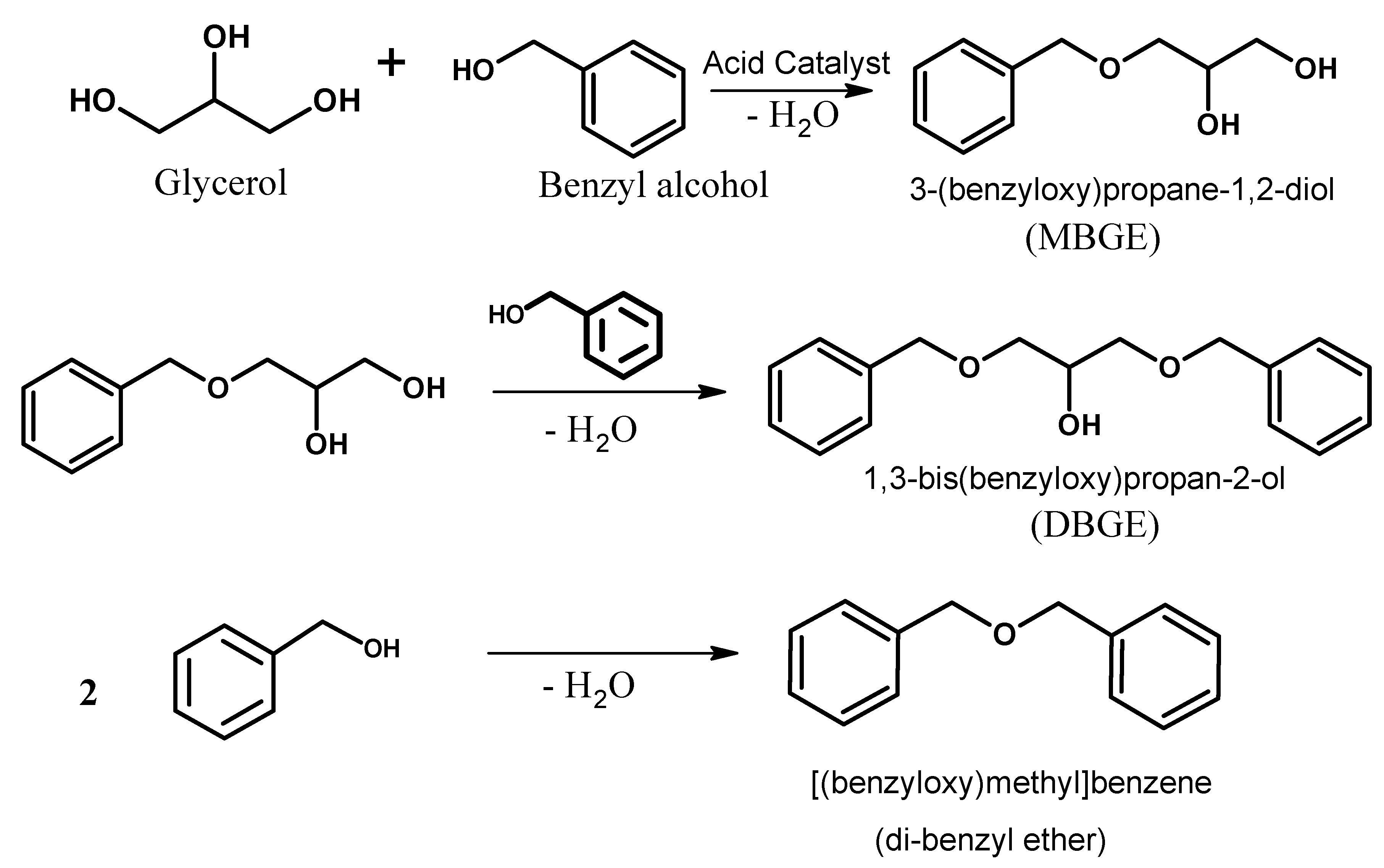
Solvent-Free Benzylation of Glycerol by Benzyl Alcohol Using Heteropoly Acid Impregnated on K-10 Clay as Catalyst
Abstract
:1. Introduction
2. Result and Discussion
2.1. Efficacy of Differrent Catalysts
2.2. Product Profile
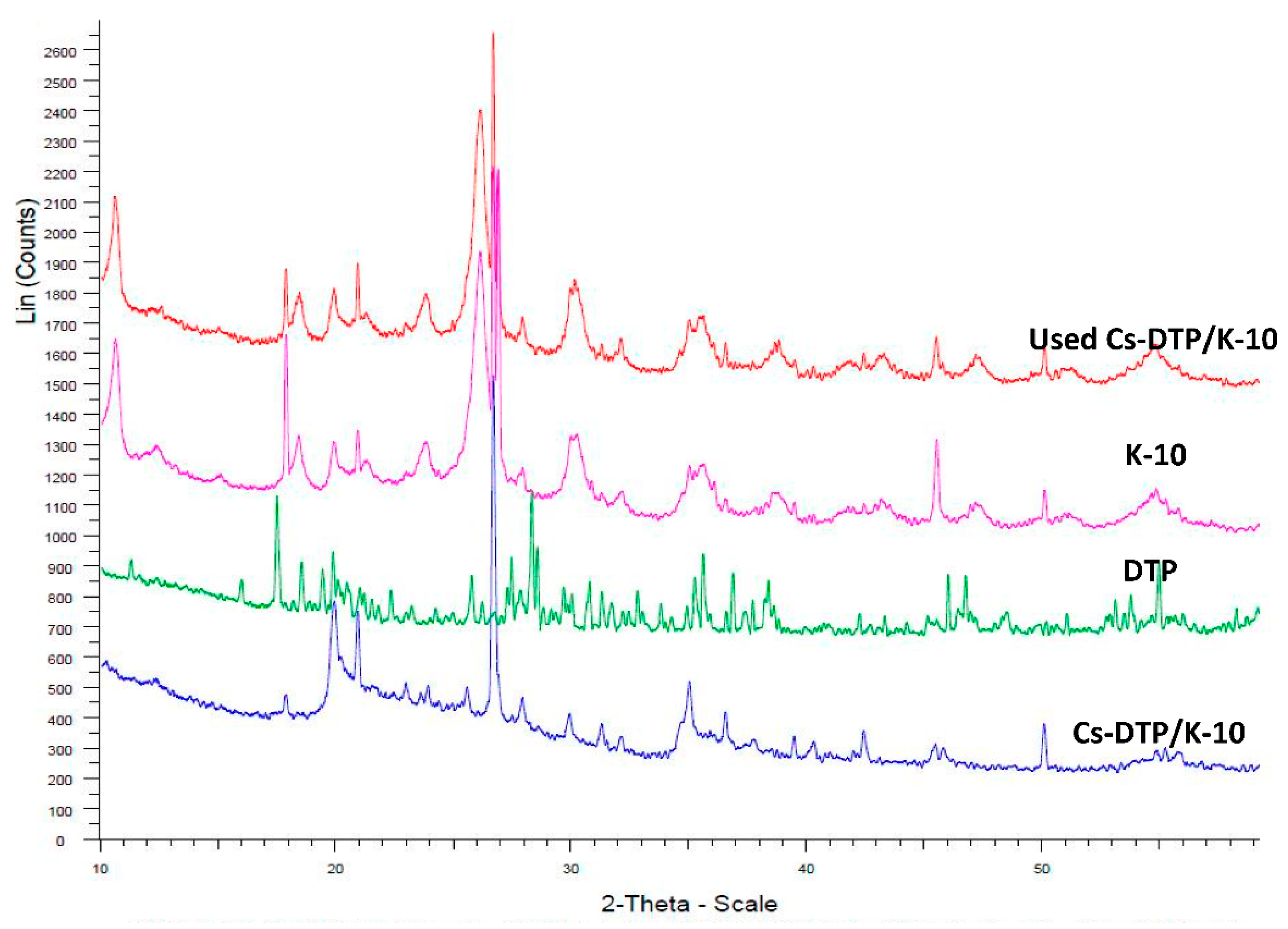
2.3. Catalyst Characterization
2.4. Parameter Optimization Study
2.4.1. Proof of Absence of External Mass Transfer Resistance
2.4.2. Effect of Catalyst Loading
2.4.3. Effect of Mole Ratio of Glycerol to Benzyl Alcohol
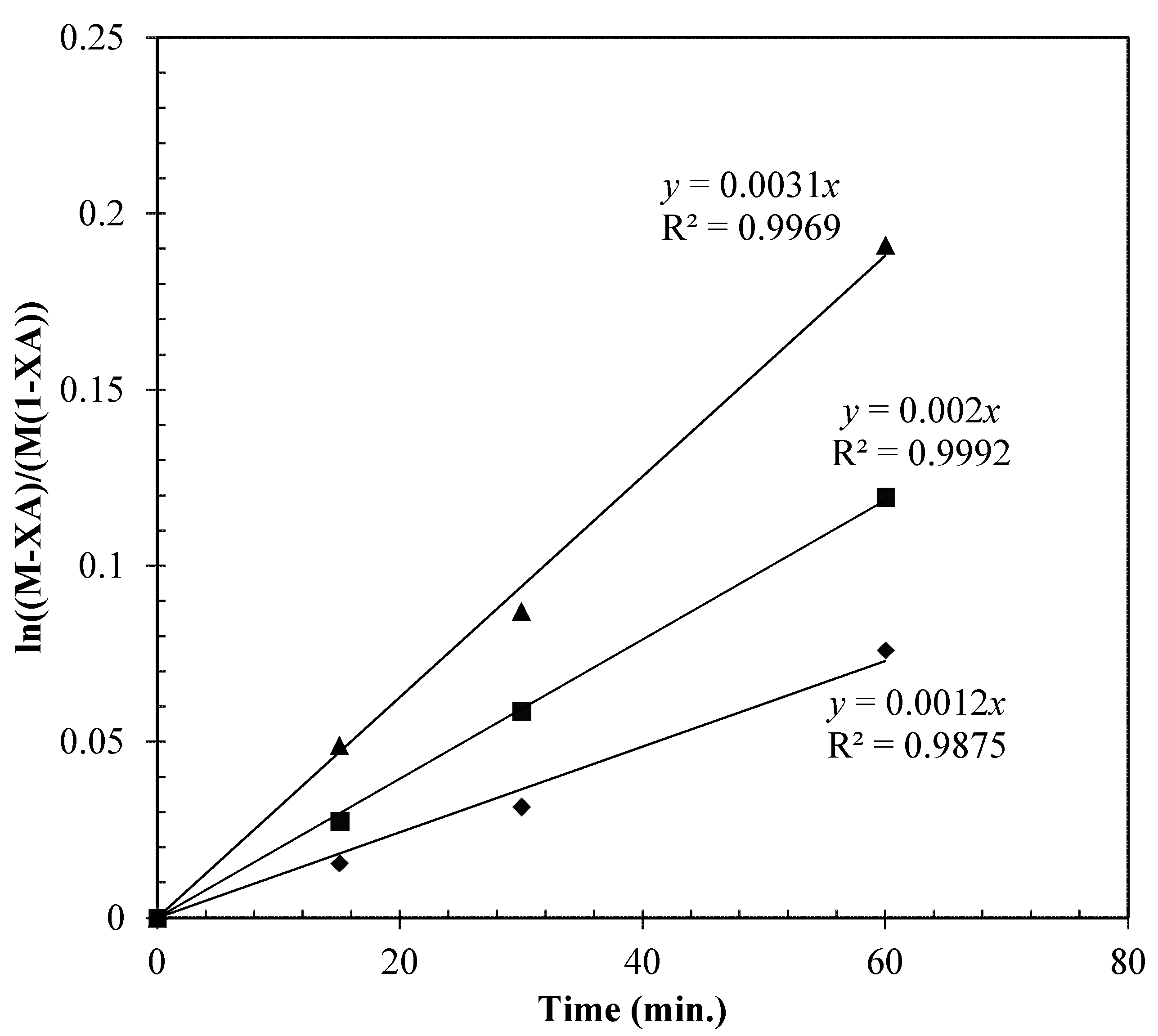
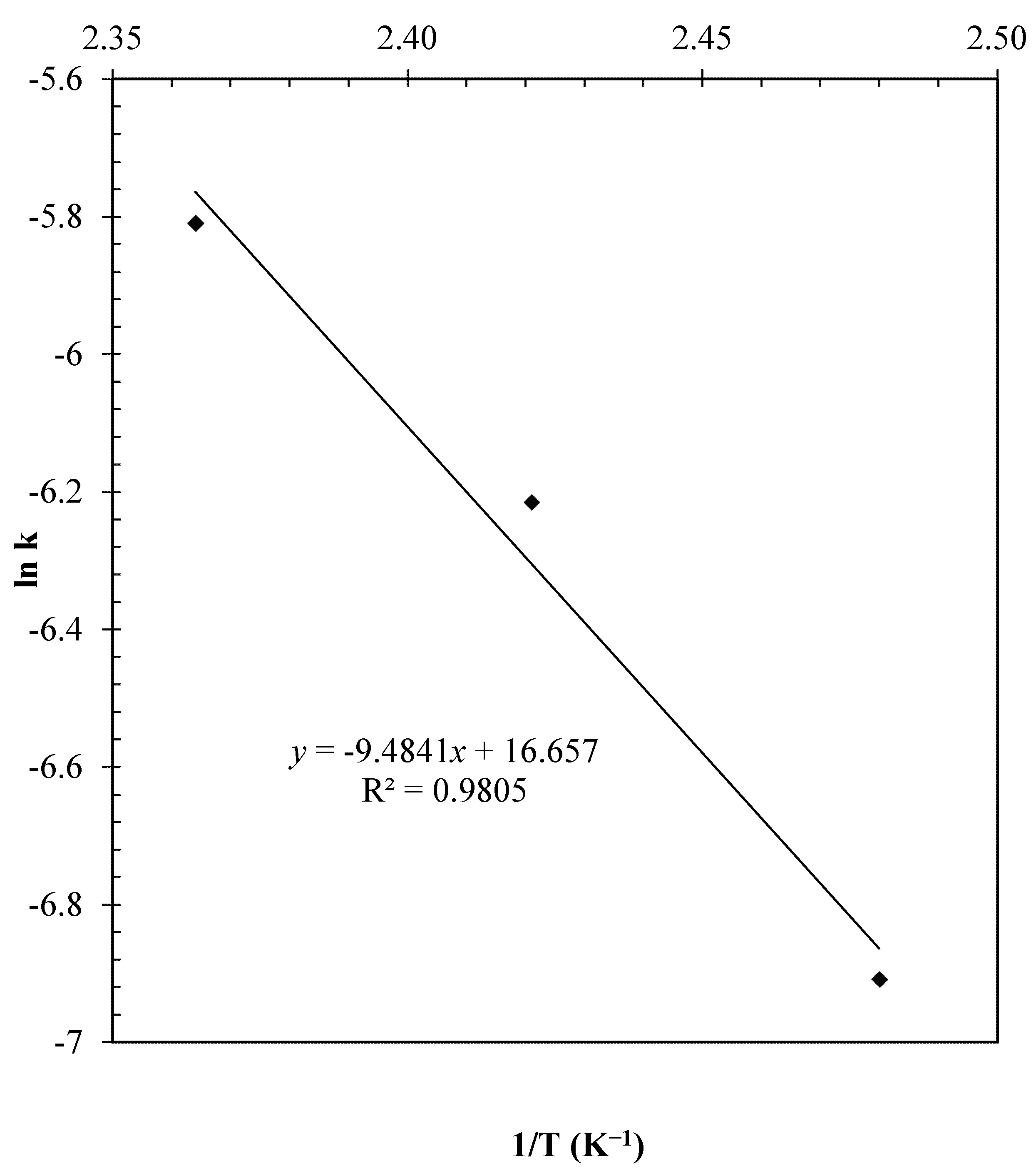
2.4.4. Effect of Temperature
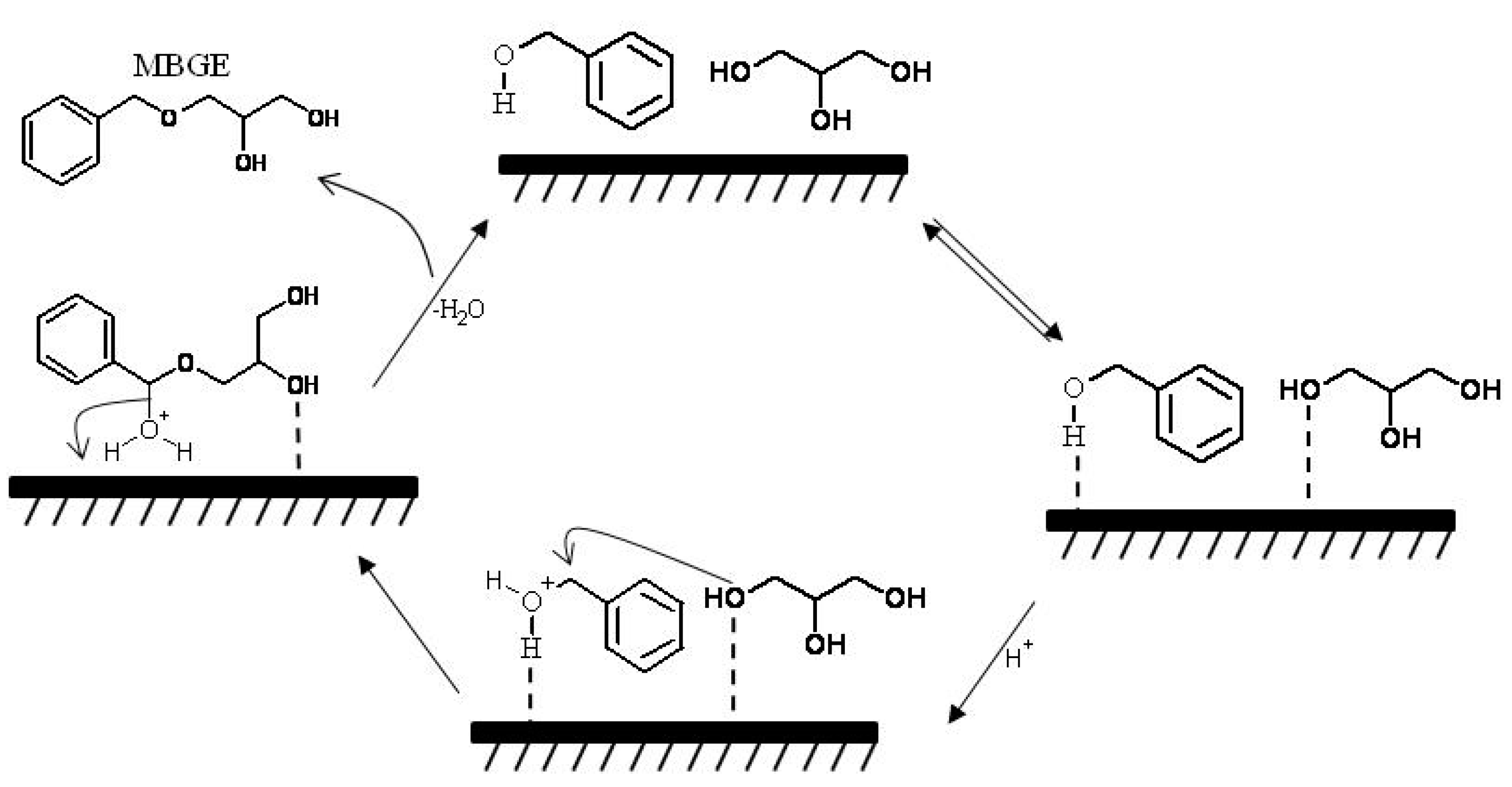
2.5. Reaction Mechanism and Kinetic Model
2.6. Catalyst Reusability Study
3. Experimental Section
3.1. Chemicals
3.2. Catalyst Synthesis
3.3. Experimentql Setup
3.4. Reaction Procedure
3.5. Method of Analysis
3.6. Catalyst Characterization
3.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.6.2. Surface Area Analysis
3.6.3. Inductively Coupled Plasma Mass Spectroscopy (ICP-MS)
3.6.4. Scanning Electron Microscopy (SEM)
3.6.5. X-ray Diffraction Study (XRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Reactant species A—Glycerol |
| AS | Chemisorbed A |
| B | Reactant species B—Benzyl alcohol |
| BS | Chemisorbed B |
| E | Product mono benzyl glycerol ether (MBGE) |
| ES | Chemisorbed product |
| W | Water |
| CA, CB | Concentration of A and B (mol/cm3) |
| CA0, CB0 | Initial concentration of A and B (mol/cm3) |
| CAS, CBS | Concentration of A and B on solid catalyst surface (mol/g cat) |
| CES, CWS | Concentration of E and W at solid catalyst surface (mol/g cat) |
| CS | Concentration of vacant sites (mol/g cat) |
| Ct | Total concentration of sites (mol/g cat) |
| rA | Rate of disappearance of A (mol/cm3/s) |
| KA | Equilibrium constant for adsorption of A on catalyst surface (L mol−1) |
| KB | Equilibrium constant for adsorption of B on catalyst surface (L mol−1) |
| k2 | Surface reaction rate constant for forward reaction (L mol−1 min−1) |
| k′2 | Surface reaction rate constant for reverse reaction (L mol−1 min−1) |
| KP | Equilibrium constant for adsorption of P on catalyst surface (L mol−1) |
| KW | Equilibrium constant for adsorption of W on catalyst surface (L min−1) |
| S | Vacant site |
| T | Time |
| W | Catalyst loading (g/cm3 of liquid phase) |
| XA | Fractional conversion of A |
References
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- He, Q.; McNutt, J.; Wang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Jerome, F.; Pouilloux, Y.; Barrault, J. Rational Design of Solid Catalysts for the Selective Use of Glycerol as a Natural Organic Building Block. ChemSusChem 2008, 1, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Eilting, J.; Irawadi, K.; Lwachinski, J.; Linder, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Behr, A.; Gomes, J.P. The refinement of renewable resources: New important derivatives of fatty acids and glycerol. Eur. J. Lipid Sci. Tech. 2010, 112, 31–50. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (Byproduct of Biodiesel Production) as a Source for Fuels and Chemicals—Mini Review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Deng, W.; Li, Y.; Zhang, Q.; Wang, Y. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J. Energy Chem. 2019, 32, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, M.; Ciriminna, R.; Rossi, M.; Pina, C.D. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar]
- Samoilov, V.O.; Ramazanov, D.N.; Nekhaev, A.I.; Maximov, A.L.; Bagdasarov, L.N. Heterogeneous catalytic conversion of glycerol to oxygenated fuel additives. Fuel 2016, 172, 310–319. [Google Scholar] [CrossRef]
- Koshchii, S.V. Optimization of synthesis of mono-o-methylglycerol isomers. Russ. J. Appl. Chem. 2002, 75, 1434–1437. [Google Scholar] [CrossRef]
- Garcia, J.I.; Garcia-Marin, H.; Mayoral, J.A.; Perez, P. Green solvents from glycerol. Synthesis and physico-chemical properties of alkyl glycerol ethers. Green Chem. 2010, 12, 426–434. [Google Scholar] [CrossRef]
- Saengarun, C.; Petsom, A.; Tungasmita, D.N. Etherification of Glycerol with Propylene or 1-Butene for Fuel Additives. Sci. World J. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; Garcia-Martinez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klepacova, K.; Mravec, D.; Bajus, M. Etherification of glycerol with tert-butyl alcohol catalysed by ion-exchange resins. Chem. Pap. 2006, 60, 224–230. [Google Scholar] [CrossRef]
- da Machado, M.S.; Sastre, E.; Perez-Pariente, J.; Cardoso, D.; De Guerenu, A.M. Selective synthesis of glycerol monolaurate with zeolitic molecular sieves. Appl. Catal. A Gen. 2000, 203, 321–328. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Romero, A.; Santos, A. Improved Etherification of Glycerol with Tert-Butyl Alcohol by the Addition of Dibutyl Ether as Solvent. Catalysts 2019, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Azzouzi, A.; Pouilloux, Y.; Jerome, F.; Barrault, J. Heterogeneously catalyzed etherification of glycerol: New pathways for transformation of glycerol to more valuable chemicals. Green Chem. 2008, 10, 164–167. [Google Scholar] [CrossRef]
- Barrault, J.; Jerome, F. Design of new solid catalysts for the selective conversion of glycerol. Eur. J. Lipid Sci. Technol. 2008, 110, 825–830. [Google Scholar] [CrossRef]
- Fahrenholtz, K.E. Adrenergic Blocking Agents. U.S. Patent No. 4,304,721, 8 December 1981. [Google Scholar]
- Kaminuma, M. Skin Preparation Composition for External Use. U.S. Patent No. 0,257,436 A1, 16 November 2006. [Google Scholar]
- Yadav, G.D.; Goel, P.K.; Joshi, A.V. Alkylation of dihydroxybenzenes and anisole with methyl-tert-butyl ether (MTBE) over solid acid catalysts. Green Chem. 2001, 3, 92–99. [Google Scholar] [CrossRef]
- Yadav, G.D.; Doshi, N.S. Alkylation of hydroquinone with methyl-tert-butyl-ether and tert-butanol. Catal. Today 2000, 60, 263–273. [Google Scholar] [CrossRef]
- Verhoef, J.M.; Kooyman, J.P.; Peters, A.J.; van Bekkum, H. A study on the stability of MCM-41-supported heteropoly acids under liquid- and gas-phase esterification conditions. Microporous Mesoporous Mater. 1999, 27, 365–371. [Google Scholar] [CrossRef]
- Chu, W.; Yang, X.; Ye, X.; Wu, Y. Vapor phase esterification catalyzed by immobilized dodecatungstosilicic acid (SiW12) on activated carbon. Appl. Catal. A Gen. 1996, 145, 125–140. [Google Scholar] [CrossRef]
- Yadav, G.D. Synergism of clay and heteropoly acids as nano-catalysts for the development of green processes with potential industrial applications. Catal. Sury. Asia 2005, 9, 117–137. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Isomerisation of citronellal to isopulegol using shape selective catalysts UDCaT-2. Langmuir 2000, 16, 4072. [Google Scholar] [CrossRef]
- Yadav, G.D.; Murkute, A.D. Novel efficient mesoporous solid acid catalyst UDCaT-4: Dehydration of 2-propanol and alkylation of mesitylene. Langmuir 2004, 20, 11607–11619. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sengupta, S. Friedel−Craft’s alkylation of diphenyl oxide with benzyl chloride over sulphated zirconia. Org. Proc. Res. Devel. 2002, 6, 256–262. [Google Scholar] [CrossRef]
- Yadav, G.D.; Kamble, S.B. Atom efficient Friedel–Crafts acylation of toluene with propionic anhydride over solid mesoporous superacid UDCaT-5. Appl. Catal. A Gen. 2012, 433, 265–274. [Google Scholar] [CrossRef]
- Malkar, R.S.; Yadav, G.D. Selectivity engineering in synthesis of thymol using sulfated ZrO2–TiO2. Ind. Eng. Chem. Res. 2017, 56, 8437–8447. [Google Scholar] [CrossRef]
- Tiwari, M.S.; Yadav, G.D. Novel aluminium exchanged dodecatungstophosphoric acid supported on K-10 clay as catalyst: Benzoylation of diphenyloxide with benzoic anhydride. RSC Adv. 2016, 6, 49091–49100. [Google Scholar] [CrossRef]
- Wagh, D.P.; Yadav, G.D. Multi-functional Fe-Al0.66DTP/MCF catalyst in cascade engineered synthesis of the drug butamben: Novelty of catalyst, reaction kinetics and mechanism. Mol. Catal. 2020, 483, 110711. [Google Scholar] [CrossRef]
- Yadav, G.D.; Salgaonkar, S.S.; Asthana, N.S. Selectivity engineering in isopropylation of benzene to cumene over cesium substituted dodecatungstophoshoric acid on K-10 clay. Appl. Catal. A Gen. 2004, 265, 153–159. [Google Scholar] [CrossRef]
- Yadav, G.D.; Asthana, N.S.; Kamble, V.S. Friedel-Crafts benzoylation of p-xylene over clay supported catalysts: Novelty of cesium substituted dodecatungstophosphoric acid on K-10 clay. Appl. Catal. A Gen. 2003, 240, 53–69. [Google Scholar] [CrossRef]
- Yadav, G.D.; Surve, P.S. Regioselective ring opening reaction of epichlorohydrin with acetic acid to 3-chloro-2-hydroxypropyl acetate over cesium modified heteropolyacid on clay support. Appl. Catal. A Gen. 2013, 468, 112–119. [Google Scholar] [CrossRef]
- Yadav, G.D.; George, G. Monoalkylation of biphenyl over modified heteropoly acids: Novelty of cesium substituted dodecatungstophosphoric acid supported on hexagonal mesoporous silica. Catal. Today 2009, 141, 130–137. [Google Scholar] [CrossRef]
- Tiwari, M.S.; Yadav, G.D. Kinetics of Friedel–Crafts benzoylation of veratrole with benzoic anhydride using Cs2.5H0.5PW12O40/K-10 solid acid catalyst. Chem. Eng. J. 2015, 266, 64–73. [Google Scholar] [CrossRef]
- Tekale, P.T.; Yadav, G.D. Esterification of propanoic acid with 1,2-propanediol: Catalysis by cesium exchanged heteropoly acid on K-10 clay and kinetic modelling. React. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Yadav, G.D.; Goel, P.K. Selective synthesis of perfumery grade cyclohexyl esters from cyclohexene and carboxylic acids over ion exchange resins: An example of 100% atom economy. Green Chem. 2000, 2, 71–77. [Google Scholar] [CrossRef]
- Silva Camila, R.B.; Goncalves Valter, L.C.; Lachter, E.R.; Mota, C.J.A. Etherification of glycerol with benzyl alcohol catalyzed by solid acids. J. Braz. Chem. Soc. 2009, 20, 201–204. [Google Scholar] [CrossRef] [Green Version]
 ) MBGE; (
) MBGE; ( ) DBE; (
) DBE; ( ) DBGE.
) DBGE.
 ) MBGE; (
) MBGE; ( ) DBE; (
) DBE; ( ) DBGE.
) DBGE.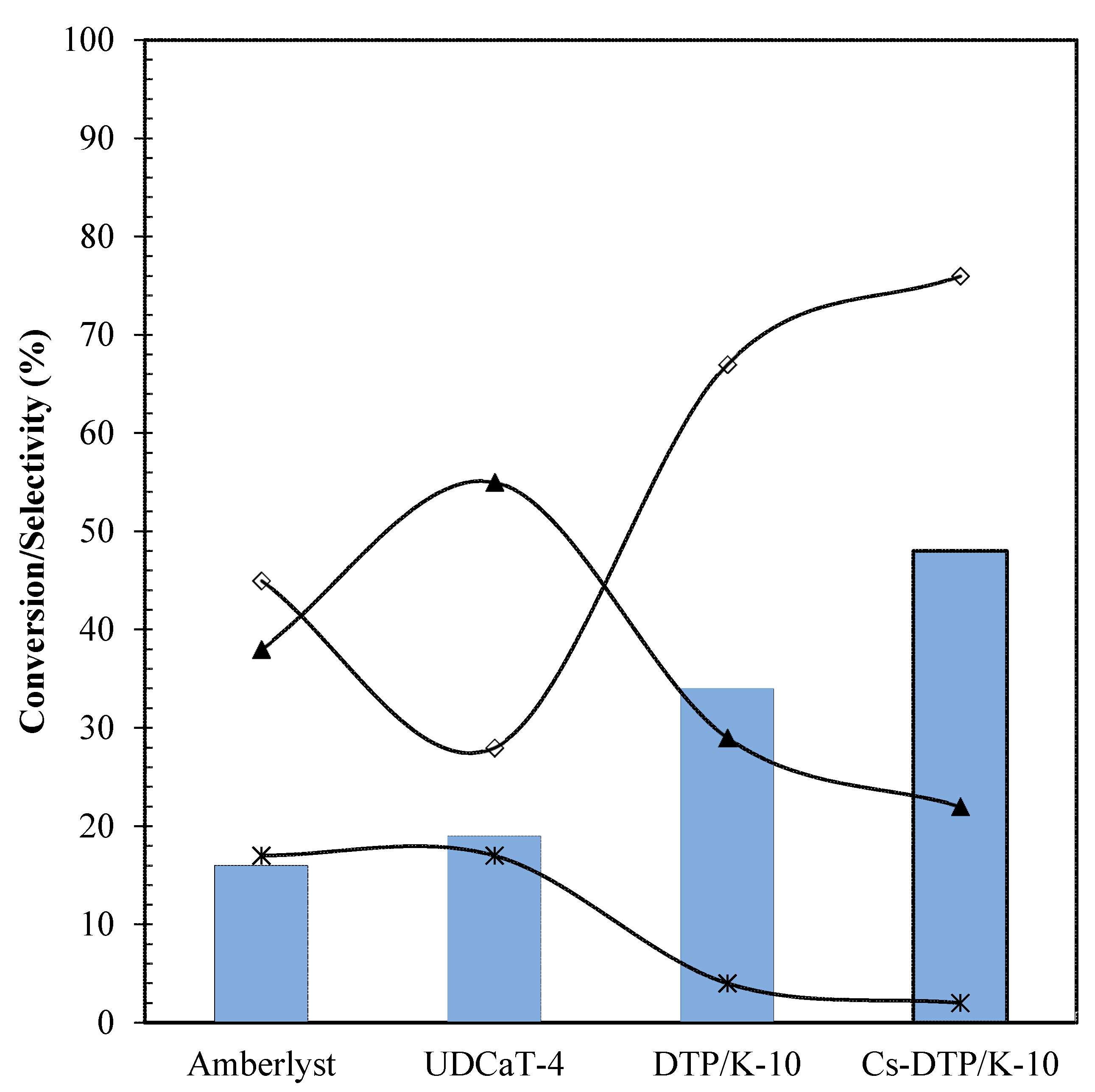





 ) MBGE; (
) MBGE; ( ) DBE; (
) DBE; ( ) DBGE.
) DBGE.
 ) MBGE; (
) MBGE; ( ) DBE; (
) DBE; ( ) DBGE.
) DBGE.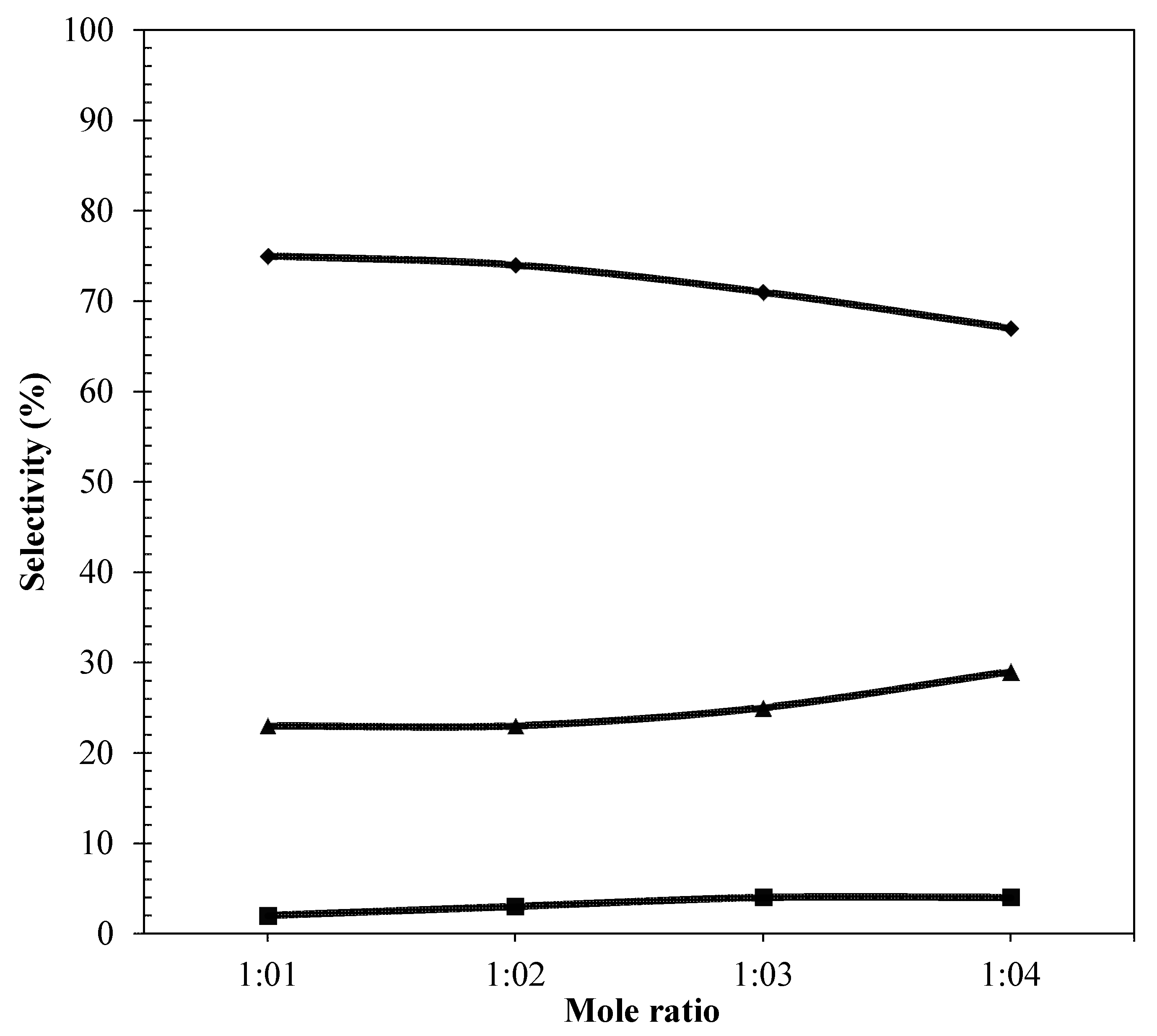
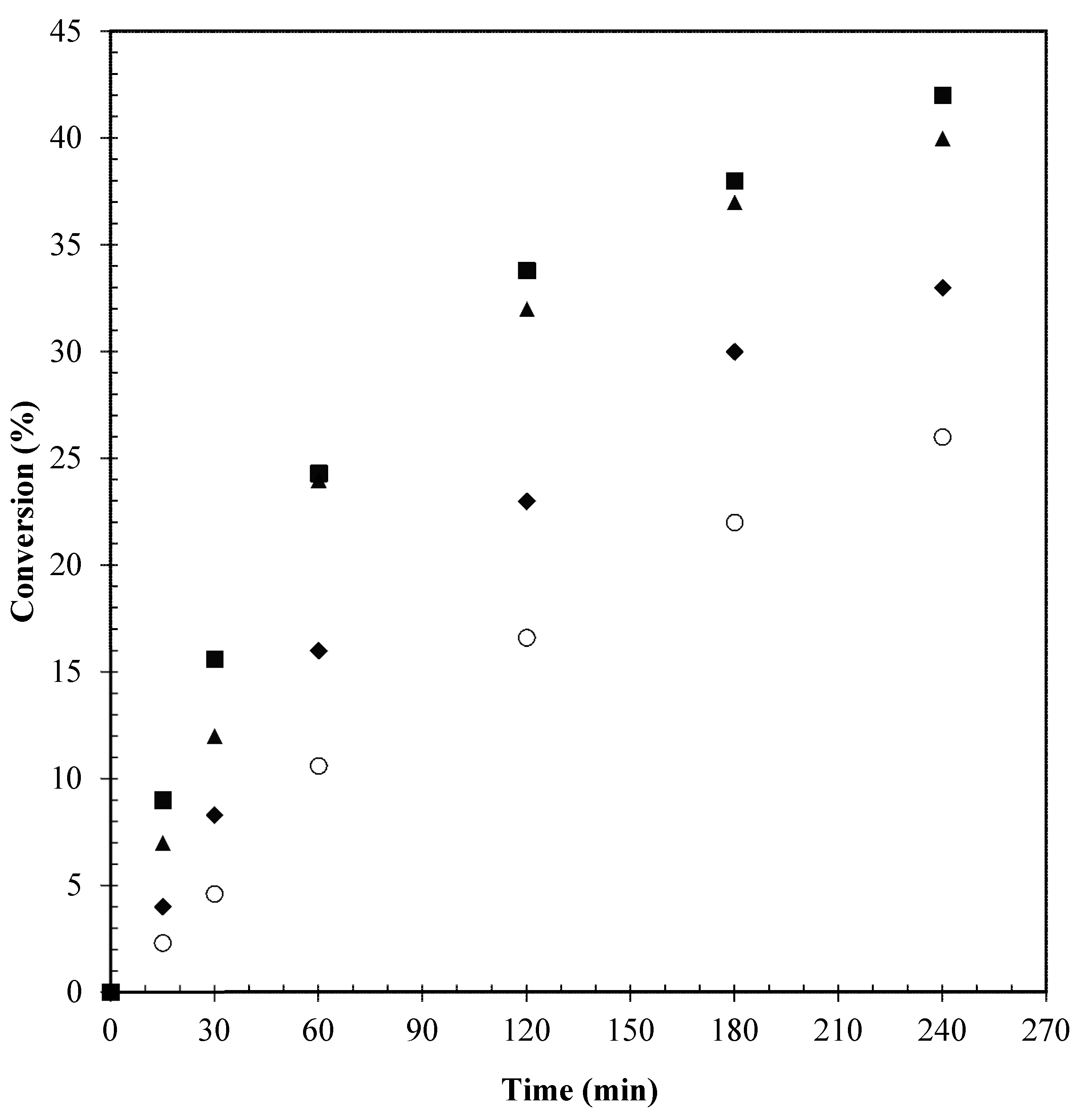
 ) Conversion; (
) Conversion; ( ) MBGE; (■) DBGE; (
) MBGE; (■) DBGE; ( ) DBE.
) DBE.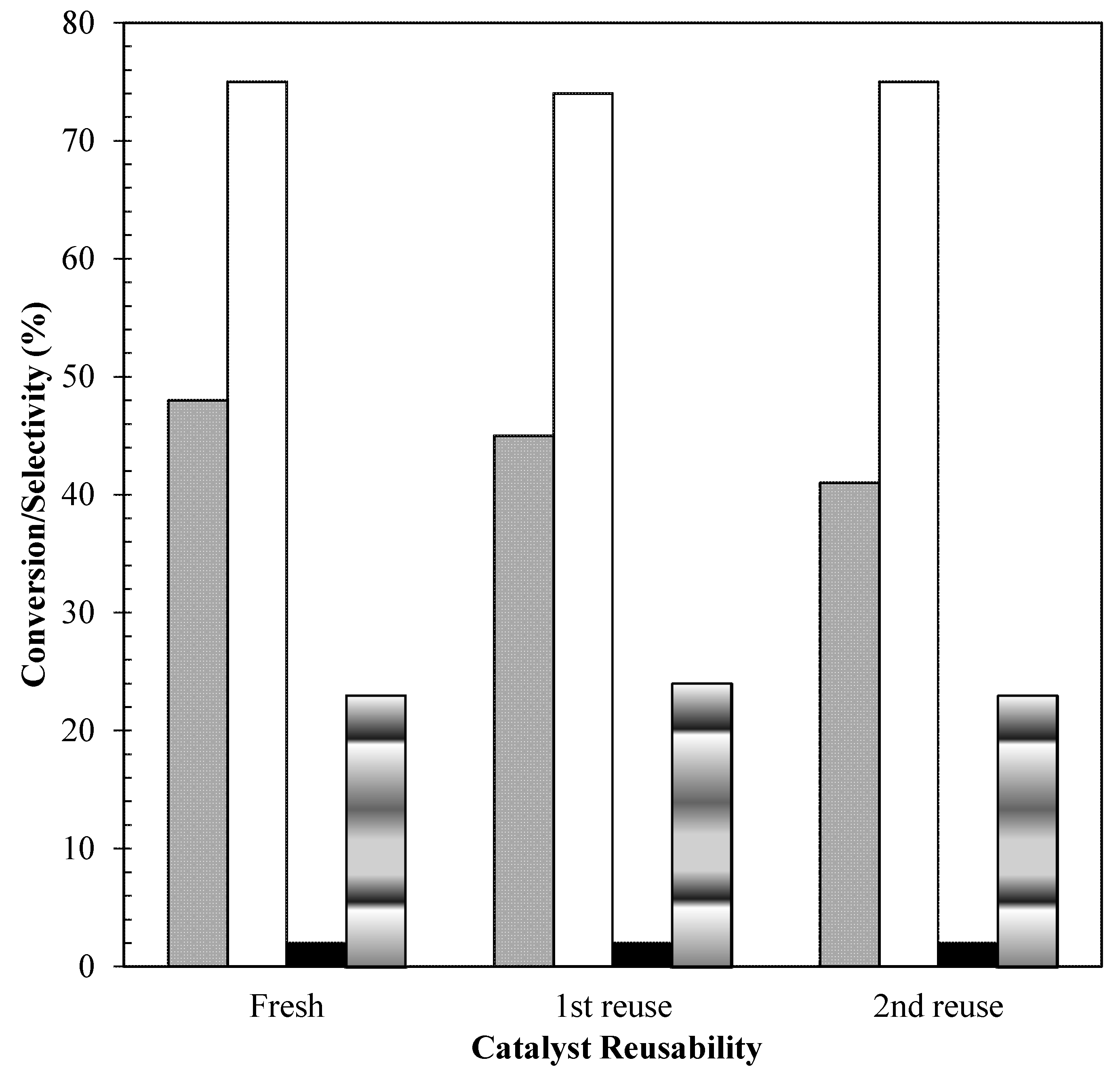
| Catalyst | BET Surface Area (m2/g) | Single Point Total Pore Volume (cm3/g) | Average Pore Diameter (4A/V by BET) (nm) |
|---|---|---|---|
| K-10 | 273.01 ± 0.84 | 0.392 | 5.749 |
| Fresh Cs-DTP/K-10 | 239.61 ± 0.45 | 0.330 | 5.518 |
| Regenerated Cs-DTP/K-10 | 223.49 ± 0.52 | 0.308 | 5.558 |
| Catalyst | Cs (ppm) | W (ppm) |
|---|---|---|
| Fresh Cs2.5H0.5PW12O40/K-10 | 18,999 | 116,708 |
| Metal content in reaction mixture | Undetected | 87.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekale, D.P.; Yadav, G.D.; Dalai, A.K. Solvent-Free Benzylation of Glycerol by Benzyl Alcohol Using Heteropoly Acid Impregnated on K-10 Clay as Catalyst. Catalysts 2021, 11, 34. https://doi.org/10.3390/catal11010034
Tekale DP, Yadav GD, Dalai AK. Solvent-Free Benzylation of Glycerol by Benzyl Alcohol Using Heteropoly Acid Impregnated on K-10 Clay as Catalyst. Catalysts. 2021; 11(1):34. https://doi.org/10.3390/catal11010034
Chicago/Turabian StyleTekale, Devendra P., Ganapati D. Yadav, and Ajay K. Dalai. 2021. "Solvent-Free Benzylation of Glycerol by Benzyl Alcohol Using Heteropoly Acid Impregnated on K-10 Clay as Catalyst" Catalysts 11, no. 1: 34. https://doi.org/10.3390/catal11010034







