Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light
Abstract
1. Introduction
2. Results and Discussion
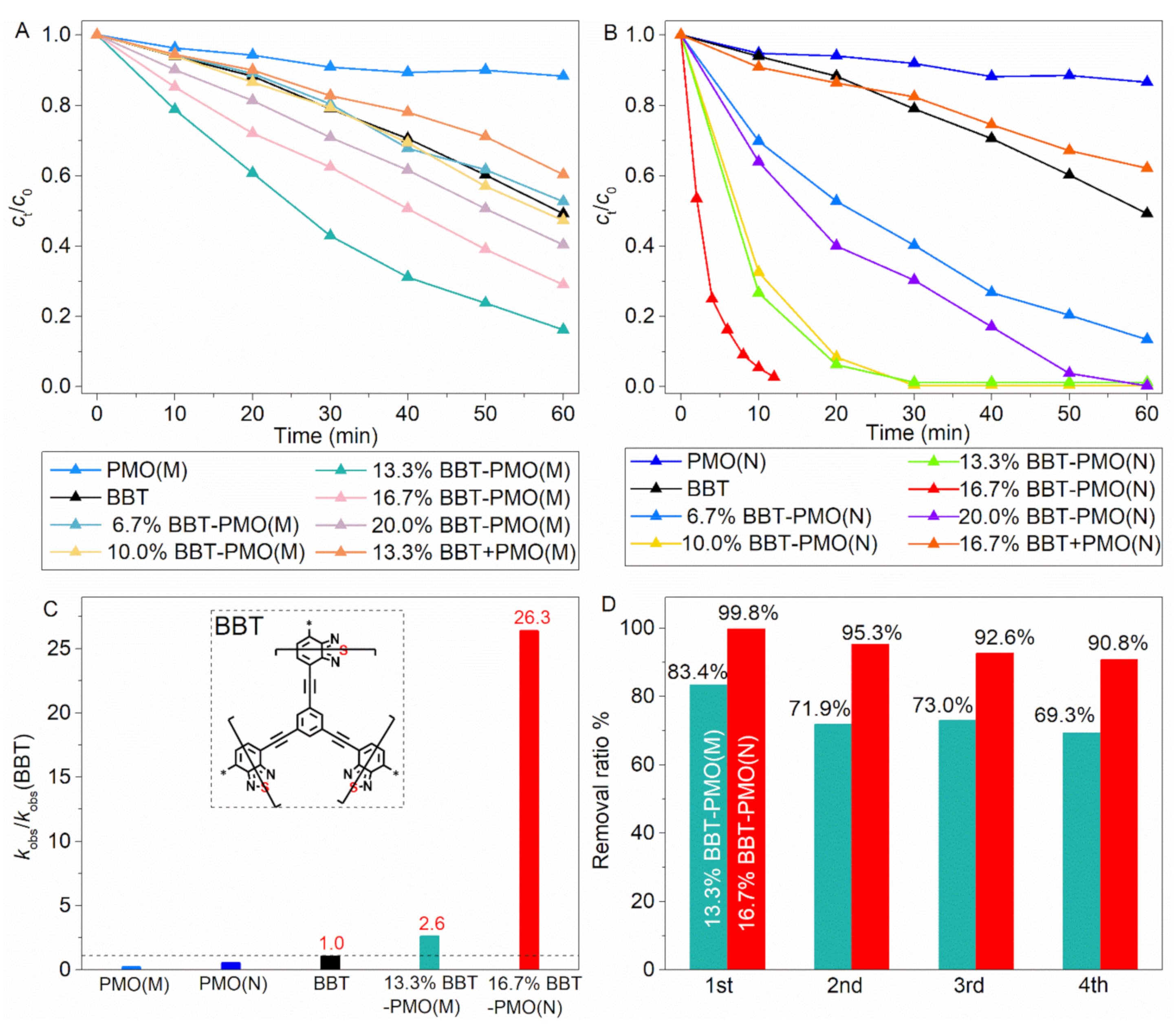
2.1. Photoreduction and Adsorption Activity of Cr(VI) over Materials
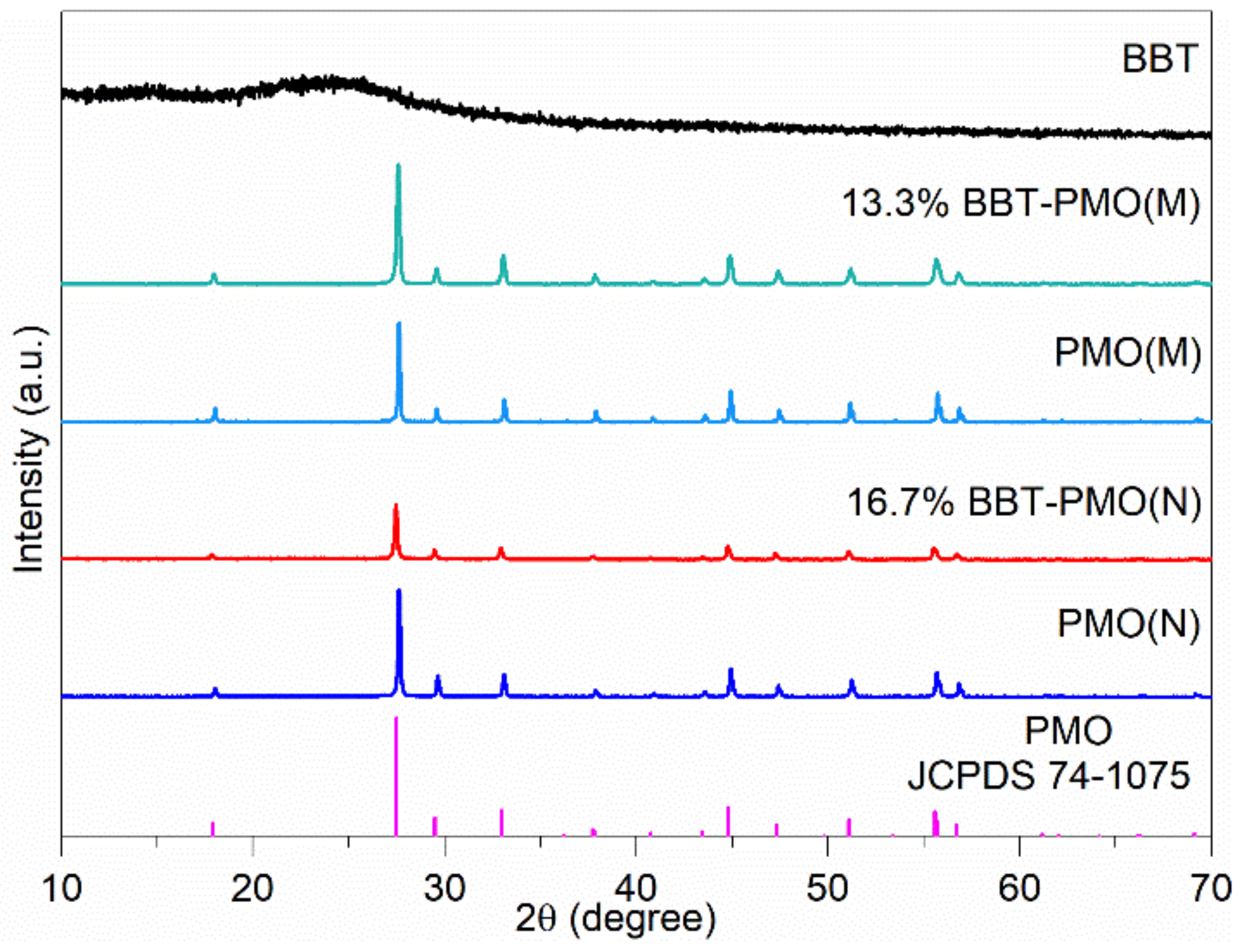
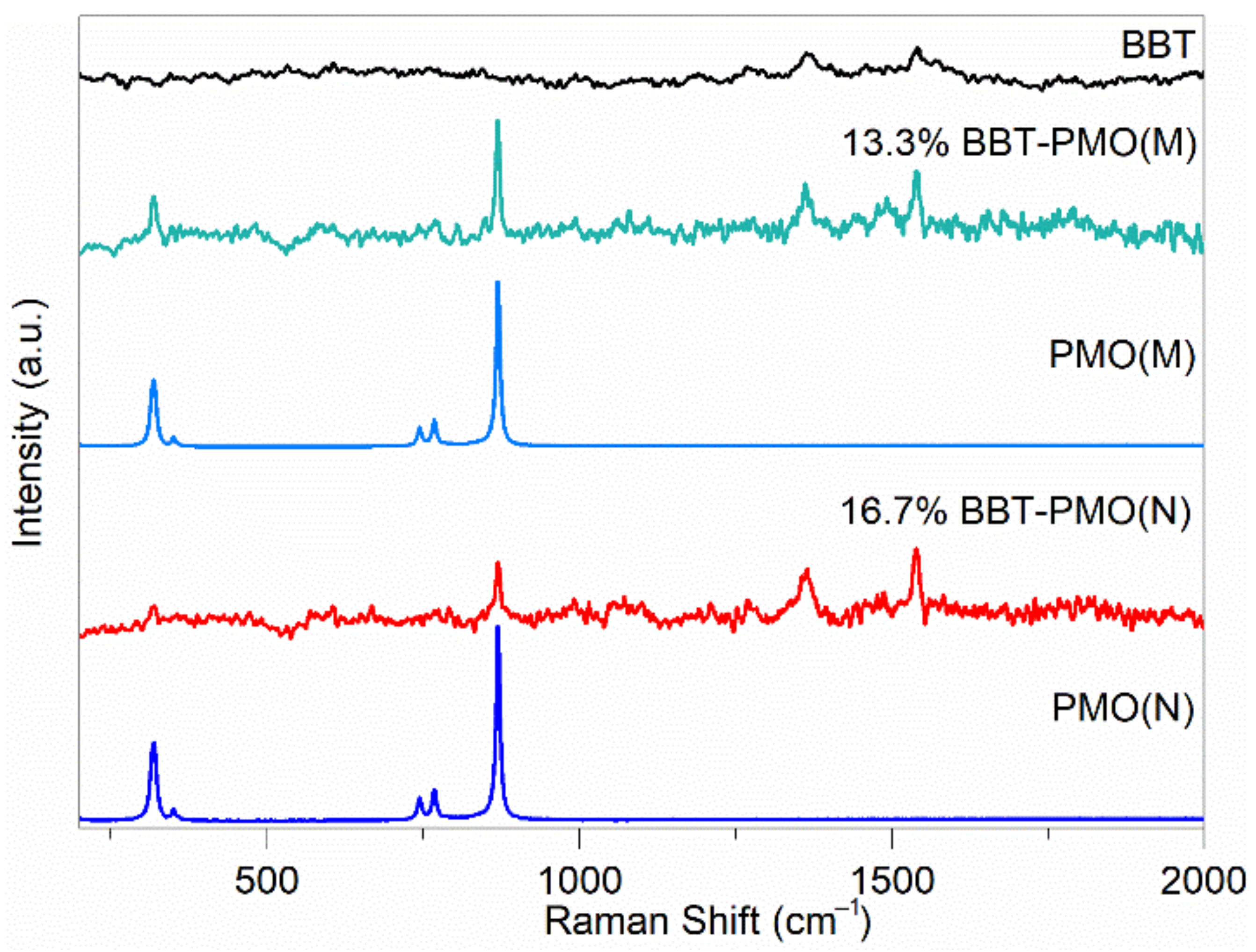
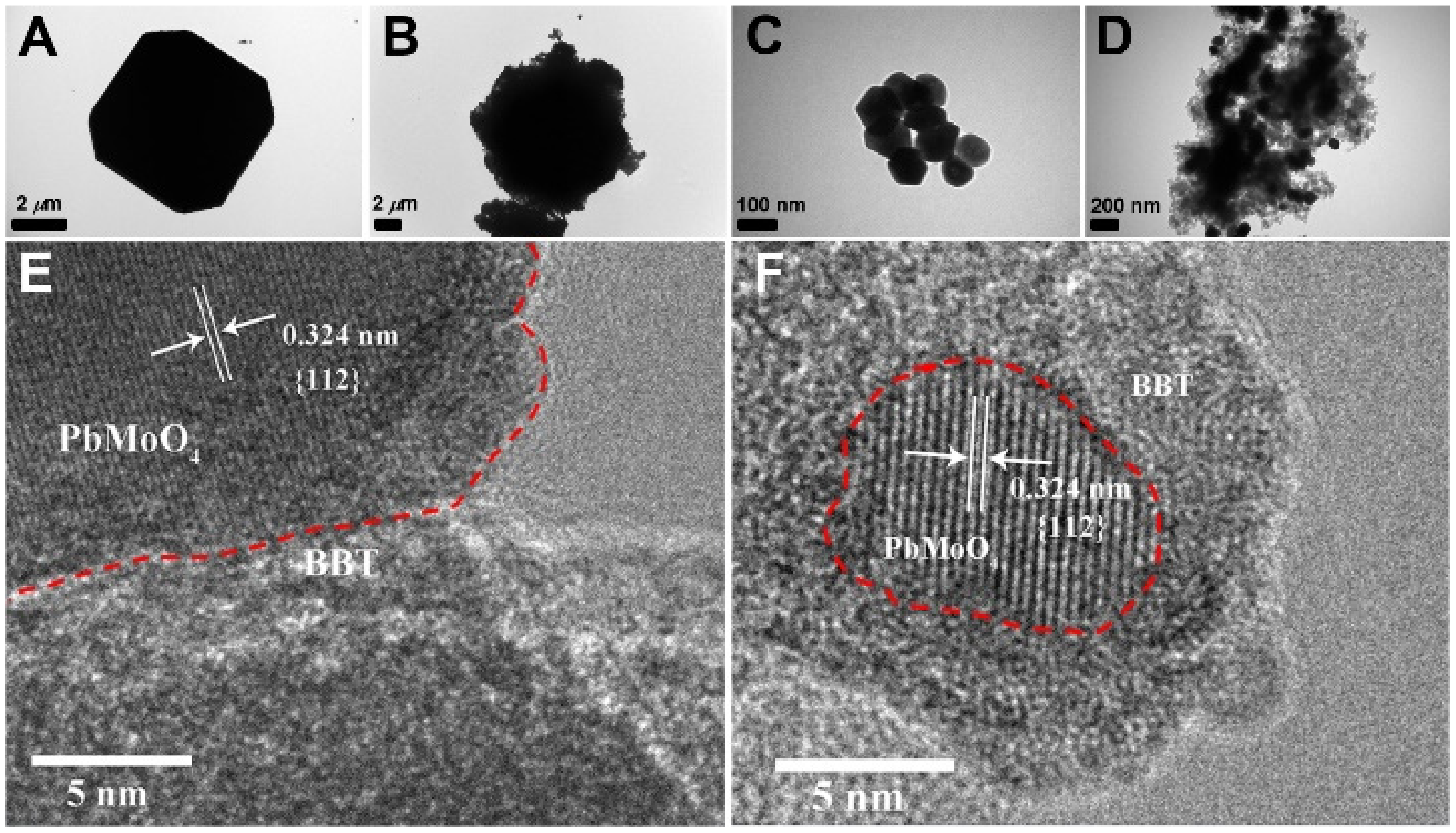
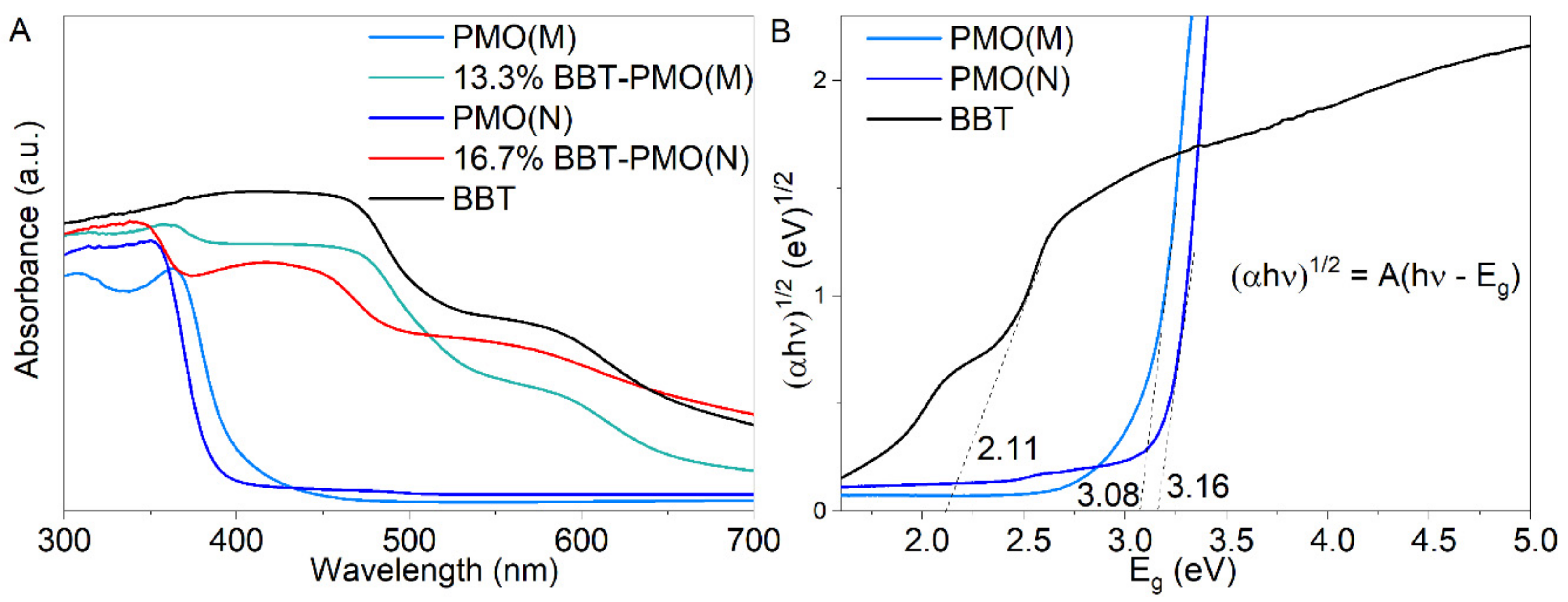
2.2. Structure and Composition of Materials
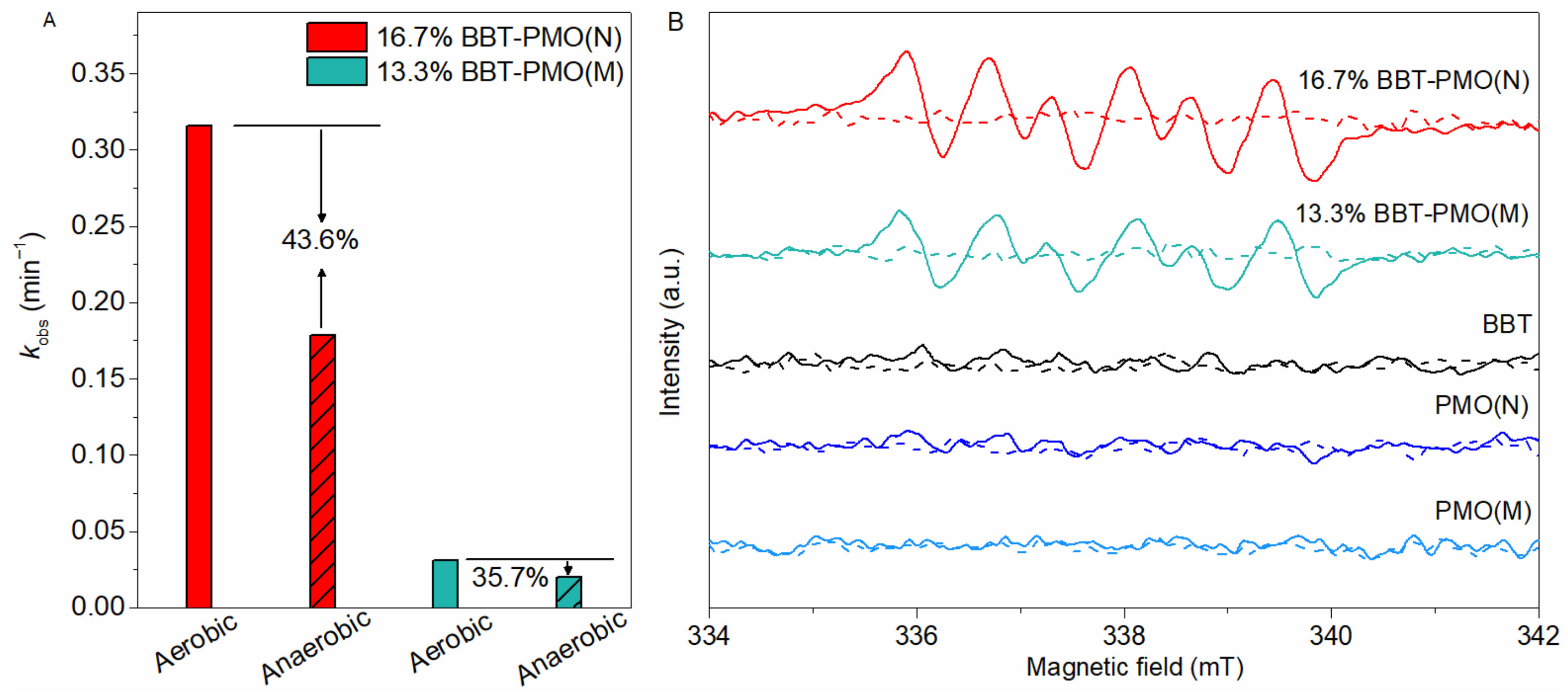
2.3. Mechanism of Photoreduction of Cr(VI) over Material
3. Materials and Methods
3.1. Synthesis of Lead Molybdate
3.2. Synthesis of BBT-PMO
3.3. Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hill, M.K. Understanding Environmental Pollution; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Salomons, W.; Förstner, U.; Mader, P. Heavy Metals: Problems and Solutions; Springer Science & Business Media: Heidelberg/Berlin, Germany, 2012. [Google Scholar]
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.R.; Vilar, V.J.P. As(III) and Cr(VI) oxyanion removal from water by advanced oxida-tion/reduction processes—A review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Hou, H.-J.; Zhang, X.-H.; Huang, D.-K.; Ding, X.; Wang, S.-Y.; Yang, X.-L.; Li, S.-Q.; Xiang, Y.-G.; Chen, H. Conjugated microporous poly(benzothiadiazole)/TiO2 heterojunction for visible-light-driven H2 production and pollutant removal. Appl. Catal. B Environ. 2017, 203, 563–571. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of cds-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photo-catalytic activity. Appl. Catal. B Environ. 2013, 142, 1–7. [Google Scholar]
- Li, K.; Peng, B.; Peng, T. Recent advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen vacancy-mediated photocatalysis of BiOCl: Reactivity, selectivity, and perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Zhan, G.; Zhang, L. Solar water splitting and Nitrogen Fixation with Layered Bismuth Oxyhalides. Accounts Chem. Res. 2017, 50, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wang, X.; Zhang, X.; Hou, H.; Dai, K.; Huang, Q.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors: A structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2018, 6, 153–159. [Google Scholar] [CrossRef]
- Yi, X.-H.; Wang, F.-X.; Du, X.-D.; Wang, P.; Wang, C.-C. Facile fabrication of BUC-21/g-C3N4 composites and their enhanced photocatalytic Cr(VI) reduction performances under simulated sunlight. Appl. Organomet. Chem. 2019, 33, e4621. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Hou, H.; Ding, X.; Li, S.; Deng, F.; Xiang, Y.; Chen, H. Highly efficient visible light induced photocatalytic activity of a novel in situ synthesized conjugated microporous poly(benzothiadiazole)-C3N4 composite. Catal. Sci. Technol. 2017, 7, 418–426. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 1–9. [Google Scholar]
- Wang, X.; Sun, M.; Murugananthan, M.; Zhang, Y.; Zhang, L. Electrochemically self-doped WO3/TiO2 nanotubes for photocatalytic degradation of volatile organic compounds. Appl. Catal. B Environ. 2020, 260, 118205. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Sun, P.; Zhang, L. ZnO/BiOI Heterostructures: Photoinduced charge-transfer property and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2011, 115, 20555–20564. [Google Scholar] [CrossRef]
- Li, K.; Chai, B.; Peng, T.; Mao, J.; Zan, L. Preparation of AgIn5S8/TiO2 Heterojunction Nanocomposite and Its Enhanced Photocatalytic H2 Production Property under Visible Light. ACS Catal. 2013, 3, 170–177. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Cheng, B. S-Scheme Heterojunction Photocatalyst. Chemistry 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, Z.; Heil, T.; Meng, A.; Cheng, B.; Cao, S.; Cheng, B.; Antonietti, M. Highly selective CO2 capture and its direct photochemical conversion on ordered 2D/1D Heterojunctions. Joule 2019, 3, 2792–2805. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Cheng, B.; Zhang, Y. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Rao, L.; Wang, P.; Huang, D.; Ding, X.; Zhang, X.; Wang, S.; Chen, H.; Zhu, Y. Conjugated Polymers with Sequential Fluorination for Enhanced Photocatalytic H2 Evolution via Proton-Coupled Electron Transfer. ACS Energy Lett. 2018, 3, 2544–2549. [Google Scholar] [CrossRef]
- Wang, S.; Hai, X.; Ding, X.; Jin, S.; Xiang, Y.; Wang, P.; Jiang, B.; Ichihara, F.; Oshikiri, M.; Meng, X.; et al. Intermolecular cascaded π-conjugation channels for electron delivery powering CO2 photoreduction. Nat. Commun. 2020, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, Y.; Qu, Y.; Ding, X.; Chen, H. Novel in situ fabrication of conjugated microporous poly(benzothiadiazole)–Bi2MoO6 Z-scheme heterojunction with enhanced visible light photocatalytic activity. J. Catal. 2017, 345, 319–328. [Google Scholar] [CrossRef]
- Kwolek, P.; Pilarczyk, K.; Tokarski, T.; Łapczyńska, M.; Pacia, M.; Szaciłowski, K. Lead molybdate—A promising material for optoelectronics and photocatalysis. J. Mater. Chem. C 2015, 3, 2614–2623. [Google Scholar] [CrossRef]
- Dai, K.; Yao, Y.; Liu, H.; Mohamed, I.; Chen, H.; Huang, Q. Enhancing the photocatalytic activity of lead molybdate by modifying with fullerene. J. Mol. Catal. A. Chem. 2013, 374–375, 111–117. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Bhatnagar, A.; Ehrampoush, M.H.; Amrollahi, M.; Jamshidi, B.; Dehvari, M.; Taghavi, M. Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: Kinetic modeling studies. Int. J. Environ. Sci. Technol. 2017, 14, 331–340. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, M.A.; Arévalo-Niño, K.; Quintero-Zapata, I.; Castro-González, I.; Almaguer-Cantú, V. Cr(VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J. Environ. Manag. 2019, 251, 109595. [Google Scholar] [CrossRef]
- Xia, J.; Jin, C.; Pan, Z.; Sun, L.; Ota, T.; Jin, Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total. Environ. 2018, 439–448. [Google Scholar] [CrossRef]
- Álvarez-González, C.A.; Martínez-Sánchez, L.; Peña-Marín, E.S.; Guerrero-Zárate, R.; Jesús-Ramírez, F.; Morales-García, V.; Uribe-López, M.; Núñez-Nogueira, G. Effects on the Growth and Digestive Enzyme Activity in Nile Tilapia Fry (Oreochromis niloticus) by Lead Exposure. Water Air Soil Pollut. 2020, 231, 1–15. [Google Scholar] [CrossRef]
- Chang, H.; Yi, H.; Ke, Q.; Zhang, J. Preparation of a AgCl/PbMoO4 Composite and Investigation of Its Photocatalytic Oxidative Desulfurization Performance. ACS Omega 2020, 5, 10927–10938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Z.; Li, X.; Yi, X.; Chu, H.; Chen, X.; Wang, C.-C. Facile fabrication of BUC-21/Bi24O31Br10 composites for enhanced photocatalytic Cr(VI) reduction under white light. Chem. Eng. J. 2020, 389, 123431. [Google Scholar] [CrossRef]
- Rudel, H.; Terytze, K. Determination of extractable chromium(vi) in soils using a photometric method. Chemosphere 1999, 39, 697–708. [Google Scholar] [CrossRef]






| s = M | s = N | |
|---|---|---|
| PMO(s) | 0.002 | 0.006 |
| BBT | 0.012 | 0.012 0.010 * |
| 6.7% BBT-PMO(s) | 0.011 | 0.03 |
| 10.0% BBT-PMO(s) | 0.012 | 0.183 |
| 13.3% BBT-PMO(s) | 0.031 | 0.15 |
| 16.7% BBT-PMO(s) | 0.02 | 0.316 |
| 20.0% BBT-PMO(s) | 0.015 | 0.041 |
| x% BBT+PMO(s) | 0.008 (x = 13.3) | 0.006 (x = 16.7) |
| BBT | PMO(M) | 13.3% BBT-PMO(M) | PMO(N) | 16.7% BBT-PMO(N) | |
|---|---|---|---|---|---|
| S | 18.69 | 2.29 | 6.17 | 8.65 | 77.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Wang, Y.; Xu, X.; Xiang, Y.; Yang, Z.; Wang, P. Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light. Catalysts 2021, 11, 106. https://doi.org/10.3390/catal11010106
Liu D, Wang Y, Xu X, Xiang Y, Yang Z, Wang P. Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light. Catalysts. 2021; 11(1):106. https://doi.org/10.3390/catal11010106
Chicago/Turabian StyleLiu, Ding, Yin Wang, Xiao Xu, Yonggang Xiang, Zixin Yang, and Pei Wang. 2021. "Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light" Catalysts 11, no. 1: 106. https://doi.org/10.3390/catal11010106
APA StyleLiu, D., Wang, Y., Xu, X., Xiang, Y., Yang, Z., & Wang, P. (2021). Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light. Catalysts, 11(1), 106. https://doi.org/10.3390/catal11010106





