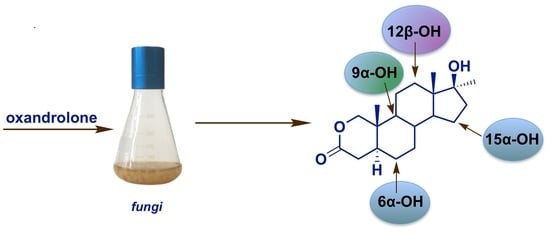
Highly Regioselective and Stereoselective Biohydroxylations of Oxandrolone
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Substrate and Microorganisms
3.2. Conditions of Cultivation and Transformation
3.3. Isolation and Identification of Products
3.4. Transformations and Spectroscopic Data of the Isolated Metabolites
3.4.1. Transformation with Fusarium culmorum
3.4.2. Transformation with Mortierella isabellina (Umbelopsis isabellina)
3.4.3. Transformation with Laetiporus sulphureus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012, 129, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Kołek, T.; Panek, A.; Milecka, N. Selective modifications of steroids performed by oxidative enzymes. Curr. Org. Chem. 2012, 16, 2551–2582. [Google Scholar] [CrossRef]
- Sedlaczek, L.; Smith, L.L. Biotransformations of steroids. Crit. Rev. Biotechnol. 1988, 7, 187–236. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.A. Steroids, the steroid community, and Upjohn in perspective: A profile of innovation. Steroids 1992, 57, 593–616. [Google Scholar] [CrossRef]
- Shull, G.M.; Kita, D.A. Microbiological conversion of steroids. I. Introduction of the 11β-hydroxyl group into C21 steroids. J. Am. Chem. Soc. 1955, 77, 763–764. [Google Scholar] [CrossRef]
- Świzdor, A.; Panek, A.; Milecka-Tronina, N. Hydroxylative activity of Aspergillus niger towards androst-4-ene and androst-5-ene steroids. Steroids 2017, 126, 101–106. [Google Scholar] [CrossRef]
- Mishra, R.; Mishra, S. Updates in bile acid-bioactive molecule conjugates and their applications. Steroids 2020, 159, 108639. [Google Scholar] [CrossRef]
- El Kihel, L. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)—Recent reports. Steroids 2012, 77, 10–26. [Google Scholar] [CrossRef]
- Kołek, T.; Milecka, N.; Świzdor, A.; Panek, A.; Białońska, A. Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellina AM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates. Org. Biomol. Chem. 2011, 9, 5414–5422. [Google Scholar] [CrossRef]
- Milecka-Tronina, N.; Kołek, T.; Świzdor, A.; Panek, A. Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids? Bioorg. Med. Chem. 2014, 22, 883–891. [Google Scholar] [CrossRef]
- Meyer, W.; Gams, W. Delimination of Umbelopsis (Mucorales, Umbelopsidaceae fam. nov.) based on ITS sequence and RFLP data. Mycol. Res. 2003, 107, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kołek, T.; Świzdor, A. Biotransformation XLV. Transformations of 4-ene-3-oxo steroids in Fusarium culmorum culture. J. Steroid Biochem. Mol. Biol. 1998, 67, 63–69. [Google Scholar] [CrossRef]
- Kołek, T. Biotransformation XLVII: Transformations of 5-ene steroids in Fusarium culmorum culture. J. Steroid Biochem. Mol. Biol. 1999, 71, 83–90. [Google Scholar] [CrossRef]
- Świzdor, A.; Kołek, T. Transformations of 4- and 17α-substituted testosterone analogues by Fusarium Culmorum. Steroids 2005, 70, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Kołek, T.; Szpineter, A. Transformations of steroid esters by Fusarium culmorum. Z. Nat. C 2006, 61, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Pappo, R.; Jung, C.J. 2-Oxasteroids: A new class of biologically active compounds. Tetrahedron Lett. 1962, 3, 365–371. [Google Scholar] [CrossRef]
- Orr, R.; Singh, M.F. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: Review of efficacy and safety. Drugs 2004, 64, 725–750. [Google Scholar] [CrossRef]
- Kratena, N.; Stöger, B.; Weil, M.; Enev, V.S.; Gärtner, P. Synthesis of two epimeric long-term metabolites of oxandrolone. Tetrahedron Lett. 2017, 58, 1316–1318. [Google Scholar] [CrossRef]
- Foster, M.A.; Taylor, A.E.; Hill, N.E.; Bentley, C.; Bishop, J.; Gilligan, L.C.; Shaheen, F.; Bion, J.F.; Fallowfield, J.L.; Woods, D.R.; et al. Mapping the steroid response to major trauma from injury to recovery: A prospective cohort study. J. Clin. Endocrinol. Metab. 2020, 105, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Ring, J.; Heinelt, M.; Sharma, S.; Letourneau, S.; Jeschke, M.G. Oxandrolone in the treatment of burn injuries: A systematic review and meta-analysis. J. Burn. Care Res. 2020, 41, 190–199. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Mohammad, M.Y.; Musharraf, S.G.; Parvez, M.; Al-Aboudi, A.; Atta-ur-Rahman. New oxandrolone derivatives by biotransformation using Rhizopus stolonifer. Steroids 2009, 74, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Guddat, S.; Fußhöller, G.; Beuck, S.; Thomas, A.; Geyer, H.; Rydevik, A.; Bondesson, U.; Hedeland, M.; Lagojda, A.; Schänzer, W.; et al. Synthesis, characterization, and detection of new oxandrolone metabolites as long-term markers in sports drug testing. Anal. Bioanal. Chem. 2013, 405, 8285–8294. [Google Scholar] [CrossRef] [PubMed]
- Rzeppa, S.; Viet, L. Analysis of sulfate metabolites of the doping agents oxandrolone and danazol using high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2016, 1029, 1–9. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency Prohibited List. 2020. Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_prohibited_list_0.pdf (accessed on 11 November 2020).
- Massé, R.; Bi, H.; Ayotte, C.; Dugal, R. Studies on anabolic steroids II—gas chromatographic/mass spectrometric characterization of oxandrolone urinary metabolites in man. Biomed. Environ. Mass Spectrom. 1989, 18, 429–438. [Google Scholar] [CrossRef]
- Desoky, E.-S.I.; Reyad, M.; Afsah, E.M.; Dawidar, A.-A.M. Synthesis and chemical reactions of the steroidal hormone 17α-methyltestosterone. Steroids 2016, 105, 68–95. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Popłoński, J.; Kozłowska, E.; Dymarska, M.; Huszcza, E.; Kostrzewa-Susłow, E. Application of α- and β-naphthoflavones as monooxygenase inhibitors of Absidia coerulea KCh 93, Syncephalastrum racemosum KCh 105 and Chaetomium sp. KCh 6651 in transformation of 17α-methyltestosterone. Bioorg. Chem. 2018, 78, 178–184. [Google Scholar] [CrossRef]
- Smith, C.; Atia-Tul-Wahab; Khan, M.S.A.; Ahmad, M.S.; Farran, D.; Choudhary, M.I.; Baydoun, E. Microbial transformation of oxandrolone with Macrophomina phaseolina and Cunninghamella blakesleeana. Steroids 2015, 102, 39–45. [Google Scholar] [CrossRef]
- Świzdor, A.; Milecka, N.; Kołek, T.; Panek, A. Process for the Preparation of 3β,15α-dihydroxy-5α-androst-17-one. Polish Patent PL 215785 B1, 31 January 2014. [Google Scholar]




| Strain | Compounds Present in the Mixture | Rt (min.) | Time of Transformation (Days) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 10 | |||
| (%) a | |||||||
| Fusarium culmorum AM282 | Oxandrolone (1) | 7.14 | 84 | 55 | 26 | 22 | - |
| 12β-Hydroxyoxandrolone (2) | 10.06 | 16 | 38 | 66 | 69 | - | |
| Mortierella isabellina AM212 | Oxandrolone (1) | 7.14 | 90 | 40 | 19 | - | - |
| 9α-Hydroxyoxandrolone (3) | 9.15 | 10 | 48 | 69 | 80 | 78 | |
| Laetiporus sulphureus AM498 | Oxandrolone (1) | 7.14 | 93 | 82 | 48 | 23 | - |
| 6α-Hydroxyoxandrolone (4) | 10.24 | - | - | 8 | 12 | 18 | |
| 15α-Hydroxyoxandrolone (5) | 9.83 | 7 | 15 | 39 | 61 | 76 | |
| Position | δC, | Type | δH, Multiplicity (J in Hz) | NOESY (H → H) a |
|---|---|---|---|---|
| 1α | 80.9, | CH2 | 3.93, d (10.7) | 5, 9 |
| 1β | 4.22, d (10.7) | 11, 19 | ||
| 3 | 170.4, | C | - | - |
| 4α | 33.7, | CH2 | 2.53, dd (5.9; 18.8) | 6 |
| 4β | 2.22, dd (13.0; 18.8) | 6, 8, 19 | ||
| 5 | 40.2, | CH | 1.72, m | 1, 7, 9 |
| 6α | 27.0, | CH2 | 1.51, m | 4 |
| 6β | 1.21 b, m | 4, 8, 19, | ||
| 7α | 31.3, | CH2 | 1.14, m | 5, 9, 14 |
| 7β | 2.02, dm | 6, 8, 15 | ||
| 8 | 35.1, | CH | 1.68, m | 6, 11, 18, 19 |
| 9 | 49.5, | CH | 0.85, m | 1, 5, 7, 12, 14 |
| 10 | 34.7, | C | - | - |
| 11α | 20.8, | CH2 | 1.46 c, m | 9 |
| 11β | 1.46 c, dm | 8, 18, 19 | ||
| 12α | 31.2, | CH2 | 1.51, dm | 9, 14, 20 |
| 12β | 1.37, m | 11, 18 | ||
| 13 | 46.5, | C | - | - |
| 14 | 57.9, | CH | 1.24 b, m | 9, 12, 20 |
| 15 | 72.3, | CH | 4.07 (td, 3.4; 9.3) | 7, 8, 11, 16, 18 |
| 16α | 50.3, | CH2 | 1,64, dd (3.4; 14.6) | 14, 20 |
| 16β | 2.42, dd (9.7; 14.6) | 8, 15, 18 | ||
| 17 | 79.1, | C | - | - |
| 18 | 15.4, | CH3 | 0.87, s | 8, 11, 19 |
| 19 | 10.2, | CH3 | 1.02, s | 1, 4, 6, 8, 11 |
| 20 | 26.1, | CH3 | 1.35, s | 12, 14, 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyczko, P.; Panek, A.; Świzdor, A. Highly Regioselective and Stereoselective Biohydroxylations of Oxandrolone. Catalysts 2021, 11, 16. https://doi.org/10.3390/catal11010016
Łyczko P, Panek A, Świzdor A. Highly Regioselective and Stereoselective Biohydroxylations of Oxandrolone. Catalysts. 2021; 11(1):16. https://doi.org/10.3390/catal11010016
Chicago/Turabian StyleŁyczko, Paulina, Anna Panek, and Alina Świzdor. 2021. "Highly Regioselective and Stereoselective Biohydroxylations of Oxandrolone" Catalysts 11, no. 1: 16. https://doi.org/10.3390/catal11010016
APA StyleŁyczko, P., Panek, A., & Świzdor, A. (2021). Highly Regioselective and Stereoselective Biohydroxylations of Oxandrolone. Catalysts, 11(1), 16. https://doi.org/10.3390/catal11010016