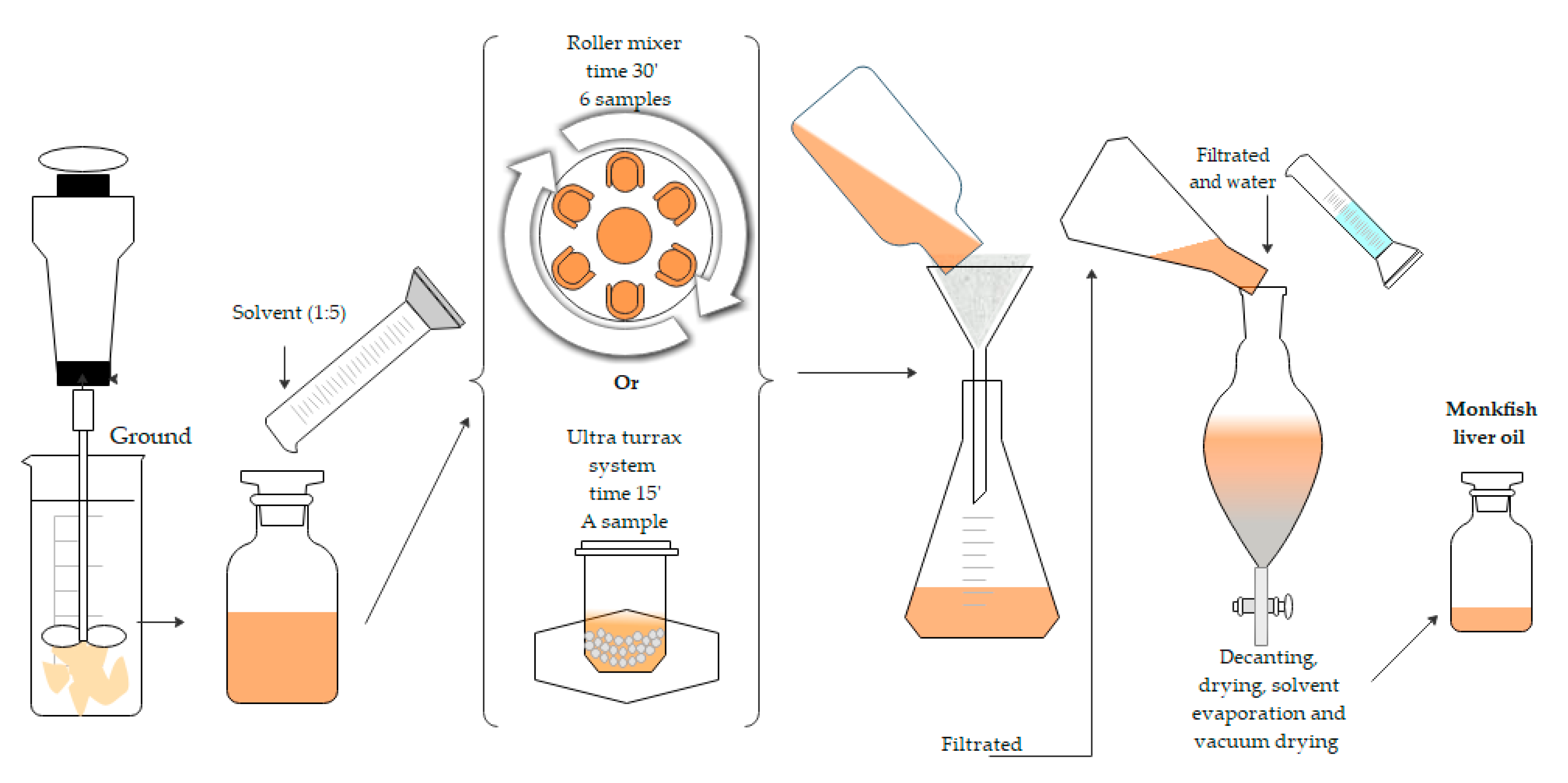
The MLO extraction was carried out following Scheme 1. Two types of agitation (RM and UT) with different extraction times were used. Different solvents were also used (FR or green solvents).
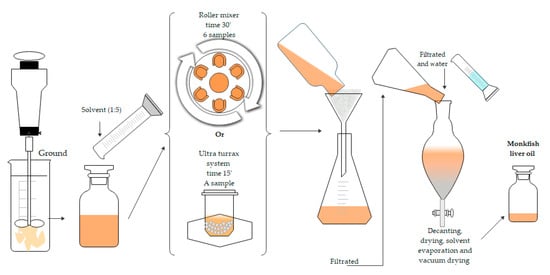
Scheme 1.
General procedure to the extraction of monkfish liver oil using two systems; Roller Mixer (RM) and ULTRA-TURRAX® (UT).
Table 2 summarizes the extraction yields obtained with the different solvents and the two agitation systems. The green solvents used correspond to an ester (DMC), two ethers (2-MeTHF and CPME) and a terpene (LMN). The extraction capacity of these green solvents was compared with the extraction capacity of a conventional extraction method (Folch reagent (FR)), which used a mixture of two traditional solvents (chloroform-methanol) in a 1:2 v/v ratio. Extraction yields were higher when the RM was used. All green solvents showed extraction yields between 96 and 100% of the maximum oil content in monkfish liver using RM. These extraction yields were higher than those by the FR solvent (89%). Authors such as Fang et al. [41] reported the importance of agitation in their tuna oil extraction experiments. No differences were observed on the NMR spectra of the MLO samples extracted with different solvents and stirring methods (RM or UT) (Figures S1 and S2).

Table 2.
Monkfish liver oil extraction with four green solvents and a conventional solvent (reference solvent) using two blending systems (mean ± standard deviations, n = 2).
This system is operated at 4000 rpm. CPME and 2-MeTHF extract the highest quantity of fish oil in the two extraction systems evaluated, which coincide with the COSMO-RS prediction. In the theoretical study, DMC shows a lower probability of oil solubility; however, in the experimental extraction, it shows better results than expected, e.g., 99% in RM. LMN presents worse extraction yields than those predicted by COSMO-RS. Nevertheless, this lower percentage of extraction could be a consequence of the harsh conditions used to recover the oil from the solution (90 °C/0.3 mbar), which could help the evaporation of part of the more volatile compounds present in the fish oil.
Considering the results obtained using the RM system, it can be said that the evaluated green solvents could be interesting alternatives to replace conventional solvents such as hexane, chloroform and methanol to develop more environmentally friendly extractive processes for oil samples [26,30]. It is particularly important to consider the ideal solvent and the best agitation method to choose the best extraction method of the MLO. An ideal alternative solvent must fulfill the following requirements: (a) it is not considered a VOC; (b) it has low toxicity for humans; (c) it has a limited impact on the environment (is eco-friendly); (d) it is obtained from renewable resources; (e) it has a high dissolving power; (f) it is easy to recover; (g) it does not change the setup process significantly [33].
In this sense, the information published in several solvent guides [25,44,45,46,47] was considered in this work. Various characteristics and properties of the solvents were evaluated according to the criteria of risk, life cycle, cost, production sources and MLO extraction performance (see Table S1) [44,45,46,47].
Considering the above information, the most appropriate solvent for MLO extraction is be 2-MeTHF. This solvent is derived from renewable resources such as corncobs and bagasse [25] and yields up to 100% with the RM extraction method. It is one of the cheapest green solvents (only beaten by LMN), and its score with respect to possible risks is acceptable (ranging from 4 to 6, except for an environmental air risk of 8).
Another interesting solvent is CPME, which provides excellent extraction yields and has a similar risk score to 2-MeTHF. However, this solvent is a byproduct of the synthesis of artificial caucho from crude oil. Moreover, it is the most expensive of all solvents studied. DMC is a solvent that presents high extraction yields with RM agitation and low risks to health and the environment (score between 1 and 5). However, its low extraction yields with UT, price (the second-highest) and the fact that it is prepared by chemical synthesis from methane gas eliminates it from consideration as an ideal solvent. LMN is acceptable in terms of risk assessment as this solvent has a similar score to CPME. However, according to its properties and what has been corroborated in experimentation, it is difficult to evaporate and presents the lowest extraction yields. Therefore, it is not considered a suitable solvent for MLO extraction in this work [25,44,45,46,47].
2.2.2. Analysis of the Extracted Oil
The extracted MLO was analyzed by 1H-NMR and GC-FID (Table 3). The 1H-NMR spectrum of MLO showed signals between 0.99 and 1.1 ppm, corresponding to omega-3 fatty acids (Figures S1 and S2). In addition, other signals corresponding to PUFA, MUFA and saturated fatty acids (SFAs) were also observed. These signals coincided with the signals reported by Catrin et al. and Bratu et al. [48,49], who determined the presence of omega-3 fatty acids in fish oil. Specific signals of DHA at 2.4 ppm corresponding to hydrogen linked to both α-carbon (=C–C–CH2–COOR) and allyl-carbon (=C–CH2–C–COOR) were also observed (Figure S3) [50].

Table 3.
Fatty acid profile (%) of monkfish liver oil (n = 3).
The fatty acid profile of the extracted oils was determined by GC-FID (Table S1 and Figure S4). MLO contained 29.8% SFAs, 43.7% monounsaturated fatty acids (MUFAs) and 26.5% PUFAs. Oleic acid was the main fatty acid with 21.1% of the total, and has been found to have similar beneficial effects on human health as omega-3 fatty acids [51]. Furthermore, the presence of 5.4% of vaccenic acid, an omega-7 isomer of the oleic acid, was determined worthy of consideration due to its association with a low risk of cardiovascular disease [52,53]. Other MUFAs present were gadoleic acid (C20:1n9), characteristic in fish oils, and erucic acid (C22:1n9), which has also been found in fish liver [41,54]. DHA and EPA were the main PUFAs in monkfish oil, with 15.2 and 4.4% of the total, respectively. The oil content of 39% w/w of fresh monkfish liver and its composition confirms the potential that this byproduct can be used as a raw material to obtain products with high added value.
2.3. Enzymatic Preparation of Fatty Acid Ethyl Esters (FAEEs)
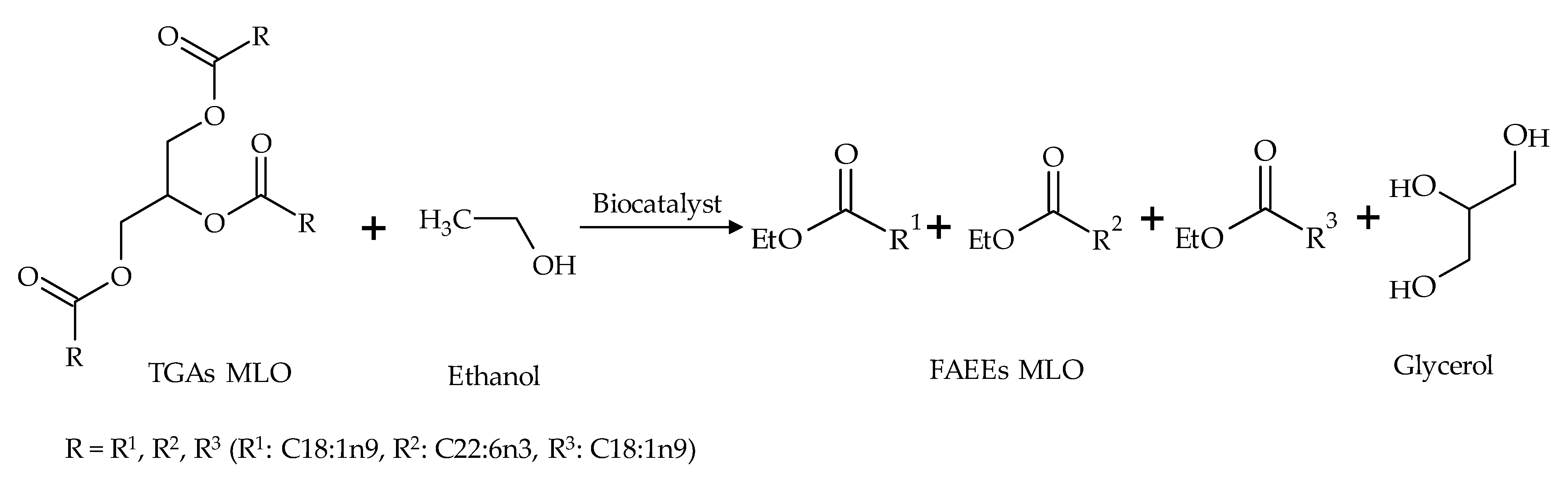
Two procedures were performed for the production of FAEEs using MLO and ethanol: (a) a one-step procedure based on a transesterification reaction (Figure 2), and (b) a two-step procedure based on sequential hydrolysis and esterification reactions (Figure 3). A commercial enzyme (Novozym 435), two resting cells (R. oryzae and A. flavus) and two mixtures of these resting cells (1:1 and 7:3 R. oryzae-A. flavus) were used as biocatalysts in a solvent-free medium.
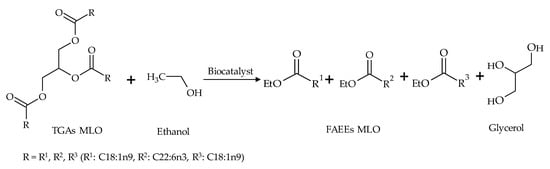
Figure 2.
Schematic reaction diagram of the synthesis of ethyl esters in the one-step transesterification reaction.
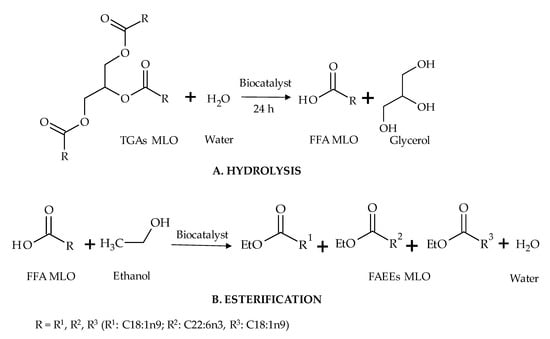
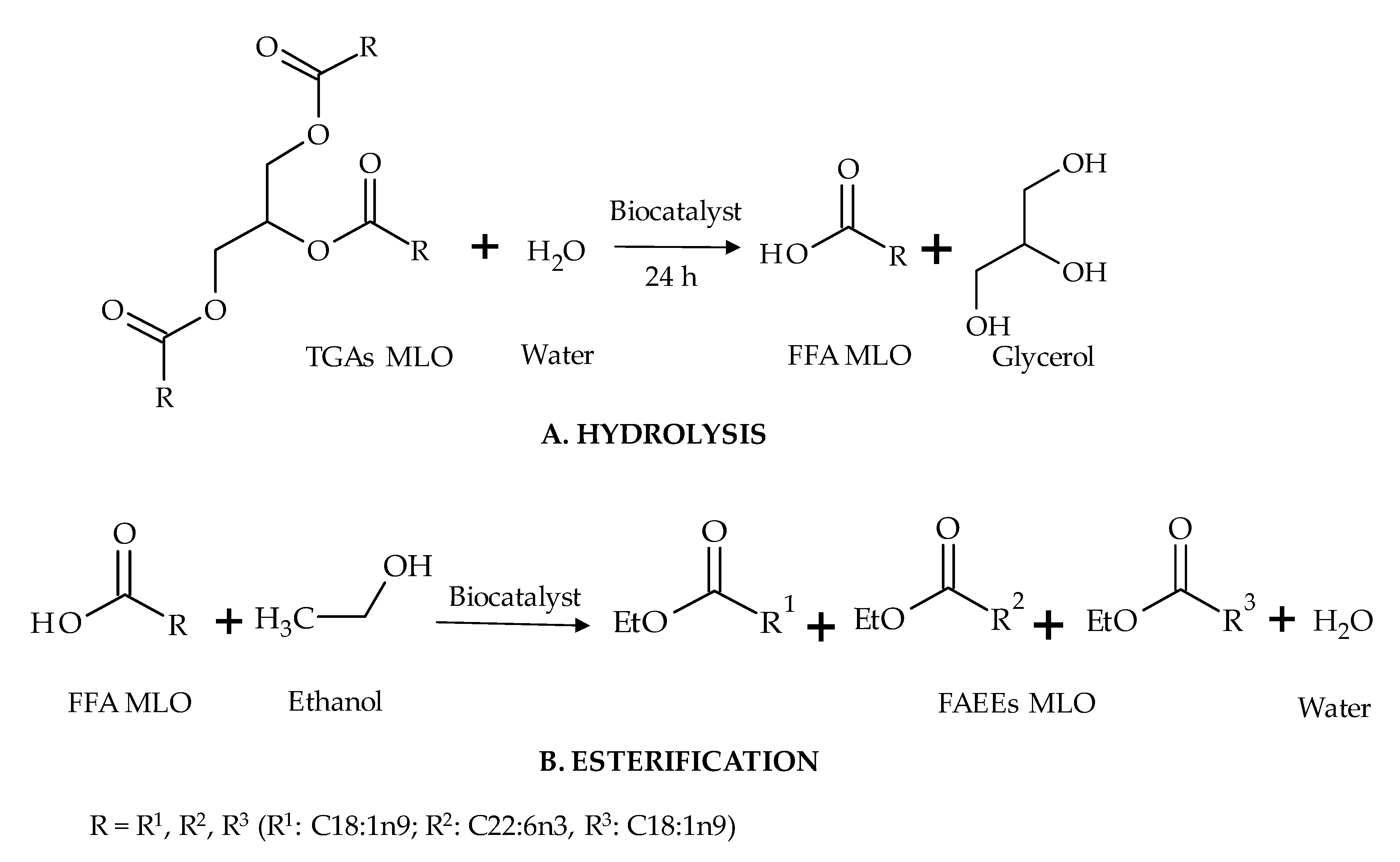
Figure 3.
Schematic reaction diagram of the synthesis of ethyl esters in the two-step reaction (A. hydrolysis followed by B. esterification reaction).
2.3.1. One-Step Synthesis: Transesterification Reactions
The results of the one-step (transesterification) and two-step reactions (hydrolysis and esterification) are shown in Table 4. The commercial enzyme showed the highest yield in the transesterification reaction. Yields were 44%, 61% and 63% for 24, 48 and 72 h, respectively. Transesterification yield with R. oryzae was the highest (53%) for 24 h, although the yield for 72 h was slightly lower (61%) than the yield achieved with Novozym (63%). A. flavus showed the lowest yields (46% for 72 h). Therefore, the resting cells from A. flavus should not be considered an alternative to the commercial enzyme for this transesterification reaction. Moreover, two mixtures of the fungal resting cells (1:1 and 7:3 R. oryzae-A. flavus) were used for the transesterification reaction. Only the 7:3 mixture led to a 57% yield after 72 h of reaction, a lower yield than that achieved using only R. oryzae. Considering these low yields, the fatty acid ethyl esters profiles of these reactions were not determined.

Table 4.
Yields (%) of the different reactions performed to prepare monkfish liver oil ethyl esters. (mean ± standard deviations, n = 3).
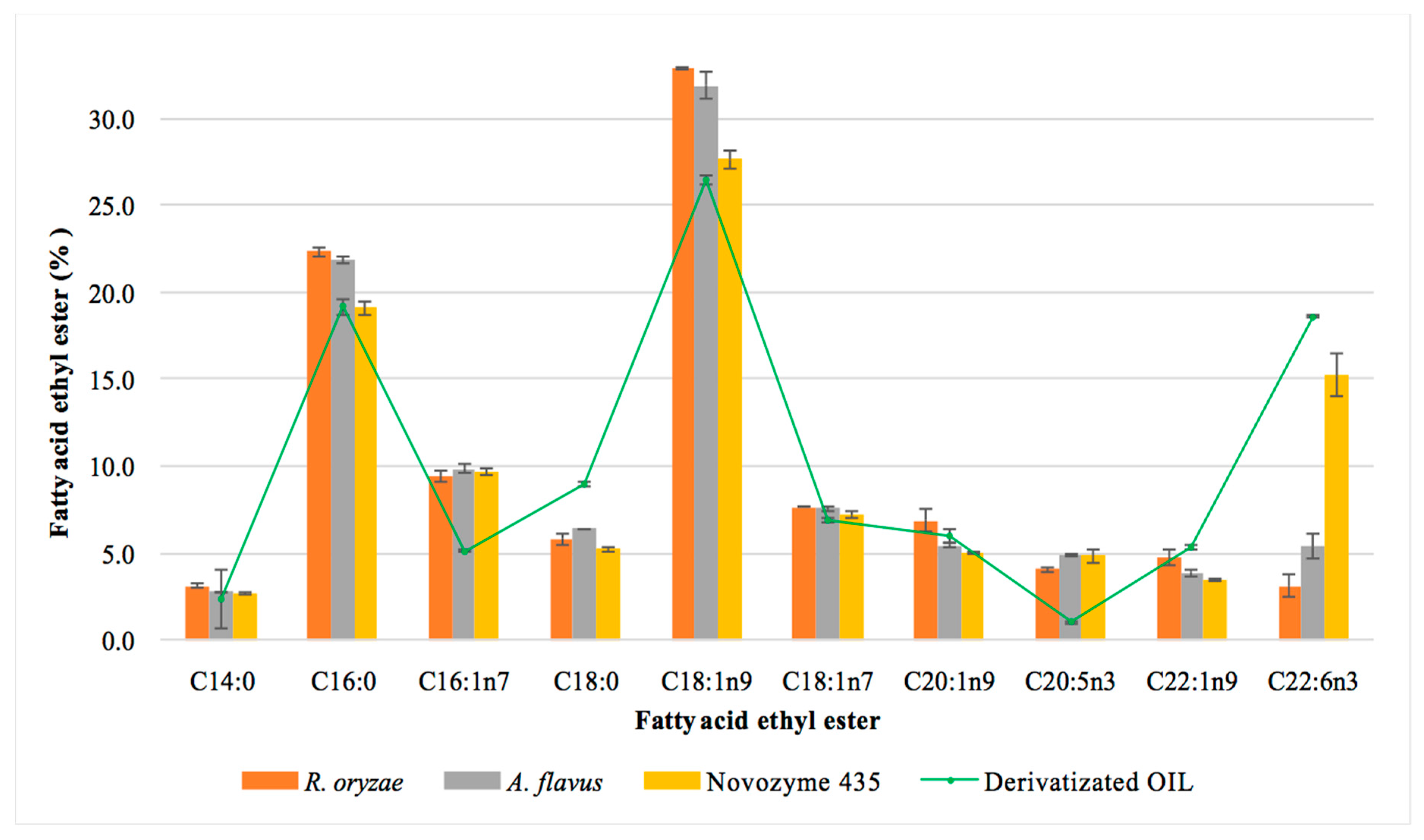
The main FAEEs obtained in the one-step reaction studied using the three biocatalysts are shown in Figure 4. The commercial enzyme and the fungal resting cells led to different fatty acid ethyl esters contents. The results corresponding to the percentage of each fatty acid is always referred to as the initial content of the corresponding fatty acid in the MLO. Thus, taking into account the total content of each fatty acid in the MLO (Table 3), and specifically the PUFAs (DHA, 15.2% and EPA, 4.4%), we can conclude that the commercial enzyme esterified 90% of DHA and 100% of EPA. This result shows that this enzyme does not discriminate between the different fatty acids to synthesize the FAEEs. Similar results have previously been described for this commercial enzyme [38,39], which indicates that it is a suitable biocatalyst for the synthesis of these omega-3 EEs. In contrast, the resting cells showed the lowest yields for the esterification of PUFAs, mainly DHA. A. flavus showed the highest yield of DHA-EE (38%) of the two resting cells studied; R. oryzae only esterified 22% of DHA present in the fish oil after a 72 h reaction; no differences were observed for the esterification of EPA. This finding suggested that lipases from R. oryzae discriminated between the different PUFAs present in the MLO. The selectivity of enzymes has been reported by different authors who suggested that some lipases could be selective for certain types of fatty acid depending on their chain length, the solvent used in its extraction and purification, the method of immobilization used for the enzyme and the reaction conditions (temperature and time) [54,55,56,57]. The selectivity showed by the resting cells from R. oryzae could facilitate the separation of the DHA from the mixture of FAEEs.
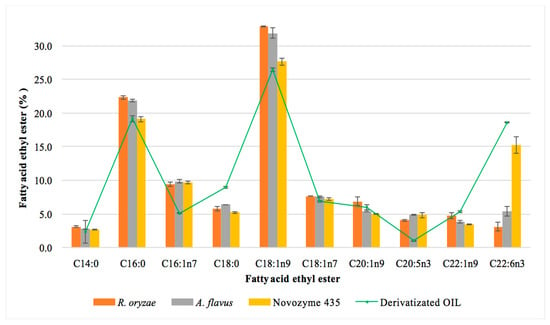
Figure 4.
Effect of the biocatalyst on the profile of the main fatty acid ethyl esters obtained in reaction 72 h. OIL: total content of the corresponding ethyl ester in the monkfish liver oil.
2.3.2. Two-Step Synthesis: Hydrolysis and Esterification Reactions
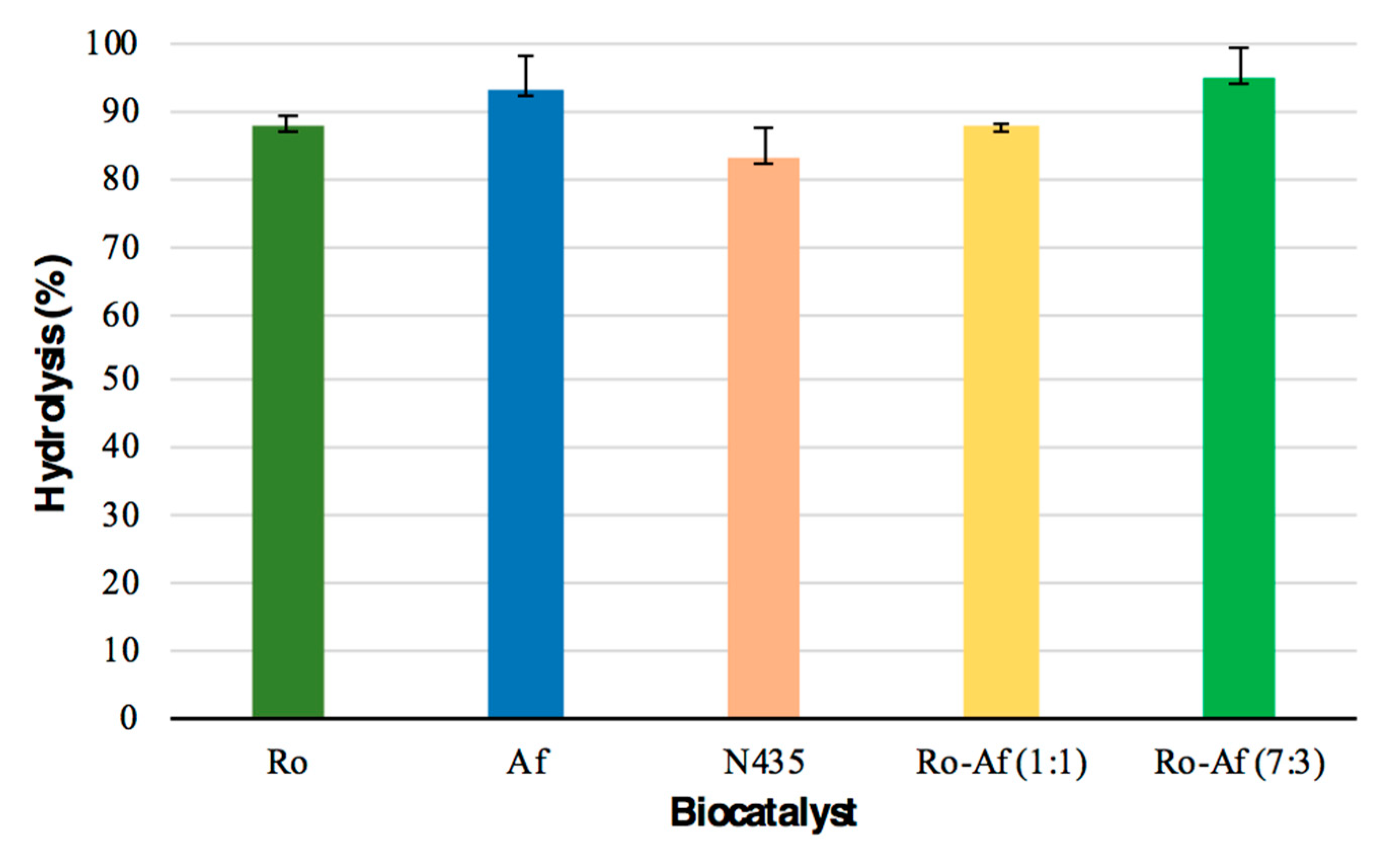
Hydrolysis, the first step of this study, was carried out using the three previous biocatalysts and two mixtures of the two resting cells (1:1 and 7:3) for 24 h (Figure 3A). The resting cells of R. oryzae mixed with A. flavus in a 7:3 ratio showed the highest percentage of hydrolysis (>95% free fatty acid (FFA)). The second biocatalyst presenting a high hydrolysis percentage was the resting cells of A. flavus (>93% FFA) (Figure 5). All resting cells studied showed a higher percentage of hydrolysis than the commercial lipase (83%), indicating that the resting cells could be a cheap alternative to immobilized commercial biocatalysts for these reactions. These hydrolysis percentages can be considered high when compared to those reported by Aranthya et al. [58] for the hydrolysis of fish oil with the enzymes of Cryptococcus sp., which yielded 25 and 66.5% of FFA for 24 and 72 h, respectively. Furthermore, it is worth noting that MLO contains more DHA than hydrolyzed cod, sardine, salmon and shark liver oils [58]. A scaling up of the hydrolytic process was carried out using the best reaction conditions achieved in the previous experiment. Starting from 25 g of fish oil, a mixture of R. oryzae and A. flavus (7:3) allowed the preparation of hydrolyzed monkfish liver oil (HMLO) with 97.8% FFA.
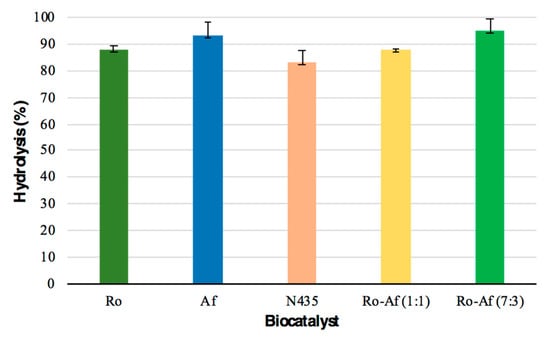
Figure 5.
Hydrolysis percentage of monkfish liver oil obtained with the different enzymes (mean ± standard deviations, n = 3, reaction 24 h). Ro, R. oryzae; Af, A. flavus; N435, Novozym 435.
The esterification reaction (Figure 3B) was performed using the hydrolyzed monkfish liver oil (HMLO) obtained in the previous experiment. In these experiments, the same positive correlation was found between reaction times and esterification performance as in the transesterification. The commercial enzyme showed the highest yield in the esterification reaction. Yields were 54, 70 and 85% for 24, 48 and 72 h, respectively. Esterification yield with R. oryzae was 65% for 72 h. A. flavus again showed the lowest yields (41% for 72 h). These results confirm that A. flavus resting cells are not very active in the esterification of the fatty acids from monkfish oil. Regarding the mixtures of the fungal resting cells, the yields were lower than the ones achieved with R. oryzae as in the transesterification reaction (see Table 4).
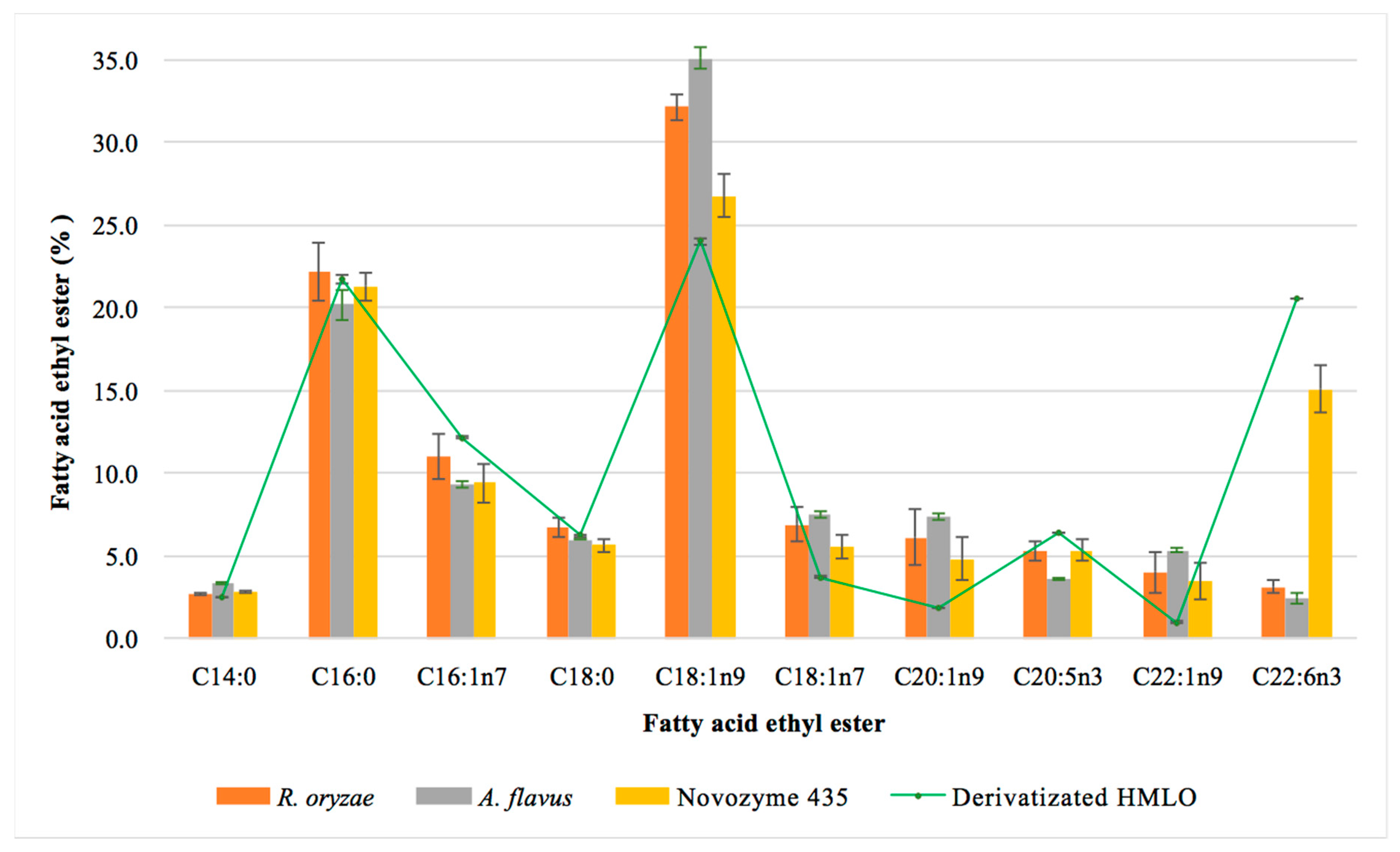
In relation to the FAEEs profiles (Figure 6), the results were similar to those shown in the one-step reactions. There was an enrichment of MUFAs such as oleic acid, gadoleic acid and vaccenic acid, which have been found beneficial to health [51]. Vaccenic acid, a source of omega-7, has been positively correlated with the presence of DHA and EPA [52,53]. The increase of palmitic acid and stearic acid observed in the assays could be due to the fatty acids present in the sunflower oil used in the production of the resting cells as lipase inductors. The microorganisms themselves can also synthesize different fatty acids, Aspergillus sp. produces long-chain fatty acids (C16:0, C16:1n7, C17:0, C18:0, C18:1n9, C18:2, C18:3 and C20:0). This fatty acid profile has been used to discriminate fungal species belonging to the Aspergillus genus [59]. In addition, the presence of some fatty acids, such as gadoleic acid (C20:1n9), may depend on the type of substrate used in the culture medium [60]. Nevertheless, the increase of these fatty acids in the final crude of the reaction cannot be higher than 4% considering the percentage of biocatalysts used (10%) and the percentage of fatty acids present in the resting cells (40%).
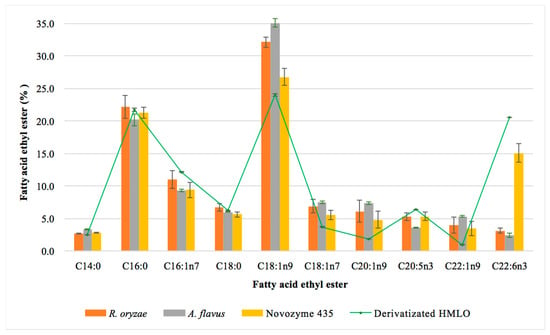
Figure 6.
Effect of the biocatalyst on the percentage of the main ethyl esters obtained in a 72 h two-step reaction. Hydrolyzed monkfish liver oil (HMLO) corresponds to the total content of the corresponding ethyl ester in the hydrolyzed monkfish liver oil.
EPA behaved similarly to one-step reactions, in which the commercial enzyme esterified up to 100% of EPA contained in HMLO, R. oryzae esterified 70% and A. flavus esterified 60%. For DHA, Novozym 435 esterified up to 90% of the DHA contained in HMLO, while the fungal resting cells did not exceed 20%. A. flavus lipase esterified up to 19% of the DHA content, while R. oryzae lipase esterified up to 16%. These low yields seem to confirm the hypothesis of DHA selectivity shown by R. oryzae. Due to the selectivity of R. oryzae lipase, DHA should be found as free fatty acid within the esterified material. The possibility of isolating these free fatty acids is also interesting as it has been shown that PUFAs ingested in the form of free fatty acids may be more bioavailable than FAEEs and therefore more assimilable in human metabolism [61].
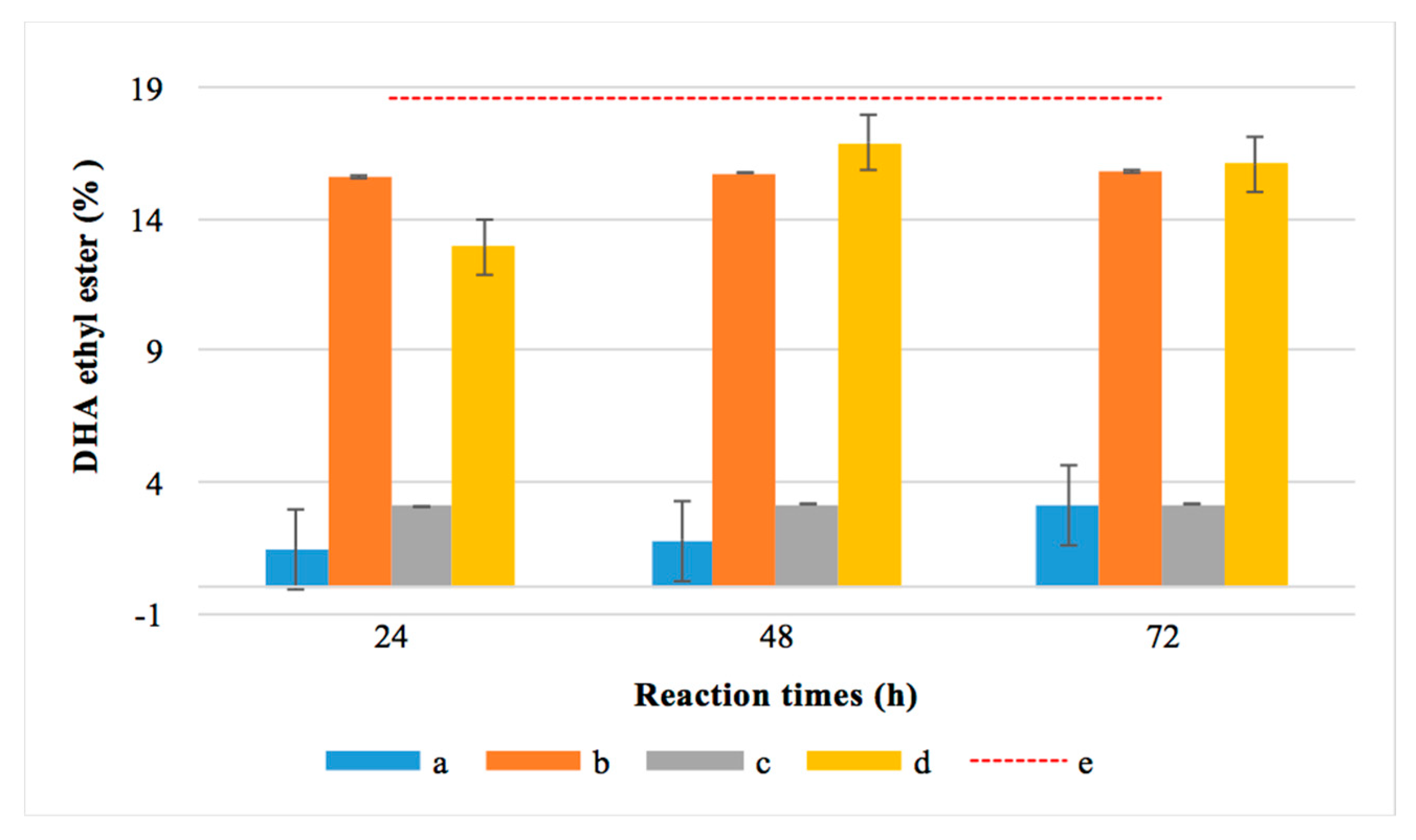
To confirm the above-mentioned hypothesis and that DHA was present as an acid in the esterified fraction, these samples were subjected to total esterification through chemical catalysis (a derivatization process) using H2SO4. Figure 7 compares the DHA-EE obtained in the one and two-step reactions at different times (24, 48 and 72 h) using R. oryzae or applying chemical catalysis.
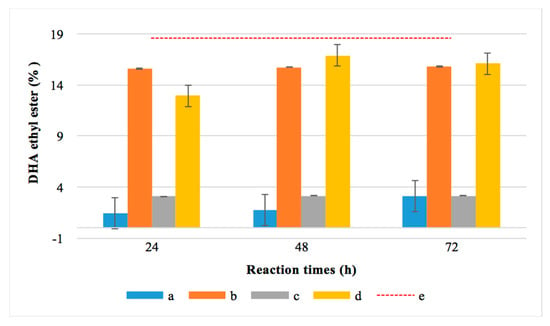
Figure 7.
Percentage of docosahexaenoic acid (DHA) before and after total chemical esterification for 24, 48 and 72 h using R. oryzae lipase and chemical catalysis. (a) Blue color: ester obtained before chemical catalysis from one-step esterification with R. oryzae. (b) Orange color: ester obtained after applying chemical catalysis to the crude resulting from one-step transesterification. (c) Grey color: ester obtained before catalysis from two-step esterification. (d) Yellow color: ester obtained after applying chemical catalysis to the crude resulting from the two-step esterification. (e) Red dotted line: total DHA content in the MLO.
As expected, chemical catalysis achieved more than 90% esterification of the DHA contained in both monkfish liver oil and the hydrolyzed monkfish liver oil. In the three reaction periods studied, it was evidenced that the lipases from R. oryzae were able to effectively discriminate between the DHA and the rest of the fatty acids present in MLO and HMLO. In the HMLO, the DHA indicated it was present as free acid since a 97.8% hydrolysis was achieved.
The selectivity demonstrated by the resting cells of this strain of R. oryzae has not previously been reported to our knowledge. However, Ashjaria et al. [62] did study the selectivity of lipases isolated from R. oryzae and immobilized by different methods in the hydrolysis of fish oil. All immobilized biocatalysts discriminated between EPA and DHA in favor of EPA.
3. Materials and Methods
3.1. Reagents and Solvents
Chloroform (purity 99%), 2-methyltetrahydrofuran, deuterated chloroform (99.9 atom % D), anhydrous sodium sulfate (Na2SO4), oleic acid (purity 90%), potassium hydrogen phosphate (K2HPO4) and magnesium sulfate (MgSO4) were purchased from Sigma-Aldrich (Sigma-Aldrich Química SA, Madrid, Spain and St. Louis, MO, USA). Cyclopentyl methyl ether (purity 99%) was from ZEON Corporation (Tokyo, Japan). Dimethyl carbonate (purity 99%), hexane (HPLC grade) and limonene (96%) were purchased from Acros Organics (Fair Lawn, NJ, USA). Ethanol absolute was purchased from Scharlau (Scharlab, Barcelona, Spain). Methanol was purchased from Fisher Scientific (Madrid, Spain). The culture media agar potato dextrosa (PDA) and yeast extract (EY) were provided by Scharlau Microbiology (Scharlab, Barcelona, Spain). Lipase-B from Candida antarctica (Novozym 435) was a gift sample from Novozymes A/S (Bagsvaerd, Denmark). The Rhizopus oryzae (CECT20476) and Aspergillus flavus (CECT20475.2.1) strains were housed in the Spanish Type Culture Collection (CECT) (Burjassot, Valencia, Spain). Monkfish liver was supplied by Congelados y Especialidades Barrufet SL (Barcelona, Spain), and sunflower oil was bought at the market.
3.2. Computational Method
This study was performed by a theoretical procedure using a computational predictive method (COSMO-RS) by considering the technical properties of the solvents and via experimentation. The comparison was made considering the amount of monkfish liver oil extracted and the technical parameters of the solvents used.
COSMO-RS Procedure
The Conductor-like Screening Model for Real Solvents (COSMO-RS) developed by Klamt and coworkers [63] is as known as a powerful method for molecular description and solvent screening based on the result of quantum chemical calculations for an understanding of the dissolving mechanism. COSMO-RS combines quantum chemical considerations (COSMO) and statistical thermodynamics (RS) to determine and predict thermodynamic properties without experimental data. In the first step, the molecule is embedded into a virtual conductor. In such an environment, the molecule induces a polarization charge density on its surface (σ-surface). Calculation can then be performed for each molecule of interest (Figure 1). The second step uses the statistical thermodynamic calculation. Blue is used to represent strongly positive polar regions, and red represents very negative polar surfaces. Green and yellow correspond to lower polarities. The thermodynamics of the molecular interactions that are based on the obtained σ-profile are then used to calculate the chemical potential of the surface segment (σ-potential) [26,31,35]. According to the composition of the monkfish liver oil determined by 1H-RMN and GC-FID, we decided to use four different models of TAG as models in the COSMO-RS analysis using the four main fatty acids from the oil.
In this work, the prediction of the relative solubility of the main triacylglycerides from monkfish liver oil in four green solvents 2-MeTHF, CPME, DMC and LMN, as well as FR, that is, a mixture of chloroform and methyl alcohol (2:1, v/v) was made by implementing this COSMO-RS model in COSMOtherm software (BIOVIA COSMOthermX19; Dassault Systèmes, France). The chemical structures of the solvents and solutes were mutually transformed into their simplified molecular-input line entry syntax (SMILES) notations, which were subsequently used to calculate the solubility parameters of solvents and compounds. The relative solubility was calculated using the following equation [63,64].
: chemical potential of pure compound j (J/mol); : chemical potential of j at infinite dilution (J/mol); : free energy of fusion of j (J/mol); : solubility of j (g/g solvent); R: gas constant; T: temperature (K).
Relative solubility is always calculated in infinite dilution. The logarithm of the best solubility is set to 0 and all other solvents are given relative to the best solvent. A solvent with a log10 (x_j) value of −1.00 yields a solubility that is decreased by a factor of 10 compared to the best solvent. Additionally, the logarithm is transformed into the probability of solubility and expressed in a percentage.
3.3. Monkfish Liver Oil Extraction
3.3.1. Extraction in a Roller Mixer
The monkfish liver oil was extracted by solid-liquid extraction with FR (chloroform-methanol 2:1 v/v) as a reference solvent and using the four green solvents (2-MeTHF, DMC, CPME and LMN). An amount of 20 g of monkfish liver previously defrosted and ground was extracted with 100 mL of each solvent at a ratio of 1:5 w/v. The mixture was stirred at 60 rpm for 30 min in a roller mixer. The sample was filtered through paper, and the solid residue was washed two times with fresh solvent. Solvents were then joined, a solution of 1% v/v of NaCl was added to the extract obtained with FR, and the mixture was shaken vigorously and left to stand for 2 h. The organic phase was recovered and dried with anhydrous sodium sulfate. It was filtered and the solid was washed with chloroform. Final solutions were evaporated under vacuum in a rotary evaporator. Samples were dried under vacuum for 2 h and were finally stored at −20 °C until analyzed (see Scheme 1). All experiments were carried out in triplicates.
To perform a better comparison of the amount of monkfish liver oil obtained by every solvent, we determined the maximum content of oil in the monkfish liver by extracting one sample five times with the FR (until no color was observed in the solvent). Solvents were joined and processed as indicated above.
3.3.2. Extraction in an ULTRA-TURRAX® System
An amount of 2 g of ground monkfish liver, 2 g of ceramics balls and 10 mL of the solvents (green solvents or FR) were added to the ULTRA-TURRAX system. The mixture was mechanically stirred at 4000 rpm for 15 min at room temperature. The mixture was then filtered and centrifuged at 5000 rpm for 5 min to separate the supernatant. The organic phase was recovered and dried with anhydrous sodium sulfate. The mixture was filtered and then the solvent was evaporated in a rotary evaporator and the oil was dried under vacuum. The samples were stored at −20 °C until analysis. All experiments were carried out in triplicate (see Scheme 1).
3.3.3. Preparation of Resting Cells
R. oryzae and A. flavus cells were grown in a synthetic liquid medium containing 2 g of asparagine, 1 g of K2HPO4, 0.5 g of MgSO4, 5 mg of thiamine hydrochloride, 1.45 mg of Fe (NO3)3·9H2O, 0.88 mg of ZnSO4·7H2O and 0.235 mg of MnSO4·H2O per litter of distilled water. The initial pH of the medium was adjusted to pH 6.0. Next, 250 mL aliquots of the medium were sterilized at 121 °C for 15 min, and 1% (v/v) of refined sunflower oil was added aseptically. The medium was inoculated with 2.5 mL of a R. oryzae and A. flavus spore suspension (1–4 × 106 spores/mL) and then incubated at 28 °C for 5 d using an orbital shaker at 200 rpm. Mycelium was harvested from the culture medium using a Büchner funnel and washed with distilled water followed by acetone. It was then dried under vacuum for 18 h and ground to a powder. The R. oryzae (CECT20476) and A. flavus (CECT20475.2.1) strains were housed in the Spanish Type Culture Collection (CECT). The enzymatic units (U) were determined beforehand on the basis of the enzymatic esterification rate of ethyl oleate from oleic acid and ethanol. The specific activity of the resting cells of R. oryzae was 1.14 U and A. flavus was 0.57 U.
3.4. Enzymatic Preparation of Ethyl Esters
3.4.1. One-Step Synthesis: Transesterification Reaction
A 1:3.2 mixture of monkfish liver oil (0.453 g; 0.5 mmol) and ethanol (0.0736 g; 1.6 mmol) was added to a reaction vial (5 mL) fitted with a PTFE-lined cap that contained 0.045 g of each biocatalyst (10% w/w based on the weight of monkfish liver oil), a commercial enzyme (Novozym 435) or resting cells (R. oryzae and A. flavus), or their mixtures (1:1 and 7:3, R. oryzae-A. flavus), respectively (see Figure 2). The mixture was stirred (220 rpm) continuously at atmospheric pressure and 28 °C. Reaction progress was evaluated for 24, 48 and 74 h. Samples were collected, filtered and the solvent was evaporated. An aliquot of 20 mg of the crude material of the reaction was dissolved in deuterated chloroform and the resulting solution was analyzed by NMR [65,66,67,68,69] and GC-FID. Experiments were carried out in triplicate.
3.4.2. Two-Step Synthesis: Hydrolysis and Esterification Reactions
In the first step, biocatalytic hydrolysis reactions (see Figure 3) were performed using the commercial enzyme, the two resting cells and their mixtures (1:1 and 7:3, R. oryzae/A. flavus). A mixture of monkfish liver oil (0.453 g; 0.5 mmol), biocatalyst (0.045 g; 10% w/w based on the weight of monkfish liver oil) and water (0.453 g; 25 mmol) was stirred (220 rpm) at 28 °C for 24 h. The sample was filtrated, centrifuged and the supernatant was collected. The resulting hydrolysate material was analyzed by NMR and GC-FID, with this process determining which of the tests offered the best results in terms of percentage of free fatty acids. With the better biocatalyst, the hydrolysis was scaled up to obtain a minimum of 30 mL of hydrolysate, with which the esterification studies were carried out. The hydrolysate material was analyzed by NMR and GC-FID to determine the hydrolysis degree and the fatty acids profile. In the second step, the hydrolyzed oil was used to perform the esterification reactions using the same biocatalysts indicated above. A mixture of hydrolyzed monkfish liver oil (0.300 g; 1 mmol), biocatalyst (0.03 g, 10% w/w based on the weight of hydrolyzed monkfish liver oil) and ethanol (0.147 g; 3.2 mmol) were stirred (220 rpm) at 28 °C. The esterification reactions were conducted for 24, 48 or 72 h. The reaction products were analyzed by NMR [65,66,67,68,69] and GC-FID. All the experiments were carried out in triplicate.
3.5. Analytical Methods
Preliminary analysis of monkfish liver oil, hydrolyzed oil and ethyl esters were carried out by 1H NMR. Spectra were recorded with a MERCURYplus NMR Spectrometer Systems VARIAN 400 MHz magnet using deuterated chloroform (99.9 atom % D) as solvent. The fatty acid profiles of the initial oil (as methyl and ethyl esters) and the ethyl esters after synthesis reactions were analyzed using an Agilent 6890 series gas chromatograph (Barcelona, Spain) coupled to a flame ionization detector (FID). The chromatographic column was a 30 m × 0.25 mm fused silica capillary coated with a 0.25 μm film thickness (50%-cyanopropyl)-methylpolysiloxane (DB-23; Agilent J&W, Madrid, Spain). The temperature program used was 180 °C for 1 min, followed by an increase of 20 °C per minute until the final temperature of 270 °C was reached, which was then maintained for 20 min. A splitless mode of 20 mL/min was applied for 9 s. Hydrogen was used as carrier gas at a constant pressure. The injection volume was 1 μL. The injection system was maintained at 270 °C and the FID at 280 °C.
4. Conclusions
During this study, different methods for MLO extraction were studied, using a traditional solvent (FR) and green solvents (2-MeTHF, CPME, DMC and LMN). In addition, two agitation systems (Roller Mixer and Ultra Turrax®) were studied. The roller mixer was the best agitation system, obtaining higher extraction percentages with all the solvents studied. All the green solvents tested showed extraction yields greater than or equal to the FR single extraction with both agitation systems. Analysis of the tested solvents (taking into account the properties of the solvents, costs, toxicity and safety use, risks to human health, environmental risks, percentage of extraction and resources) showed that 2-MeTHF was the best option for MLO extraction under the studied parameters. The lipidic profile of the MLO illustrated that it could be considered as a suitable source for obtaining PUFAs and DHA.
The results obtained in the esterification trials showed that the percentages of MLO esters in one-step transesterification were 63, 61 and 46% using Novozym 435, R. oryzae lipase and A. flavus lipase, respectively. In the two-step reactions, yields were 85, 65 and 41% using the commercial enzyme, R. oryzae and A. flavus, respectively. Consequently, the latter was definitely not a good biocatalyst for these reactions. Moreover, R. oryzae resting cells (CECT20476) showed the lowest yields of DHA-EE in one or two-processed steps, suggesting selectivity towards this fatty acid. The resting cells of the filamentous fungi used in this study, unlike commercial enzymes, did not undergo any kind of extraction, purification and immobilization process. Therefore, they were very cheap biocatalysts compared to commercial ones. Furthermore, several authors reported that enzymatic activity and selectivity of lipases could be affected by the immobilization process. Similarly, it was clear that commercial lipase esterified the omega-3 fatty acids optimally, with yields above 90% for DHA and 100% for EPA contained in the MLO. The resting cells of the R. oryzae and A. flavus tested showed a good percentage of hydrolysis (88% and 93%, respectively), higher than those of the commercial enzyme (61%). These results open a new path to studying the enrichment of PUFA using these resting cells.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/1/100/s1, Figure S1: 1H-NMR spectrum of monkfish liver oil (MLO) extracted with Roller mixer (RM) and different solvents; Figure S2: 1H-NMR spectrum of hydrolysed monkfish liver oil (HMLO) extracted with ULTRA-TURRAX® (UT) and different solvents; Figure S3: 1H-NMR spectrum of MLO extracted by Folch method (FM) and with RM agitation; Figure S4: Chromatogram (GC-FID on DB-23, 30 m) chemically esterified monkfish liver oil; Table S1: Green solvent properties, extraction yields (MLO), substance information and cost analysis.
Author Contributions
The individual contributions of each author is the following: R.C.-G. and E.Y.-V. conceptualization; J.A.-O. performed the experiments; R.C.-G. and M.T. methodology; J.A.-O. and E.Y.-V. validation, formal analysis and investigation; M.B. resources; J.A.-O. writing—original draft preparation; E.Y.-V. and R.C.-G. writing—review and editing, E.Y.-V. and R.C.-G. supervision; M.B. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Interreg POCTEFA program (EFA253/16 BIOPLAST) and the PhD student aid program “Jade Plus” financed by Banco Santander of which Johanna Aguilera Oviedo is a beneficiary.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
This work was supported in part by the Spanish government through the Interreg POCTEFA program (EFA253/16 BIOPLAST) and the PhD student aid program “Jade Plus” financed by Banco Santander of which Johanna Aguilera Oviedo is a beneficiary. The authors would like to thank the Vice-rector of Research of the University of Lleida for the scholarship granted to Johanna Aguilera Oviedo. The authors would also like to thank Congelados y Especialidades Barrufet SL for supplying the monkfish livers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Punia, S.; Sandhub, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A Review. Pharma Nutr. 2019, 10, 100162. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P.S. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Von Schacky, C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc. Health Risk Manag. 2006, 2, 251–262. [Google Scholar] [CrossRef]
- Golpour, P.; Nourbakhsh, M.; Mazaherioun, M.; Janani, L.; Yaghmaei, P. Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: A double-blind randomised placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2020, 162, 108120. [Google Scholar] [CrossRef]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef]
- Volpato, M.; Hull, M.A. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Rev. 2018, 37, 545–555. [Google Scholar] [CrossRef]
- Nindrea, R.D.; Aryandono, T.; Lazuardi, L.; Dwiprahasto, I. Protective Effect of Omega-3 Fatty Acids in Fish Consumption Against Breast Cancer in Asian Patients: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2019, 20, 327–332. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A., Jr.; Meyer, B.J.; Tsang, B.; Von Hurst, P.R. Inflammation (IL-1β) Modifies the Effect of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids on Core Symptoms of Autism Spectrum Disorder—An Exploratory Pilot Study. Nutrients 2020, 12, 661. [Google Scholar] [CrossRef]
- Swanson, D.; Block, B.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Kwatra, B.A. Review on Potential Properties and Therapeutic Applications of DHA and EPA. Int. J. Pharm. Pharm. Res. 2019, 16, 140–176. [Google Scholar]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in Fish Extraction, Fractionation, Importance in Health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Sargent, J.R.; Bell, M.V.; Bell, J.G.; Henderson, R.J.; Tocher, D.R. Origins and functions of n-3 polyunsaturated fatty acids in marine organisms. In Phospholipids: Characterization, Metabolism and Novel Biological Applications; Cevc, G., Paltauf, F., Eds.; AOCS Press: Champaign, IL, USA, 1995; pp. 248–258. [Google Scholar]
- Espinosa, S.; Diaza, M.S.; Brignolea, E.A. Food additives obtained by supercritical extraction from natural sources. J. Supercrit. Fluids 2008, 45, 213–219. [Google Scholar] [CrossRef]
- Pike, I.H.; Jackson, A. Fish oil: Production and use now and in the future. Lipid Technol. 2010, 22, 59–61. [Google Scholar] [CrossRef]
- Angulo, B.; Fraile, J.M.; Gil, L.; Herrerías, C.I. Comparison of chemical and enzymatic methods for the transesterification of waste fish oil Fatty Ethyl Esters with different Alcohols. ACS Omega 2020, 5, 1479–1487. [Google Scholar] [CrossRef]
- Informe Realizado por la Asociación Empresarial de Acuicultura de España (APROMAR). 2019, pp. 1–91. Available online: http://apromar.es/sites/default/files/2019/InformeAcui/APROMAR%20Informe%20ACUICULTURA%202019%20v-1-2.pdf (accessed on 15 August 2020).
- Informe EL MERCADO PESQUERO DE LA UE 2019. 2019, p. 1. Available online: https://www.eumofa.eu/documents/20178/314856/ES_El+mercado+pesquero+de+la+UE_2019.pdf/ (accessed on 29 August 2020).
- Erasmus, V.N.; Kadhila, T.; Gabriel, N.N.; Thyberg, K.L.; Ilungu, S.; Machado, T. Assessment and quantification of Namibian seafood waste production. Ocean Coast. Manag. 2021, 199, 105402. [Google Scholar] [CrossRef]
- Iñarra, B.; Bald, C.; San Martín, D.; Orive, M.; Cebrián, M.; Zufía, J. Guía para la Valorización de Subproductos de la Acuicultura. AZTI, Derio. 2018, pp. 1–44. Available online: https://www.azti.es/wp-content/uploads/2018/12/AZTI_guia_VALACUI101218online.pdf (accessed on 15 August 2020).
- Fisher, R.A.; DuPaul, B. A Fisherman’s Guide: Getting the Most out of Monkfish. Marine. Resource Advisory No. 37; Virginia Institute of Marine Science, College of William and Mary: Gloucester Point, VA, USA, 1990; pp. 1–7. [Google Scholar] [CrossRef]
- Manipulación de la Pesca del día en Embarcaciones de Red de Enmalle; Servicio Central de Publicaciones Gobierno Vasco, Departamento de Medio Ambiente, Planificación Territorial y Pesca; ISBN 978-84-457-3186-433-34. Colección ITSASO 37; 2011; pp. 33–34. Available online: http://www.euskadi.net/ejgvbiblioteka (accessed on 15 July 2020).
- Guía de Buenas Prácticas de Higiene para Buques Palanqueros y Buques Factoría Congeladores. Organización de Productores de Buques Congeladores de Merlúcidos, Cefalópodos y Especies Varias. OPPC-3. 2017, pp. 78–80. Available online: http://www.arvi.org/publicaciones/GUIA_DE_BUENAS_PRACTICAS_DE_HIGIENE.PDF (accessed on 15 July 2020).
- Pacetti, D.; Alberti, F.; Boselli, E.; Frega, N.G. Characterisation of furan fatty acids in Adriatic fish. Food Chem. 2010, 122, 209–215. [Google Scholar] [CrossRef]
- Folch, A.J.; Lees, M.; Sloane-Stanlet, G.H. Simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. Available online: http://www.jbc.org/ (accessed on 16 May 2019). [CrossRef]
- Cascant, M.M.; Breil, B.; Garrigues, S.; De la Guardia, M.; Fabiano-Tixier, A.S.; Chemat, F. A green analytical chemistry approach for lipid extraction: Computation methods in the selection of green solvents as alternative to hexane. Anal. Bioanal. Chem. 2017, 409, 3527–3539. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela- Garayoa, R.; Billy, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Codex Alimentarius. NORMA PARA ACEITES DE PESCADO: CXS 329-2017. Adoptada en. 2017. Available online: www.codexalimentarios.org (accessed on 20 August 2020).
- Tanzi, D.C.; Vian, M.A.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as Green Solvents for Extraction of Oil from Microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano-Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Selka, A.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Touaibiac, M.; Chemat, F. Solvent from forestry biomass. Pinane a stable terpene derived from pine tree byproducts to substitute n-hexane for the extraction of bioactive compounds. Green Chem. 2016, 18, 6596–6608. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Ferreira, G.F.; Moreira, L.S.; Wolf, M.R.M.; Filho, R.M. Comparison of several methods for effective lipid extraction from wet microalgae using green solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Review. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Sicaire, A.G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Ma, X.; Huang, H.; Wang, Y. Two-Stage Enzymatic Preparation of Eicosapentaenoic Acid (EPA) And Docosahexaenoic Acid (DHA) Enriched Fish Oil Triacylglycerols. J. Agric. Food Chem. 2018, 66, 218–227. [Google Scholar] [CrossRef]
- Miyashita, K.; Uemura, M.; Hosokawa, M. Effective Prevention of Oxidative Deterioration of Fish Oil: Focus on Flavor Deterioration. Annu. Rev. Food Sci. Technol. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Moreno-Perez, S.; Machado, D.F.; Pires, J.; Luna, P.; Señorans, F.J.; Guisan, J.M.; Fernandez-Lorente, G. Critical Role of Different Immobilized Biocatalysts of a Given Lipase in the Selective Ethanolysis of Sardine Oil. J. Agric. Food Chem. 2017, 65, 117–122. [Google Scholar] [CrossRef]
- Castejón, N.; Señoráns, F.J. Strategies for Enzymatic Synthesis of Omega-3 Structured Triacylglycerols from Camelina sativa Oil Enriched in EPA and DHA. Eur. J. Lipid Sci. Technol. 2019, 121, 1800412. [Google Scholar] [CrossRef]
- Ranjan-Moharana, T.; Byreddy, A.R.; Puri, M.; Barrow, C.; Rao, N.M. Selective Enrichment of Omega-3 Fatty Acids in Oils by Phospholipase A1. PLoS ONE 2016, 11, e0151370. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gu, S.; Liu, S.; Zhang, J.; Ding, Y.; Liu, J. Extraction of oil from high-moisture tuna liver by subcritical dimethyl ether: Feasibility and optimization by the response surface method. RSC Adv. 2018, 8, 2723–2733. [Google Scholar] [CrossRef]
- Routray, W.; Dave, D.; Ramakrishnan, V.V.; Murphy, W. Production of High Quality Fish Oil by Enzymatic Protein Hydrolysis from Cultured Atlantic Salmon By-Products: Investigation on Effect of Various Extraction Parameters Using Central Composite Rotatable Design. Waste Biomass Valor 2018, 9, 2003–2014. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Fabiano-Tixier, A.S.; Lino, C.; Avellone, G.; Chemat, F.; Pagliaro, M. Omega-3 Extraction from Anchovy Fillet Leftovers with Limonene: Chemical, Economic, and Technical Aspects. ACS Omega 2019, 4, 15359–15363. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jimenez-Gonzalez, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide-embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehadad, S.; Dunne, P.J. CHEM21 selection guide of classical- and less classical-solvent. Green Chem. 2016, 18, 288. [Google Scholar] [CrossRef]
- De Jesus, S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Catrin, E.T.; Brecker, L.; Wagner, K.H. 1H NMR spectroscopy as tool to follow changes in the fatty acids of fish oils. Eur. J. Lipid Sci. Technol. 2008, 110, 141–148. [Google Scholar] [CrossRef]
- Bratu, A.; Mihalache, M.; Hanganu, A.M.; Chira, N.A.; Todaşcă, M.C.; Roşca, S. Quantitative determination of fatty acids from fish oils using GC-MS method and 1H NMR spectroscopy. U.P.B. Sci. Bull. 2013, 75, 24–31. [Google Scholar]
- Nestor, G.; Bankefors, J.; Schlechtriem, G.; Brannas, E.; Pickova, J.; Sandstrom, C. High-Resolution 1H Magic Angle Spinning NMR Spectroscopy of Intact Arctic Char (Salvelinus Alpinus) Muscle. Quantitative Analysis of n-3 Fatty Acids, EPA and DHA. J. Agric. Food Chem. 2010, 58, 10799–10803. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Khaneghah, A.M.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Djoussé, L.; Matsumoto, C.; Hanson, N.Q.; Weir, N.L.; Tsai, M.Y.; Gaziano, J.M. Plasma cis-vaccenic acid and risk of heart failure with antecedent coronary heart disease in male physicians. Clin. Nutr. 2014, 33, 478–482. [Google Scholar] [CrossRef]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Sissener, N.H.; Ørnsrud, R.; Sanden, M.; Frøyland, L.; Remø, S.; Lundebye, A.K. Erucic Acid (22:1n-9) in Fish Feed, Farmed, and Wild Fish and Seafood Products. Nutrients 2018, 10, 1443. [Google Scholar] [CrossRef]
- Shimada, Y.; Maruyama, K.; Sugihara, A.; Moriyama, S.; Tominaga, Y. Purification of Docosahexaenoic Acid from Tuna Oil by a Two-Step Enzymatic Method: Hydrolysis and Selective Esterification. JAOCS 1997, 74, 1441. [Google Scholar] [CrossRef]
- Gudmundur, G.; Haraldsson, G.G.; Kristinsson, B.; Sigurdardottir, R.; Gudmundson, G.G.; Breivikb, H. The Preparation of Concentrates of Eicosapentaenoic Acid and Docosahexaenoic Acid by Lipase-Catalyzed Transesterification of Fish Oil with Ethanol. JAOCS 1997, 74, 1419–1424. [Google Scholar]
- Xu, Y.; Wang, D.; Mu, X.Q.; Zhao, K.A.; Zhang, K.C. Biosynthesis of ethyl esters of short-chain fatty acids using whole-cell lipase from Rhizopus chinesis CCTCC M201021 in non-aqueous phase. J. Mol. Catal. B Enzym. 2002, 18, 29–37. [Google Scholar] [CrossRef]
- Aarthya, M.; Saravananb, P.; Ayyaduraia, N.; Gowthamana, M.K.; Kaminia, N.R. A two-step process for production of omega 3-polyunsaturated fatty acid concentrates from sardine oil using Cryptococcus sp. MTCC 5455lipase. J. Mol. Catal. B Enzym. 2016, 125, 25–33. [Google Scholar] [CrossRef]
- Fraga, M.E.; Santana-N, D.M.; Gatti, J.M.; Direito, G.M.; Cavaglieri, L.R.; Rosa-R, C.A. Characterization of Aspergillus species based on fatty acid profiles. Mem. Inst. Oswaldo Cruz Rio Janeiro 2008, 103, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Asci, F.; Aydin, B.; Akkus, G.U.; Unal, A.; Erdogmus, S.F.; Korcan, S.E.; Jahan, I. Fatty acid methyl ester analysis of Aspergillus fumigatus isolated from fruit pulps for biodiesel production using GC-MS spectrometry. Bioengineered 2020, 11, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Neubronner, J.; Kressel, G.; Merkel, M.; Schacky, C.V.; Hahn, A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in stat in-treated dyslipidemic subjects: Results from a six-month randomized controlled trial. Prostaglandins Leukot Essent Fat. Acids 2011, 85, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Ashjaria, M.; Mohammadic, M.; Badria, R. Selective concentration of eicosapentaenoic acid and docosahexaenoic acid from fish oil with immobilized/stabilized preparations of Rhizopus oryzae lipase. J. Mol. Catal. B Enzym. 2015, 122, 147–155. [Google Scholar] [CrossRef]
- Klamt, A. Prediction of the mutual solubilities of hydrocarbons and water with COSMO-RS. Fluid Phase Equilib. 2003, 206, 223–235. [Google Scholar] [CrossRef]
- BIOVIA COSMOtherm 2020 User Guide; Dassault Systèmes: Vélizy-Villacoublay, France, 2019.
- Canela-Garayoa, R.; Yara-Varón, E.; Balcells, M.; Torres, M.; Eras, J. Nuclear Magnetic Resonance Spectroscopy: An Alternative Fast Tool for Quantitative Analysis of the Solvent-free Ethanolysis of Coconut Oil Using Fungal Resting Cells. New Biotechnol. 2014, 31, S89. [Google Scholar] [CrossRef]
- Prakash, R.; Aulakh, S.S. Transesterification of used edible and non-edible oils to alkyl esters by Aspergillus sp. as a whole cell catalyst. J. Basic Microbiol. 2011, 51, 607–613. [Google Scholar] [CrossRef]
- De Jesus, M.D.P.M.; De Melo, L.N.; Da Silva, J.P.V.; Crispim, A.C.; Figueiredo, I.M.; Bortoluzzi, J.H.; Meneghetti, S.M.P. Evaluation of proton nuclear magnetic resonance spectroscopy for determining the yield of fatty acid ethyl esters obtained by transesterification. Energy Fuels 2015, 29, 7343–7349. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).