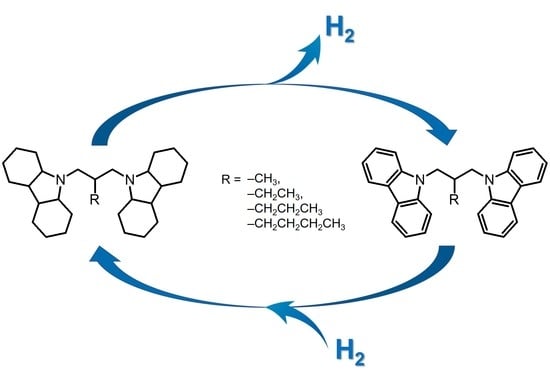
Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
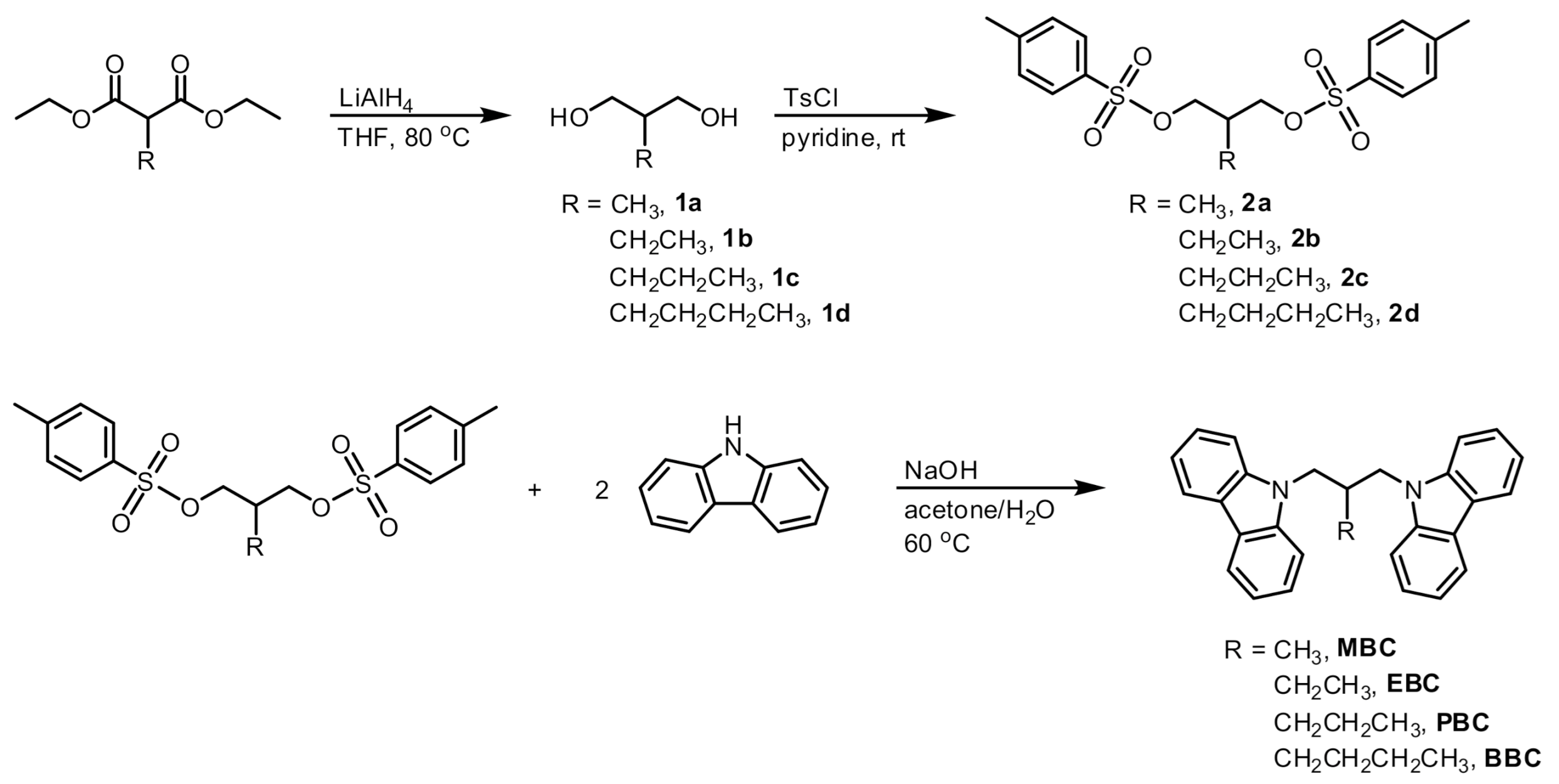
2.2. Synthesis
2.3. TGA and DSC Measurements
2.4. Catalytic Hydrogenation Reaction
2.5. Catalytic Dehydrogenation Reaction
3. Results and Discussion
3.1. Synthesis
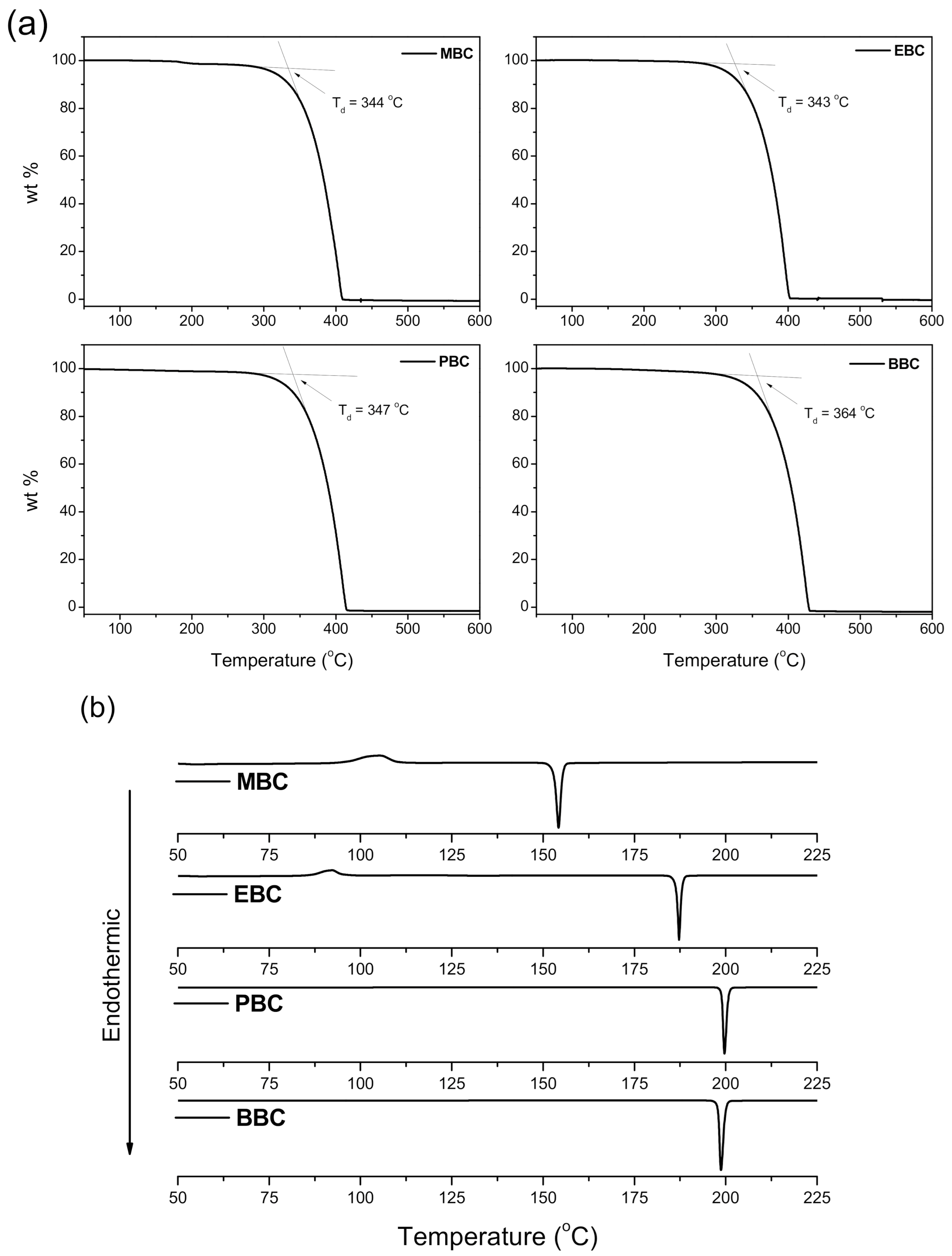
3.2. Thermal Properties
3.3. Catalytic Hydrogenation Reactions
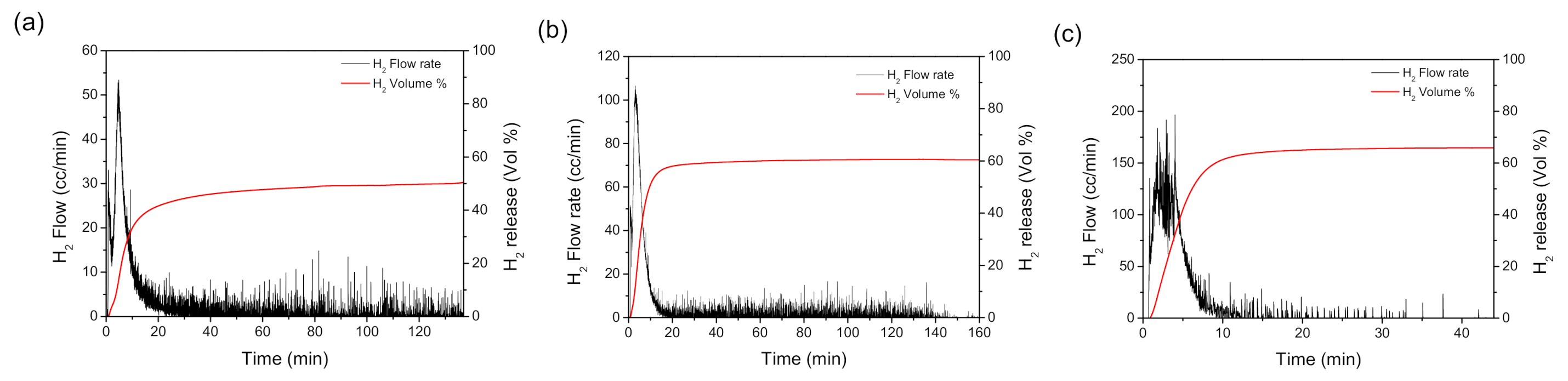
3.4. Catalytic Dehydrogenation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, H.; Guo, F.; Luo, M.; Yao, Q.; Lu, Z.-H. La(OH)3-decorated NiFe nanoparticles as efficient catalyst for hydrogen evolution from hydrous hydrazine and hydrazine borane. Int. J. Hydrog. Energy 2020, 45, 11641–11650. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrog. Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Wan, C.; Cheng, D.-G.; Chen, F.; Zhan, X. Fabrication of CeO2 nanotube supported Pt catalyst encapsulated with silica for high and stable performance. Chem. Commun. 2015, 51, 9785–9788. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, M.; Lin, H.; Wei, K.; Guo, X.; Yu, C.; Yom, T.; Xi, Z.; Yin, Z.; Sun, S. Efficient Hydrogen Generation from Ammonia Borane and Tandem Hydrogenation or Hydrodehalogenation over AuPd Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 2814–2821. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Xu, P.; Liu, P.; Zhao, Y.; Yang, J. Development of high pressure gaseous hydrogen storage technologies. Int. J. Hydrog. Energy 2012, 37, 1048–1057. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Mehrpooya, M. Introducing and energy analysis of a novel cryogenic hydrogen liquefaction process configuration. Int. J. Hydrog. Energy 2017, 42, 6033–6050. [Google Scholar] [CrossRef]
- Gleichweit, C.; Amende, M.; Bauer, U.; Schernich, S.; Höfert, O.; Lorenz, M.P.A.; Zhao, W.; Müller, M.; Koch, M.; Bachmann, P.; et al. Alkyl chain length-dependent surface reaction of dodecahydro-N-alkylcarbazoles on Pt model catalysts. J. Chem. Phys. 2014, 140, 204711. [Google Scholar] [CrossRef] [Green Version]
- Makowski, P.; Thomas, A.; Kuhn, P.; Goettmann, F. Organic materials for hydrogenstorage applications: From physisorption on organic solids to chemisorption in organic molecules. Energy Environ. Sci. 2009, 2, 480–490. [Google Scholar] [CrossRef]
- Cacciola, G.; Giordano, N.; Restuccia, G. Cyclohexane as a liquid phase carrier in hydrogen storage and transport. Int. J. Hydrog. Energy 1984, 9, 411–419. [Google Scholar] [CrossRef]
- Maria, G.; Marin, A.; Wyss, C.; Muller, S.; Newson, E. Modelling and scaleup of the kinetics with deactivation of methylcyclohexane dehydrogenation for hydrogen energy storage. Chem. Eng. Sci. 1996, 51, 2891–2896. [Google Scholar] [CrossRef]
- Pradhan, A.U.; Shukla, A.; Pande, J.V.; Karmarkar, S.; Biniwale, R.B. A feasibility analysis of hydrogen delivery system using liquid organic hydrides. Int. J. Hydrog. Energy 2011, 36, 680–688. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrog. Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Cresswell, D.; Garforth, A. Hydrogen storage in liquid organic hydride: Producing hydrogen catalytically from methylcyclohexane. Energy Fuels 2011, 25, 4217–4234. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hydrogen storage in liquid organic heterocycles. Energy Environ. Sci. 2008, 1, 134–138. [Google Scholar] [CrossRef]
- Clot, E.; Eisenstein, O.; Crabtree, R.H. Computational structure—Activity relationships in H2 storage: How placement of N atoms affects release temperatures in organic liquid storage materials. Chem. Commun. 2007, 22, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Eblagon, K.M.; Tam, K.; Yu, K.M.K.; Tsang, S.C.E. Comparative study of catalytic hydrogenation of 9-ethylcarbazole for hydrogen storage over noble metal surfaces. J. Phys. Chem. C 2012, 116, 7421–7429. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tam, K.; Tsang, S.C.E. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications. Energy Environ. Sci. 2012, 5, 8621–8630. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tam, K.; Yu, K.M.K.; Zhao, S.-L.; Gong, X.-Q.; He, H.; Ye, L.; Wang, L.-C.; Ramirez-Cuesta, A.J.; Tsang, S.C. Study of catalytic sites on ruthenium for hydrogenation of N-ethylcarbazole: Implications of hydrogen storage via reversible catalytic hydrogenation. J. Phys. Chem. C 2010, 114, 9720–9730. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Rentsch, D.; Friedrichs, O.; Remhof, A.; Zuettel, A.; Ramirez-Cuesta, A.J.; Tsang, S.C. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier. Int. J. Hydrog. Energy 2010, 35, 11609–11621. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Analysis of H2 release from organic polycyclics over Pd catalysts using DFT. J. Phys. Chem. C 2013, 117, 194–204. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage. Appl. Catal. A Gen. 2012, 419, 67–72. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 2715–2722. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts. J. Catal. 2011, 279, 36–47. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Kinetics of hydrogen uptake and release from heteroaromatic compounds for hydrogen storage. Ind. Eng. Chem. Res. 2010, 49, 1018–1026. [Google Scholar] [CrossRef]
- Wang, Z.; Tonks, I.; Belli, J.; Jensen, C.M. Dehydrogenation of N-ethyl perhydrocarbazole catalyzed by PCP pincer iridium complexes: Evaluation of a homogenous hydrogen storage system. J. Organomet. Chem. 2009, 694, 2854–2857. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Zhao, L.; Smith, K.J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst. Appl. Catal. A Gen. 2009, 362, 155–162. [Google Scholar] [CrossRef]
- Preuster, P.; Fang, Q.; Peters, R.; Deja, R.; Nguyen, V.N.; Blum, L.; Stolten, D.; Wasserscheid, P. Solid oxide fuel cell operating on liquid organic hydrogen carrier-based hydrogen e making full use of heat integration potentials. Int. J. Hydrog. Energy 2018, 43, 1758–1768. [Google Scholar] [CrossRef]
- Müller, K.; Thiele, S.; Wasserscheid, P. Evaluations of concepts for the integration of fuel cells in liquid organic hydrogen carrier systems. Energy Fuels 2019, 33, 10324–10330. [Google Scholar] [CrossRef]
- Heublein, N.; Stelzner, M.; Sattelmayer, T. Hydrogen storage using liquid organic carriers: Equilibrium simulation and dehydrogenation reactor design. Int. J. Hydrog. Energy 2020, 45, 24902–24916. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.-J.; Zhang, J.-L.; Tao, Y.; Chen, R.-F. Synthesis and characterization of heteroatom substituted carbazole derivatives: Potential host materials for phosphorescent organic light-emitting diodes. New J. Chem. 2013, 37, 977–985. [Google Scholar] [CrossRef]
- Ohta, H.; Tetsukawa, H.; Noto, N. Enantiotopically selective oxidation of α,ω-diols with enzyme systems of microorganisms. J. Org. Chem. 1982, 47, 2400–2404. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, S.; Cheng, H. A comparative study of catalytic dehydrogenationof perhydro-N-ethylcarbazole over noble metalcatalys. Int. J. Hydrog. Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]

| MBC | EBC | PBC | BBC | |
|---|---|---|---|---|
| Structure of H2-lean form | 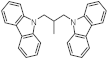 |  | 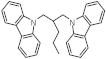 | 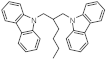 |
| Structure of H2-rich form |  | 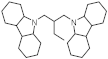 |  | 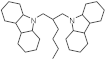 |
| Melting points of H2-lean form (°C) | 154 | 187 | 200 | 198 |
| Decomposition temperature of H2-lean form (°C) | 344 | 343 | 347 | 364 |
| H2 capacity (wt %) | 5.86 | 5.67 | 5.49 | 5.31 |
| Catalyst | Reaction Condition | Feed Catalyst (g)/LOHC (g) | Ideal wt % | Experimental wt % | |
|---|---|---|---|---|---|
| MBC | Ru/Al2O3 | 190 °C/24 h | 0.30/3.00 | 5.86 wt % | 5.92 ± 0.24 wt % |
| 0.187 g | 0.189 ± 0.008 g | ||||
| EBC | Ru/Al2O3 | 190 °C/24 h | 0.30/3.01 | 5.67 wt % | 5.81 ± 0.24 wt % |
| 0.181 g | 0.185 ± 0.008 g | ||||
| PBC | Ru/Al2O3 | 190 °C/24 h | 0.30/3.01 | 5.49 wt % | 5.34 ± 0.24 wt % |
| 0.174 g | 0.170 ± 0.008 g | ||||
| BBC | Ru/Al2O3 | 190 °C/24 h | 0.42/4.25 | 5.31 wt % | 5.61 ± 0.24 wt % |
| 0.254 g | 0.269 ± 0.008 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Shin, B.S.; Kang, J.W.; Han, W.-S. Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials. Catalysts 2021, 11, 123. https://doi.org/10.3390/catal11010123
Jung J, Shin BS, Kang JW, Han W-S. Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials. Catalysts. 2021; 11(1):123. https://doi.org/10.3390/catal11010123
Chicago/Turabian StyleJung, Joori, Byeong Soo Shin, Jeong Won Kang, and Won-Sik Han. 2021. "Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials" Catalysts 11, no. 1: 123. https://doi.org/10.3390/catal11010123
APA StyleJung, J., Shin, B. S., Kang, J. W., & Han, W. -S. (2021). Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials. Catalysts, 11(1), 123. https://doi.org/10.3390/catal11010123