The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts
Abstract
1. Introduction
2. Results and Discussions
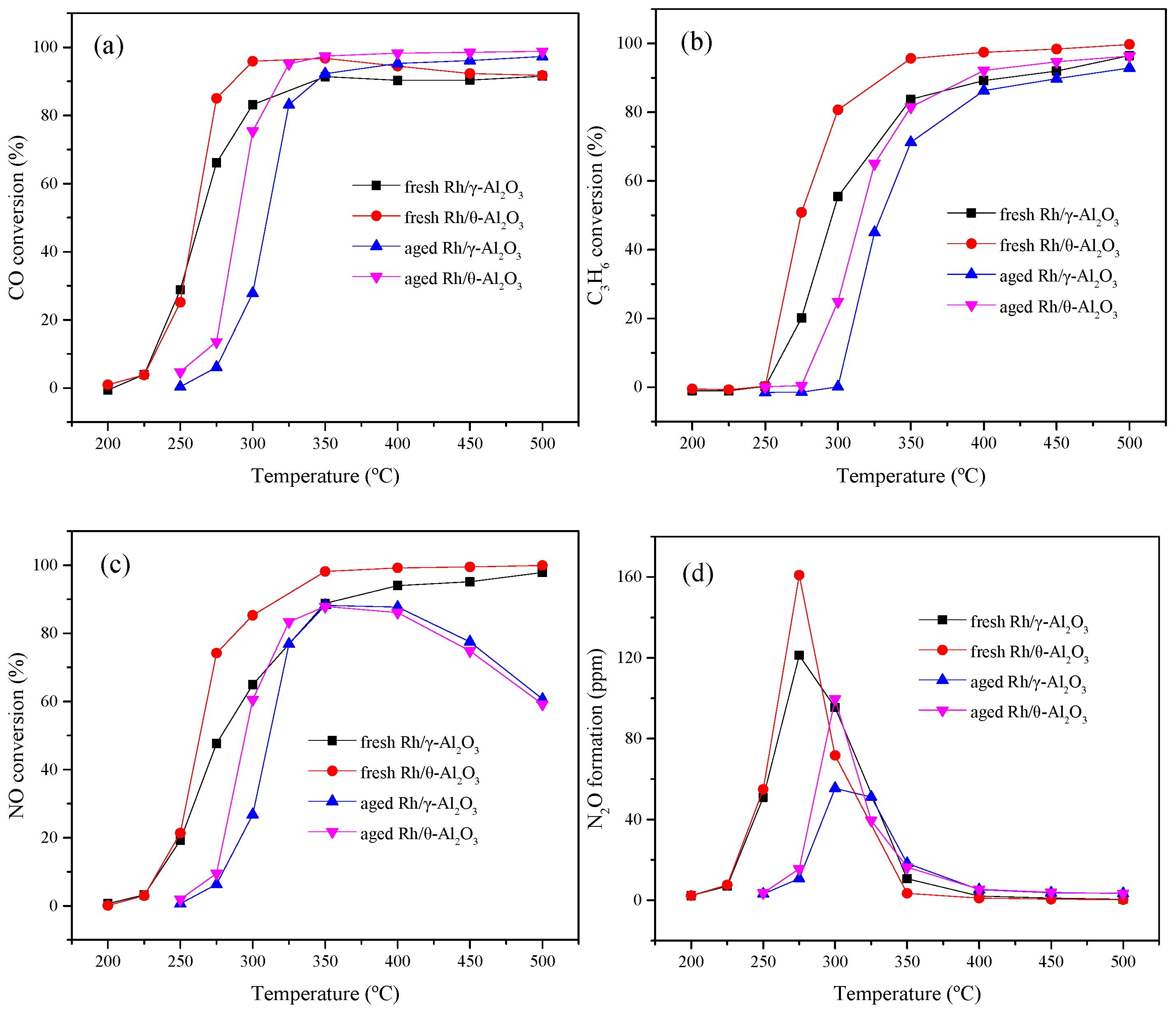
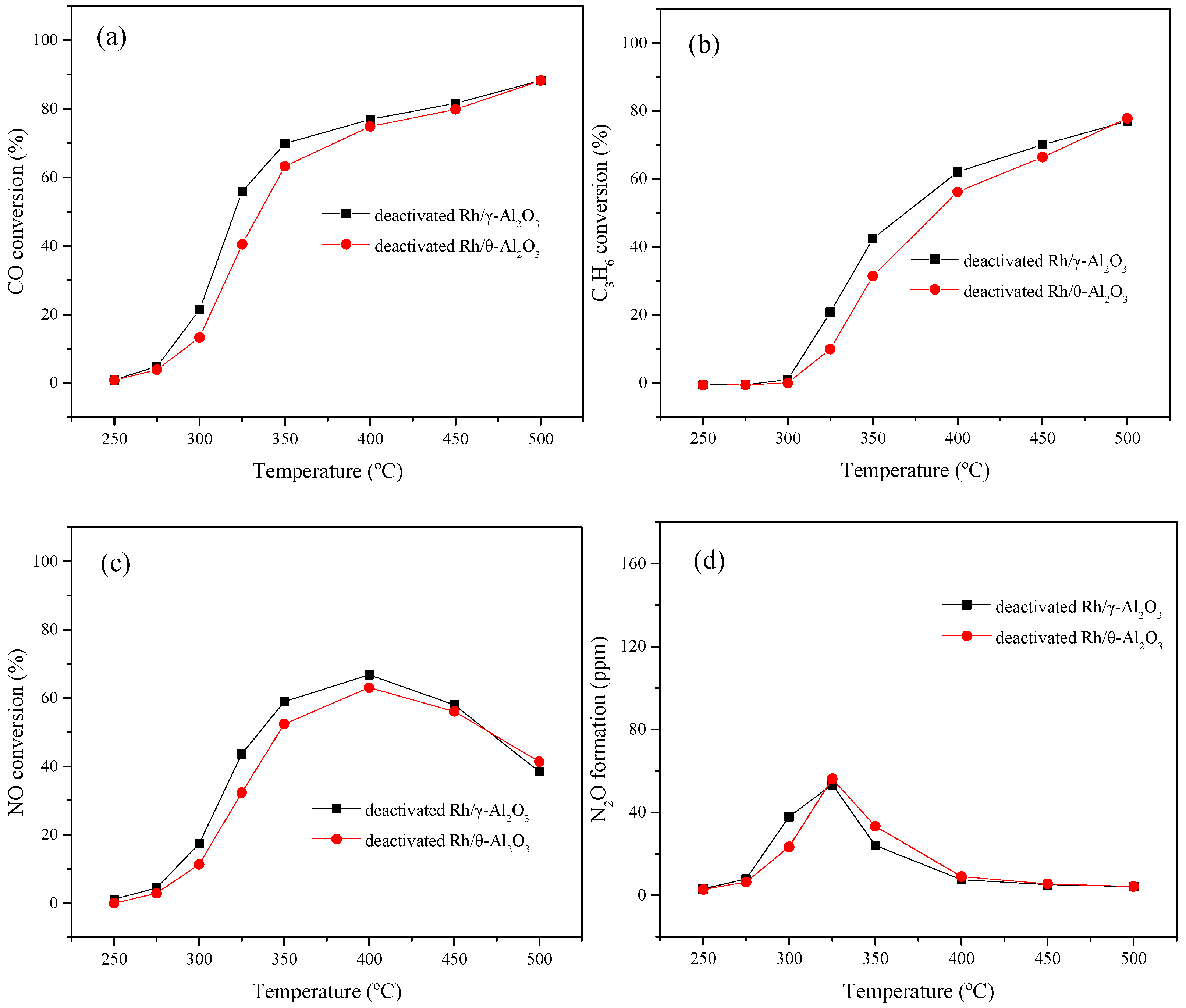
2.1. The Effect of γ, θ-Phase Alumina on the Hydrothermal Stability of Rh/γ, θ-Al2O3 Catalysts
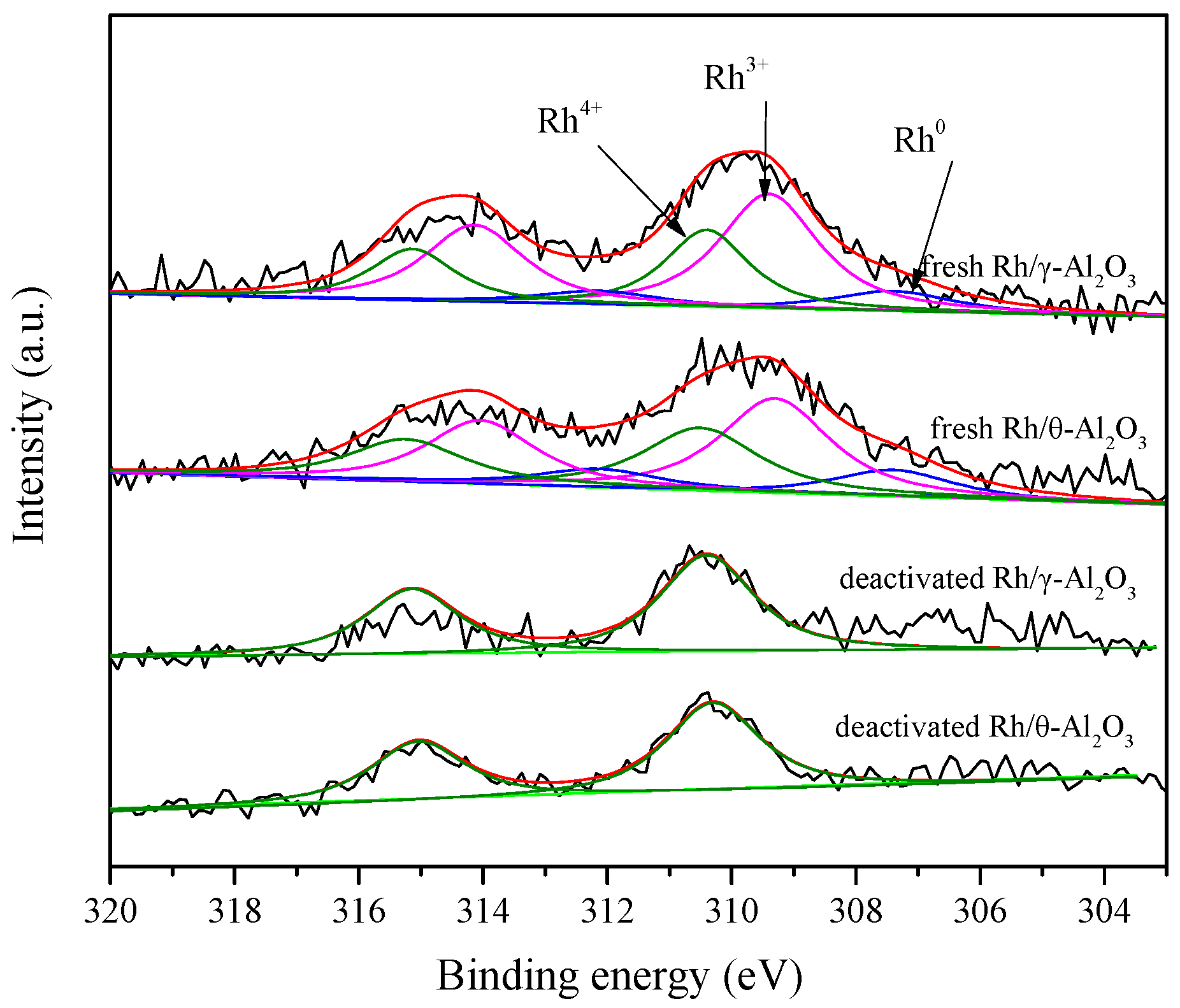
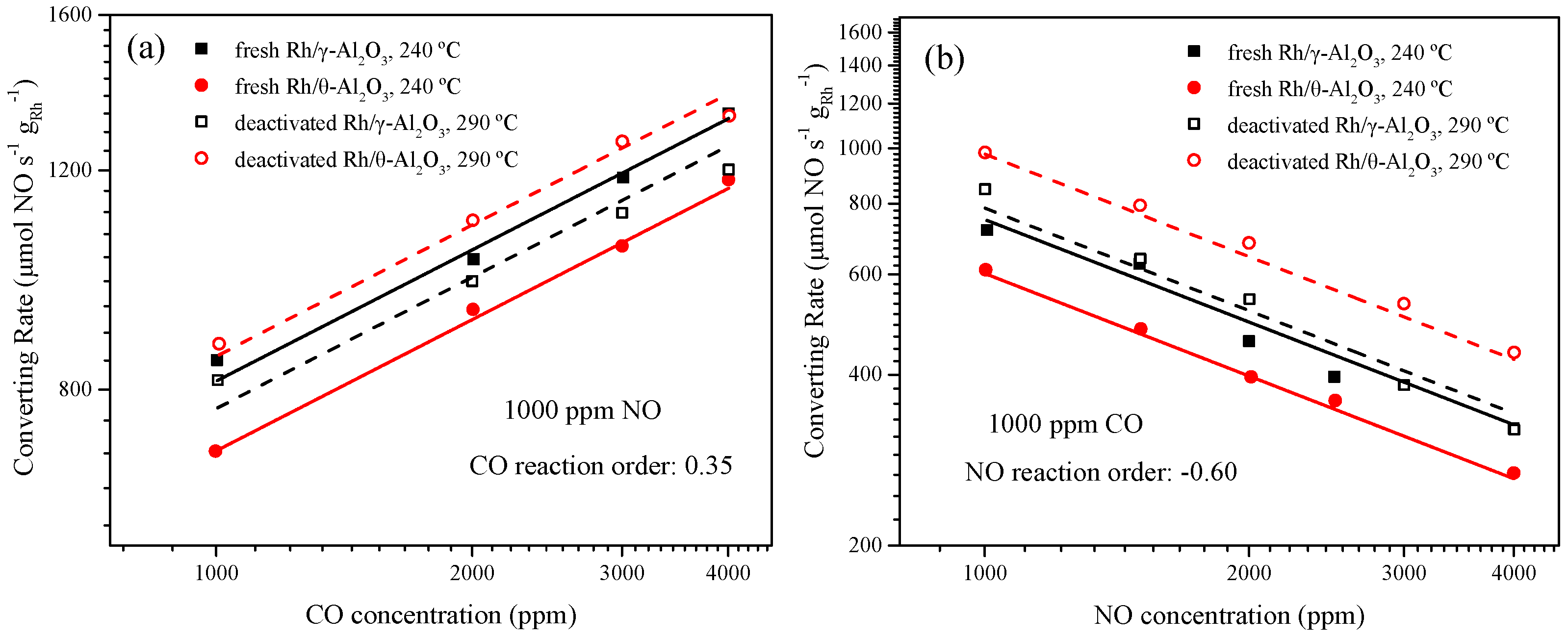
2.2. The Researches of Non- and Strongly-Interacting Rh
2.3. The Amount of Non-Interacting Rh in Aged Samples
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Activity Tests
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelef, M.; Graham, G.W. Why rhodium in automotive three-way catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- Samed, A.J.; Yamamoto, Y.; Hidaka, M.; Hinokuma, S.; Machida, M. An attempt to stabilize supported Ru catalysts against oxidative volatilization. Catal. Commun. 2017, 91, 6–9. [Google Scholar] [CrossRef]
- Asokan, C.; Yang, Y.; Dang, A.; Getsoian, A.B.; Christopher, P. Low-temperature ammonia production during NO reduction by CO is due to atomically dispersed rhodium active sites. ACS Catal. 2020, 10, 5217–5222. [Google Scholar] [CrossRef]
- Loferski, P.J. Minerals Yearbook: Platinum-Group Metals. US Geological Survey 2012. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/platinum/myb1-2012-plati.pdf (accessed on 12 January 2021).
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and historical journal to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K. An investigation of the use of zirconia as a support for rhodium catalysts. Appl. Catal. A Gen. 1996, 143, 317–335. [Google Scholar] [CrossRef]
- Wong, C.; McCabe, R.W. Effects of high-temperature oxidation and reduction on the structure and activity of Rh/Al2O3 and Rh/SiO2 catalysts. J. Catal. 1989, 119, 47–64. [Google Scholar] [CrossRef]
- McCabe, R.W.; Usmen, R.K.; Ober, K.; Gandhi, H.S. The effect of alumina phase structure on the dispersion of rhodium/alumina catalysts. J. Catal. 1995, 151, 385–393. [Google Scholar] [CrossRef]
- Yao, H.C.; Japar, S.; Shelef, M. Surface interactions in the system Rh/Al2O3. J. Catal. 1977, 50, 407–418. [Google Scholar] [CrossRef]
- Yao, H.C.; Stepien, H.K.; Gandhi, H.S. Metal-support interaction in automotive exhaust catalysts: Rh-washcoat interaction. J. Catal. 1980, 61, 547–550. [Google Scholar] [CrossRef]
- Pu, S.; Li, L.; Ma, J.; Lu, F.; Li, J. Disperse fine equiaxed alpha alumina nanoparticles with narrow size distribution synthesised by selective corrosion and coagulation separation. Sci. Rep. 2015, 5, 11575. [Google Scholar] [CrossRef]
- Amrute, A.P.; Łodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schüth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar] [CrossRef]
- Daturi, M.; Binet, C.; Lavalley, J.C.; Galtayries, A.; Sporken, R. Surface investigation on CexZr1-xO2 compounds. Phys. Chem. Chem. Phys. 1999, 1, 5717–5724. [Google Scholar] [CrossRef]
- Huang, T.; Shen, M.; Cheng, G.; Wang, Y.; Wang, J.; Li, W.; Oh, S.H.; Qi, G.; Yang, M.; Wang, J. Possible negative influences of increasing content of cerium on activity and hydrothermal stability of Rh/ceria-zirconia three-way-catalysts. J. Rare Earths 2020. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; Gao, Y.; Liu, S.; Weng, D.; Ran, R. NO reduction by CO over Rh/Al2O3 and Rh/AlPO4 catalysts: Metal-support interaction and thermal aging. J. Colloid. Interface. Sci. 2013, 408, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Nakahara, Y.; Sato, T.; Iwakura, H.; Takeshita, S.; Minami, S.; Yoshida, H.; Machida, M. Rh/ZrP2O4 as an efficient automotive catalyst for NOx reduction under slightly lean conditions. ACS Catal. 2015, 5, 1986–1994. [Google Scholar] [CrossRef]
- Buwono, H.P.; Yamamoto, M.; Kakei, R.; Hinokuma, S.; Yoshida, H.; Machida, M. Redox dynamics of Rh supported on ZrP2O4 and ZrO2 analyzed by time-resolved in situ optical spectroscopy. J. Phys. Chem. C 2017, 121, 17982–17989. [Google Scholar] [CrossRef]
- Machida, M.; Murakami, K.; Hinokuma, S.; Uemura, K.; Ikeue, K.; Matsuda, M.; Chai, M.; Nakahara, Y.; Sato, T. AlPO4 as a support capable of minimizing threshold loading of Rh in automotive catalysts. Chem. Mater. 2009, 21, 1796–1798. [Google Scholar] [CrossRef]
- Getsoian, A.B.; Theis, J.R.; Paxton, W.A.; Lance, M.J.; Lambert, C.K. Remarkable improvement in low temperature performance of model three-way catalysts through solution atomic layer deposition. Nat. Catal. 2019, 2, 614–622. [Google Scholar] [CrossRef]
- Rogozhnikov, V.N.; Snytnikov, P.V.; Salanov, A.N.; Kulikov, A.V.; Ruban, N.V.; Potemkin, D.I.; Sobyanin, V.A.; Kharton, V.V. Rh/θ-Al2O3/FeCrAlloy wire mesh composite catalyst for partial oxidation of natural gas. Mater. Lett. 2019, 236, 316–319. [Google Scholar] [CrossRef]
- Heo, I.; Yoon, D.Y.; Cho, B.K.; Nam, I.S.; Choung, J.W.; Yoo, S. Activity and thermal stability of Rh-based catalytic system for an advanced modern TWC. Appl. Catal. B Environ. 2012, 121, 75–87. [Google Scholar] [CrossRef]
- Su, X.; Chen, S.; Zhou, Z. Synthesis and characterization of monodisperse porous α-Al2O3 nanoparticles. Appl. Surf. Sci. 2012, 258, 5712–5715. [Google Scholar] [CrossRef]
- Hwang, C.P.; Yeh, C.T.; Zhu, Q. Rhodium-oxide species formed on progressive oxidation of rhodium clusters dispersed on alumina. Catal. Today 1999, 51, 93–101. [Google Scholar] [CrossRef]
- Chen, J.G.; Colaianmi, M.L.; Chen, P.J.; Yates, J.T.; Fisher, G.B. Thermal behavior of a Rh/Al2O3 model catalyst: Disappearance of surface Rh upon heating. J. Phys. Chem. 1990, 94, 5059–5062. [Google Scholar] [CrossRef]
- Zimowska, M.; Wagner, J.B.; Dziedic, J.; Camra, J.; Borzęcka-Prokop, B.; Najbar, M. Some aspects of metal-support strong interactions in Rh/Al2O3 catalyst under oxidising and reducing conditions. Chem. Phys. Lett. 2006, 417, 137–142. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Cao, Y.; Wu, X.; Weng, D.; Fan, J.; Wu, X. Insight into the effects of different ageing protocols on Rh/Al2O3 catalyst. Appl. Surf. Sci. 2014, 308, 230–236. [Google Scholar] [CrossRef]
- Weng-Sieh, Z.; Gronsky, R.; Bell, A.T. Microstructural evolution of γ-alumina-supported Rh upon aging in air. J. Catal. 1997, 170, 62–74. [Google Scholar] [CrossRef]
- Muller, O.; Roy, R. Formation and stability of the platinum and rhodium oxides at high oxygen pressures and the structures of Pt3O4, β-PtO2 and RhO2. J. Less-Common Met. 1968, 16, 129–146. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K.; Cruise, N.A. An investigation of the deactivation of Rh/alumina catalysts under strong oxidising conditions. Appl. Catal. A Gen. 1996, 147, 375–394. [Google Scholar] [CrossRef]
- Cao, Y.; Ran, R.; Wu, X.; Zhao, B.; Weng, D. Improved activity and durability of Rh-based three-way catalyst under diverse aging atmospheres by ZrO2 support. J. Environ. Sci. 2017, 52, 197–203. [Google Scholar] [CrossRef]
- Granger, P.; Lecomte, J.J.; Dathy, C.; Leclercq, L.; Leclercq, G. Kinetics of CO+NO reaction over rhodium and platinum-rhodium on alumina. J. Catal. 1998, 175, 194–203. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Yang, V.N.; Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 2015, 137, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically active Au-O(OH)x-species stabilized by alkali ions on zeolites and mesoporous oxides. Science 2014, 346, 1498–1501. [Google Scholar] [CrossRef]
- Luo, M.F.; Song, Y.P.; Lu, J.Q.; Wang, X.Y.; Pu, Z.Y. Identification of CuO species in high surface area CuO-CeO2 catalysts and their catalytic activities for CO oxidation. J. Phys. Chem. C 2007, 111, 12686–12692. [Google Scholar] [CrossRef]
- Fernández, E.; Liu, L.; Boronat, M.; Arenal, R.; Concepcion, P.; Corma, A. Low-temperature catalytic NO reduction with CO by subnanometric Pt clusters. ACS Catal. 2019, 9, 11530–11541. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Shen, G.; Wang, J.; Wang, Y.; Zhang, W.; Wang, J.; Shen, M. The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts. Catalysts 2021, 11, 99. https://doi.org/10.3390/catal11010099
Cheng G, Shen G, Wang J, Wang Y, Zhang W, Wang J, Shen M. The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts. Catalysts. 2021; 11(1):99. https://doi.org/10.3390/catal11010099
Chicago/Turabian StyleCheng, Guanghao, Gurong Shen, Jun Wang, Yunhao Wang, Weibo Zhang, Jianqiang Wang, and Meiqing Shen. 2021. "The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts" Catalysts 11, no. 1: 99. https://doi.org/10.3390/catal11010099
APA StyleCheng, G., Shen, G., Wang, J., Wang, Y., Zhang, W., Wang, J., & Shen, M. (2021). The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts. Catalysts, 11(1), 99. https://doi.org/10.3390/catal11010099




