In Situ TEM Study of Rh Particle Sintering for Three-Way Catalysts in High Temperatures
Abstract
1. Introduction
2. Results and Discussion
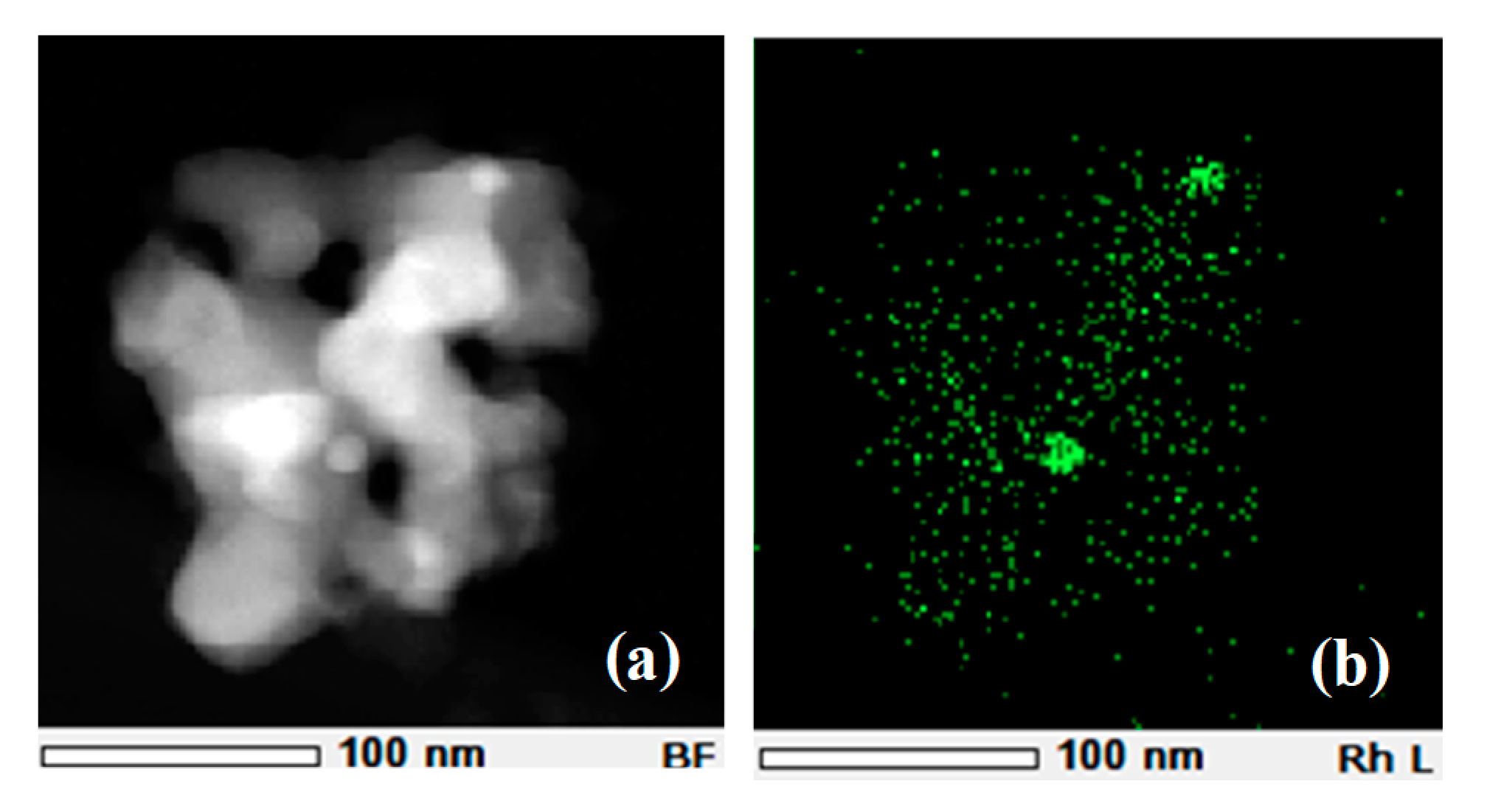
2.1. Postmortem Analysis of Engine-Aged Catalyst by Ex Situ TEM Observations
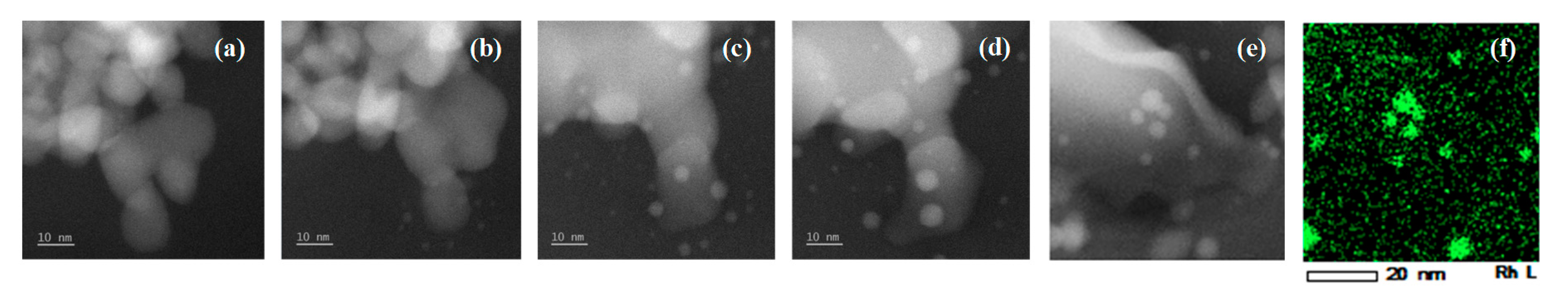
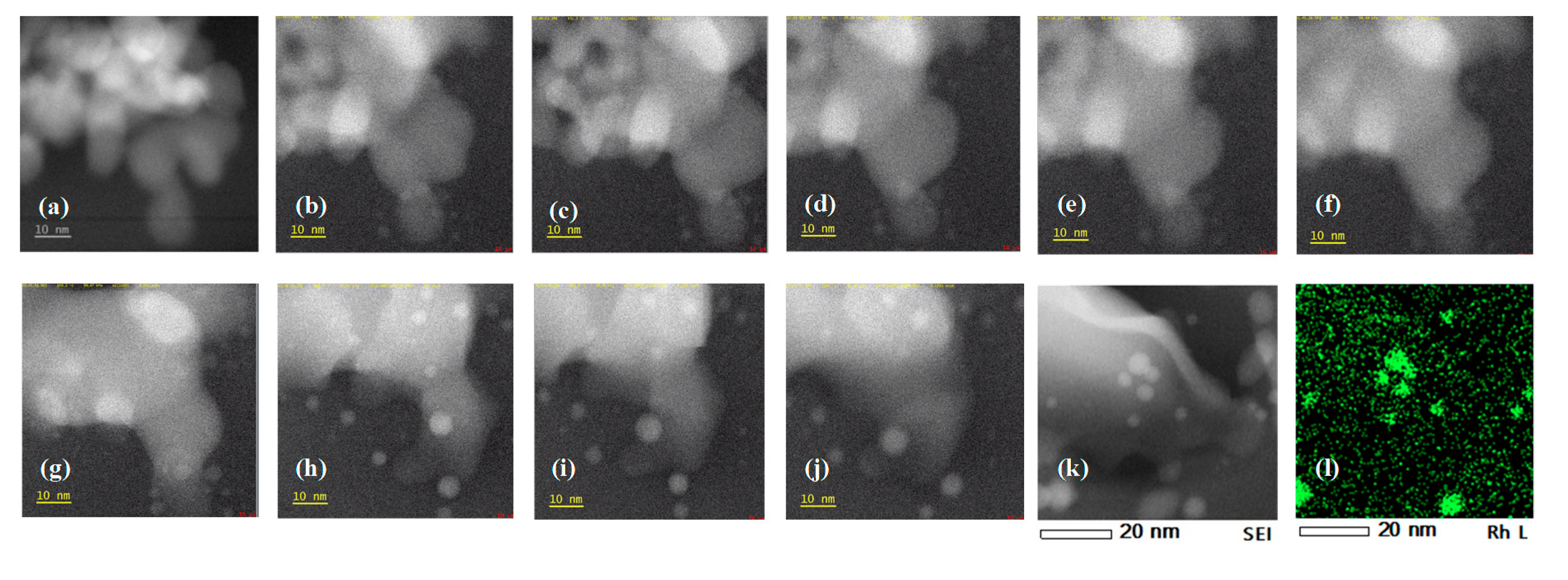
2.2. In Situ TEM Observations
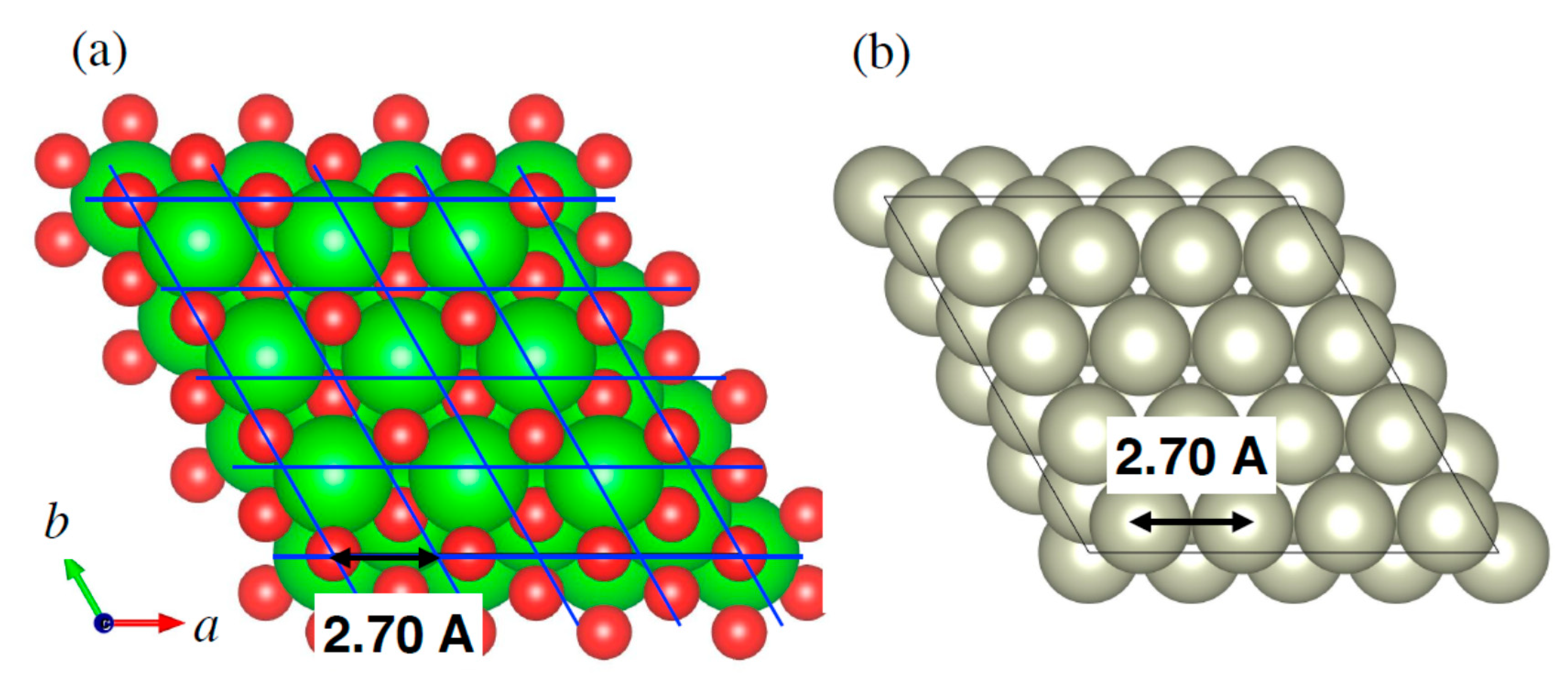
2.3. Effects of Rh–ZrO2 Interaction on the In Situ Sintering Behavior
2.4. In Situ TEM Observation in N2 and O2 Atmospheres
2.5. DFT Calculation Results
3. Experimental
3.1. Catalyst Preparation
3.2. In Situ and Ex Situ STEM Observations
3.3. DTA-TG Measurement
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Tech. Pap. 2020. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Tanabe, T.; Nagai, Y.; Dohmae, K.; Sobukawa, H.; Shinjoh, H. Sintering and redispersion behavior of Pt on Pt/MgO. J. Catal. 2008, 257, 117–124. [Google Scholar] [CrossRef]
- Wang, N.; Li, S.; Zong, Y.; Yao, Q. Sintering inhibition of flame-made Pd/CeO2 nano catalyst for low temperature methane combustion. J. Aerosol. Sci. 2017, 105, 64–72. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Qu, P.; Hu, W.; Shen, P.; Zhang, G.; Jiao, Y.; Zhong, L.; Chen, Y. Promotion of yttrium (Y) on the water resistance and hydrothermal stability of Pd/ZrO2 catalyst coated on the monolith for complete methane oxidation. J. Taiwan Inst. Chem. Eng. 2019, 103, 44–56. [Google Scholar] [CrossRef]
- Tanabe, T.; Morikawa, A.; Hatanaka, M.; Takahashi, N.; Nagai, Y.; Sato, A.; Kuno, O.; Suzuki, H.; Shinjoh, H. The interaction between supported Rh- and Nd2O3-enriched surface layer on ZrO2 for Rh sintering suppression. Catal. Today 2012, 184, 219–226. [Google Scholar] [CrossRef]
- Yati, I.; Ridwan, M.; Jeong, G.E.; Lee, Y.; Choi, J.W.; Yoon, C.W.; Suh, D.J.; Ha, J.M. Effects of sintering-resistance and large metal–support interface of alumina nanorod-stabilized Pt nanoparticle catalysts on the improved high temperature water gas shift reaction activity. Catal. Commun. 2014, 56, 11–16. [Google Scholar] [CrossRef]
- Morikawa, A.; Tanabe, T.; Hatanaka, M.; Takahashi, N.; Sato, A.; Kuno, O.; Suzuki, H.; Shinjoh, H. Inhibition of Rh sintering and improved reducibility of Rh on ZrO2 nanocomposite with an Al2O3diffusion barrier. Appl. Catal. A: Gen. 2015, 493, 33–39. [Google Scholar] [CrossRef]
- Suzuki, A.; Nakamura, K.; Sato, R.; Okushi, K.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Kubo, M.; Williams, M.C.; et al. Multi-scale theoretical study of support effect on sintering dynamics of Pt. Surf. Sci. 2009, 603, 3049–3056. [Google Scholar] [CrossRef]
- Koga, H.; Hayashi, A.; Ato, Y.; Tada, K.; Hosokawa, S.; Tanaka, T.; Okumura, M. Effect of ceria and zirconia supports on NO reduction over platinum-group metal catalysts: A DFT study with comparative experiments. Catal. Today 2019, 332, 236–244. [Google Scholar] [CrossRef]
- Gerceker, D.; Önal, I. A DFT study on CO oxidation on Pd4 and Rh4 clusters and adsorbed Pd and Rh atoms on CeO2 and Ce0.75Zr0.25O2supports for TWC applications. Appl. Surf. Sci. B 2013, 285, 927–936. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Ostwald ripening in a Pt/SiO2 model catalyst studied by in situ TEM. J. Catal. 2011, 281, 147–155. [Google Scholar] [CrossRef]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ Transmission Electron Microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef]
- Fujita, T.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Abe, H. In-Situ TEM Study of a Nanoporous Ni–Co Catalyst Used for the Dry Reforming of Methane. Metals 2017, 7, 406. [Google Scholar] [CrossRef]
- Hirata, H.; Kishita, K.; Nagai, Y.; Dohmae, K.; Shinjoh, H.; Matsumoto, S. Characterization and dynamic behavior of precious metals in automotive exhaust gas purification catalysts. Catal. Today 2011, 164, 467–473. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct Observations of Oxygen-induced Platinum Nanoparticle Ripening Studied by In Situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriako, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, S.; Katz, M.B.; Graham, G.W.; Pan, X. In Situ Observation of Rh-CaTiO3 Catalysts during Reduction and Oxidation Treatments by Transmission Electron Microscopy. ACS Catal. 2017, 7, 1579–1582. [Google Scholar] [CrossRef]
- Toya, Y.; Nakayama, H.; Hara, H.; Nagata, M. Optimization of TWC Design for Various Engine Operation Conditions. Sae Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Ye, F.; Xu, M.; Dai, S.; Tieu, P.; Ren, X.; Pan, X. In Situ TEM Studies of Catalysts Using Windowed Gas Cells. Catalysts 2020, 10, 779. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]













| 1 | 900 ℃ | 950 ℃ | 1000 ℃ | 1050 ℃ |
|---|---|---|---|---|
| Rh/ZrO2 | ZrO2 | Rh | ||
| Rh/Y-ZrO2 | Y-ZrO2 | Rh | ||
| Rh/CeO2 | CeO2 | Rh | ||
| Rh/ɤ-Al2O3 | Al2O3/Rh |
| Support | Junction Energy (eV) |
|---|---|
| ZrO2 | −0.39 |
| Y-ZrO2 | −1.06 |
| Y-ZrO2-δ | −0.67 |
| CeO2 | −0.52 |
| Material | Composition | BET Surface Area (m2/g) | Supplier |
|---|---|---|---|
| ZrO2 | 100% ZrO2 | 93.8 | DKKK (a) |
| Y-ZrO2 | 22.7mol% Y2O3-ZrO2 | 71.7 | DKKK |
| CeO2 | 100% CeO2 | 100.6 | DKKK |
| ɤ-Al2O3 | 100% Al2O3 | 141.0 | Sasol(Sandton, South Africa) |
| Conditions | STEM | EDS |
|---|---|---|
| Acceleration voltage (kV) | 200 | 200 |
| Irradiation current (pA/cm2) | 68 | 68 |
| Image size in pixels | 1024 × 1024 | 256 × 256 |
| Pixel Size (nm/pixel) | 0.064 | 0.26 |
| Dwell time | 10 μs | 0.2 ms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, H.; Nagata, M.; Abe, H.; Shimizu, Y. In Situ TEM Study of Rh Particle Sintering for Three-Way Catalysts in High Temperatures. Catalysts 2021, 11, 19. https://doi.org/10.3390/catal11010019
Nakayama H, Nagata M, Abe H, Shimizu Y. In Situ TEM Study of Rh Particle Sintering for Three-Way Catalysts in High Temperatures. Catalysts. 2021; 11(1):19. https://doi.org/10.3390/catal11010019
Chicago/Turabian StyleNakayama, Hiroki, Makoto Nagata, Hideki Abe, and Yukihiro Shimizu. 2021. "In Situ TEM Study of Rh Particle Sintering for Three-Way Catalysts in High Temperatures" Catalysts 11, no. 1: 19. https://doi.org/10.3390/catal11010019
APA StyleNakayama, H., Nagata, M., Abe, H., & Shimizu, Y. (2021). In Situ TEM Study of Rh Particle Sintering for Three-Way Catalysts in High Temperatures. Catalysts, 11(1), 19. https://doi.org/10.3390/catal11010019






