Heterogeneous Electro-Fenton as “Green” Technology for Pharmaceutical Removal: A Review
Abstract
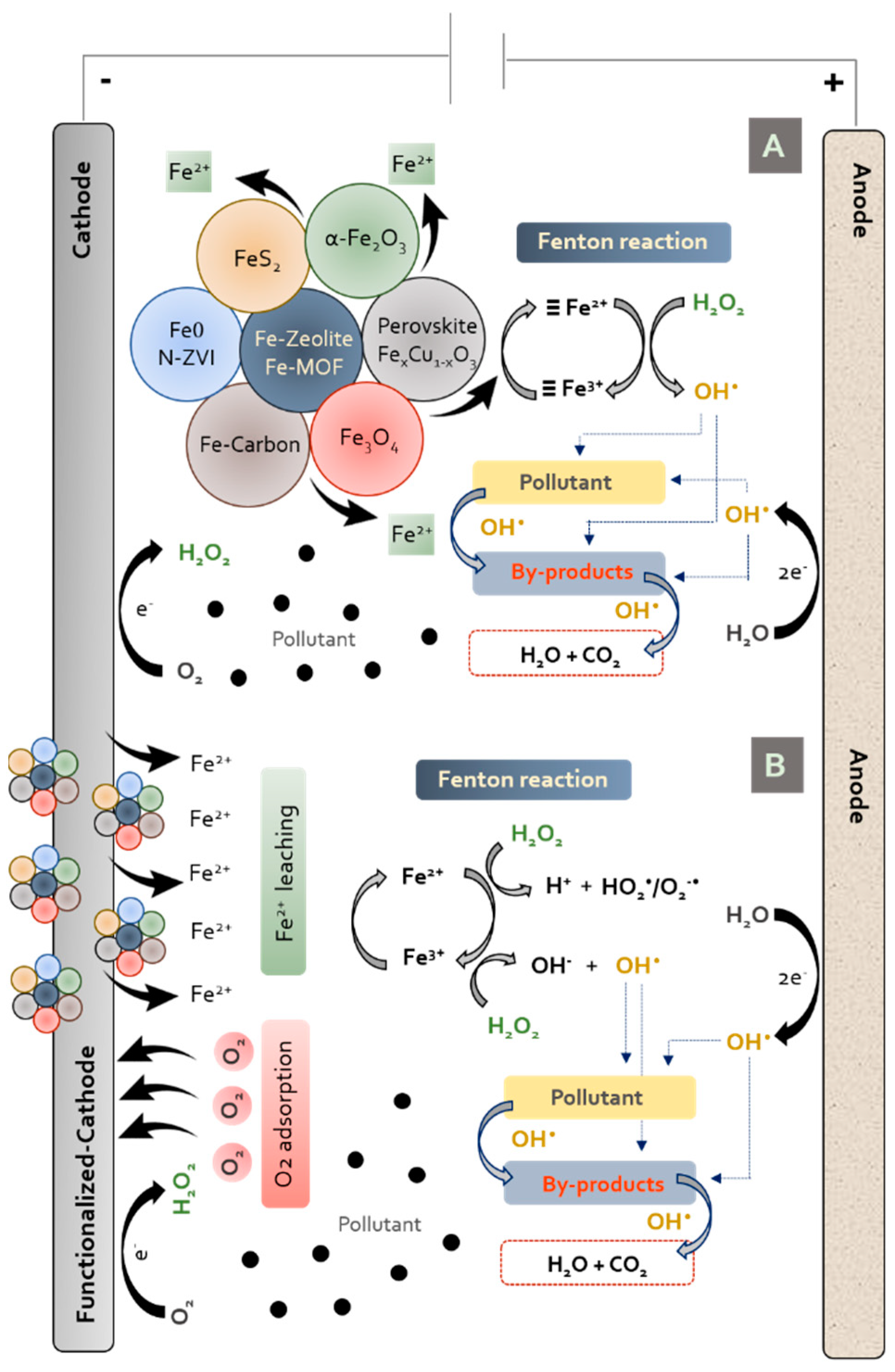
:1. Introduction
2. Fundamentals of Heterogeneous Electro-Fenton Process
3. Experimental Variables and Parameters of Heterogeneous Electro-Fenton Process
3.1. Effect of Catalyst Concentration
3.2. Influence of Applied Current Intensity
3.3. Solution pH Effect
3.4. Oxidation Efficiency and Energy Consumption
4. Heterogeneous Catalysts
5. Heterogeneous Electro-Fenton Process with Iron Functionalised-Cathode
6. Application of Electro-Fenton Process for the Removal of Pharmaceutical Compounds
6.1. Antibiotics
6.2. β-Blockers
6.3. NSAIDs
7. Photoelectro-Fenton Process
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Resolution A/70/L.1 Adopted by the General Assembly on 25 September 2015. Transforming Our World: The 2030 Agenda for Sustainable Development, 2020 (2015). Available online: https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed on 30 October 2020).
- Geissen, V.; Mol, H.G.J.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van Der Ploeg, M.; Van De Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Uresti, D.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.; Faria, J.L.; Hentati, O.; Silva, A.M.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Bradley, P.M.; Journey, C.A.; Button, D.T.; Carlisle, D.M.; Clark, J.M.; Mahler, B.J.; Nakagaki, N.; Qi, S.L.; Waite, I.R.; VanMetre, P.C. Metformin and Other Pharmaceuticals Widespread in Wadeable Streams of the Southeastern United States. Environ. Sci. Technol. Lett. 2016, 3, 243–249. [Google Scholar] [CrossRef]
- Bu, Q.; Cao, Y.; Yu, G.; He, X.; Zhang, H.; Sun, J.; Yun, M.; Cao, Z. Identifying targets of potential concern by a screening level ecological risk assessment of human use pharmaceuticals in China. Chemosphere 2020, 246, 125818. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ. 2018, 615, 297–306. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, J.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Oturan, N.; Bechelany, M.; Cretin, M.; Oturan, M.A. Highly efficient and stable FeIIFeIII LDH carbon felt cathode for removal of pharmaceutical ofloxacin at neutral pH. J. Hazard. Mater. 2020, 393, 122513. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, M.; Pan, Y.; Du, X.; Wang, Q. MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-Fenton process in degradation of sulfamethazine: Approach and mechanism. Chem. Eng. J. 2021, 403, 126361. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C. Template-free microspheres decorated with Cu-Fe-NLDH for catalytic removal of gentamicin in heterogeneous electro-Fenton process. J. Environ. Manag. 2019, 248, 109236. [Google Scholar] [CrossRef] [PubMed]
- Droguett, C.; Salazar, R.; Brillas, E.; Sirés, I.; Carlesi, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total Environ. 2020, 740, 140154. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Olvera-Vargas, H.; Oturan, M.A.; Huguenot, D.; Gadri, A.; Ammar, S.; Brillas, E.; Oturan, M.A. Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Appl. Catal. B Environ. 2017, 209, 637–647. [Google Scholar] [CrossRef]
- Monteil, H.; Péchaud, Y.; Oturan, N.; Oturan, M.A. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chai, S.; Zhao, H.; Wang, Y. Three-Dimensional Homogeneous Ferrite-Carbon Aerogel: One Pot Fabrication and Enhanced Electro-Fenton Reactivity. ACS Appl. Mater. Interfaces 2013, 5, 842–852. [Google Scholar] [CrossRef]
- Luo, T.; Feng, H.; Tang, L.; Lu, Y.; Tang, W.; Chen, S.; Yu, J.; Xie, Q.; Ouyang, X.; Chen, Z. Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3: Mechanism and degradation pathway. Chem. Eng. J. 2020, 382, 122970. [Google Scholar] [CrossRef]
- Segura, S.G.; Brillas, E. Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode. Water Res. 2011, 45, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Meijide, J.; Pazos, M.; Sanromán, M. Heterogeneous electro-Fenton catalyst for 1-butylpyridinium chloride degradation. Environ. Sci. Pollut. Res. 2017, 26, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Bounab, L.; Iglesias, O.; González-Romero, E.; Pazos, M.; Sanromán, M. Effective heterogeneous electro-Fenton process of m-cresol with iron loaded actived carbon. RSC Adv. 2015, 5, 31049–31056. [Google Scholar] [CrossRef] [Green Version]
- Vilar, V.J.; Moreira, F.C.; Ferreira, A.C.; Sousa, M.; Gonçalves, C.; Alpendurada, M.; Boaventura, R.A. Biodegradability enhancement of a pesticide-containing bio-treated wastewater using a solar photo-Fenton treatment step followed by a biological oxidation process. Water Res. 2012, 46, 4599–4613. [Google Scholar] [CrossRef] [PubMed]
- Meijide, J.; Rodríguez, S.; Sanromán, M.A.; Pazos, M. Comprehensive solution for acetamiprid degradation: Combined electro-Fenton and adsorption process. J. Electroanal. Chem. 2018, 808, 446–454. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Oturan, N.; Ammar, S.; Gadri, A.; Oturan, M.A.; Brillas, E. Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis. Environ. Chem. Lett. 2017, 15, 689–693. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Campos, S.; Salazar, R.; Arancibia-Miranda, N.; Rubio, M.; Aranda, M.; García, A.; Sepúlveda, P.; Espinoza, L.C. Nafcillin degradation by heterogeneous electro-Fenton process using Fe, Cu and Fe/Cu nanoparticles. Chemosphere 2020, 247, 125813. [Google Scholar] [CrossRef]
- Carrasco-Díaz, M.R.; Castillejos-López, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chem. Eng. J. 2016, 304, 408–418. [Google Scholar] [CrossRef]
- Özcan, A.; Özcan, A.A.; Demirci, Y.; Şener, E. Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxacin. Appl. Catal. B Environ. 2017, 200, 361–371. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Pazos, M.; Sanromán, M. Prompt removal of antibiotic by adsorption/electro-Fenton degradation using an iron-doped perlite as heterogeneous catalyst. Process. Saf. Environ. Prot. 2020, 144, 100–110. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@PC core–shell hybrid for sulfamethazine degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nidheesh, P. Importance of Graphene in the Electro-Fenton Process. ACS Omega 2020, 5, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Bina, B.; Ebrahimi, A. A novel three-dimensional electro-Fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process. Saf. Environ. Prot. 2018, 117, 200–213. [Google Scholar] [CrossRef]
- Mi, X.; Li, Y.; Ning, X.; Jia, J.; Wang, H.; Xia, Y.; Sun, Y.; Zhan, S. Electro-Fenton degradation of ciprofloxacin with highly ordered mesoporous MnCo2O4-CF cathode: Enhanced redox capacity and accelerated electron transfer. Chem. Eng. J. 2019, 358, 299–309. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Le, T.X.H.; Bechelany, M.; Oturan, M.A.; Papirio, S.; Esposito, G.; Van Hullebusch, E.; Cretin, M.; Oturan, M.A. Electrochemical mineralization of sulfamethoxazole over wide pH range using FeIIFeIII LDH modified carbon felt cathode: Degradation pathway, toxicity and reusability of the modified cathode. Chem. Eng. J. 2018, 350, 844–855. [Google Scholar] [CrossRef]
- Nsubuga, H.; Basheer, C.; Haider, M.B. An enhanced beta-blockers degradation method using copper-boron-ferrite supported graphite electrodes and continuous droplet flow-assisted electro-Fenton reactor. Sep. Purif. Technol. 2019, 221, 408–420. [Google Scholar] [CrossRef]
- Pourzamani, H.; Hajizadeh, Y.; Mengelizadeh, N. Application of three-dimensional electrofenton process using MWCNTs-Fe3O4 nanocomposite for removal of diclofenac. Process. Saf. Environ. Prot. 2018, 119, 271–284. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, M.A.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Rahmatinia, Z.; Rahmatinia, M. Removal of the metronidazole from aqueous solution by heterogeneous electro-Fenton process using nano-Fe3O4. Data Brief. 2018, 19, 2139–2145. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
- Rosales, E.; Diaz, S.; Pazos, M.; Sanromán, M.A. Comprehensive strategy for the degradation of anti-inflammatory drug diclofenac by different advanced oxidation processes. Sep. Purif. Technol. 2019, 208, 130–141. [Google Scholar] [CrossRef]
- Sadeghi, M.; Mehdinejad, M.H.; Mengelizadeh, N.; Mahdavi, Y.; Pourzamani, H.; Hajizadeh, Y.; Zare, M.R. Degradation of diclofenac by heterogeneous electro-Fenton process using magnetic single-walled carbon nanotubes as a catalyst. J. Water Process. Eng. 2019, 31, 100852. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Nsubuga, H.; Basheer, C.; Jalilov, A.; Haider, M.B.; Al-Saadi, A.A. Droplet flow-assisted heterogeneous electro-Fenton reactor for degradation of beta-blockers: Response surface optimization, and mechanism elucidation. Environ. Sci. Pollut. Res. 2019, 26, 14313–14327. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H.; Zhou, M. Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(II)/Fe(III) cycle. Sep. Purif. Technol. 2020, 248, 117022. [Google Scholar] [CrossRef]
- Ye, Z.; Schukraft, G.E.; L’Hermitte, A.; Xiong, Y.; Brillas, E.; Petit, C.; Sirés, I. Mechanism and stability of an Fe-based 2D MOF during the photoelectro-Fenton treatment of organic micropollutants under UVA and visible light irradiation. Water Res. 2020, 184, 115986. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Wei, Y.-Q.; Liu, B.; Lin, Y.; Li, G.; Zhang, F. Enhanced degradation of ibuprofen by heterogeneous electro-Fenton at circumneutral pH. Chemosphere 2018, 209, 998–1006. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Mi, X.; Zhan, S.; Hu, W. Evaluation of ciprofloxacin destruction between ordered mesoporous and bulk NiMn2O4/CF cathode: Efficient mineralization in a heterogeneous electro-Fenton-like process. Environ. Sci. Nano 2019, 6, 661–671. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629. [Google Scholar] [CrossRef]
- Johansson, C.H.; Janmar, L.; Backhaus, T. Toxicity of ciprofloxacin and sulfamethoxazole to marine periphytic algae and bacteria. Aquat. Toxicol. 2014, 156, 248–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Zeng, Z.; Li, X.; Niu, C.-G.; Xiao, R.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Hu, L.; et al. Multi-walled carbon nanotube/amino-functionalized MIL-53(Fe) composites: Remarkable adsorptive removal of antibiotics from aqueous solutions. Chemosphere 2018, 210, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.; Brillas, E.; Garrido, J.A.; Rodríguez, R.M.; Sirés, I. Routes for the electrochemical degradation of the artificial food azo-colour Ponceau 4R by advanced oxidation processes. Appl. Catal. B Environ. 2016, 180, 227–236. [Google Scholar] [CrossRef]
- Benito-Peña, E.; Partal-Rodera, A.; León-González, M.; Bondi, M.C. Evaluation of mixed mode solid phase extraction cartridges for the preconcentration of beta-lactam antibiotics in wastewater using liquid chromatography with UV-DAD detection. Anal. Chim. Acta 2006, 556, 415–422. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Elmolla, E.S.; Chaudhuri, M. Degradation of the antibiotics amoxicillin, ampicillin and cloxacillin in aqueous solution by the photo-Fenton process. J. Hazard. Mater. 2009, 172, 1476–1481. [Google Scholar] [CrossRef]
- Panizza, M.; Dirany, A.; Sirés, I.; Haidar, M.; Oturan, N.; Oturan, M.A. Complete mineralization of the antibiotic amoxicillin by electro-Fenton with a BDD anode. J. Appl. Electrochem. 2014, 44, 1327–1335. [Google Scholar] [CrossRef]
- Berendsen, B.; Stolker, L.; De Jong, J.; Nielen, M.; Tserendorj, E.; Sodnomdarjaa, R.; Cannavan, A.; Elliott, C. Evidence of natural occurrence of the banned antibiotic chloramphenicol in herbs and grass. Anal. Bioanal. Chem. 2010, 397, 1955–1963. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Fidaleo, M.; Fidaleo, M.; Lavecchia, R. Degradation and antibiotic activity reduction of chloramphenicol in aqueous solution by UV/H2O2 process. J. Environ. Manag. 2014, 133, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yan, C.; Li, M.; Wang, X.; Bi, W.; Dong, W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Microwave assisted preparation of microporous activated carbon from Siris seed pods for adsorption of metronidazole antibiotic. Chem. Eng. J. 2013, 214, 310–318. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Amendola, L.; Molaioni, F.; Botrè, F. Detection of beta-blockers in human urine by GC-MS-MS-EI: Perspectives for the antidoping control. J. Pharm. Biomed. Anal. 2000, 23, 211–221. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, M.A.; Raffy, S.; Esposito, G.; Van Hullebusch, E.D.; Cretin, M.; Oturan, M.A. Use of Sub-stoichiometric Titanium Oxide as a Ceramic Electrode in Anodic Oxidation and Electro-Fenton Degradation of the Beta-blocker Propranolol: Degradation Kinetics and Mineralization Pathway. Electrochim. Acta 2017, 242, 344–354. [Google Scholar] [CrossRef]
- Ramil, M.; El Aref, T.; Fink, G.; Scheurer, M.; Ternes, T.A. Fate of Beta Blockers in Aquatic-Sediment Systems: Sorption and Biotransformation. Environ. Sci. Technol. 2010, 44, 962–970. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Cooper, W.J.; Song, W. Photochemical fate of beta-blockers in NOM enriched waters. Sci. Total Environ. 2012, 426, 289–295. [Google Scholar] [CrossRef]
- Rigobello, E.S.; Dantas, A.D.B.; Di Bernardo, L.; Vieira, E.M. Removal of diclofenac by conventional drinking water treatment processes and granular activated carbon filtration. Chemosphere 2013, 92, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Singh, P.; Aboul-Enein, H.Y.; Sharma, B. Chiral Analysis of Ibuprofen Residues in Water and Sediment. Anal. Lett. 2009, 42, 1747–1760. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Geissen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Cho, H.-H.; Huang, H.; Schwab, K. Effects of Solution Chemistry on the Adsorption of Ibuprofen and Triclosan onto Carbon Nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Gao, G.; Liu, H. Electrochemical Carbon Nanotube Filter for Adsorption, Desorption, and Oxidation of Aqueous Dyes and Anions. J. Phys. Chem. C 2011, 115, 3621–3629. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar] [CrossRef]


| Pharmaceutical. | Class | Group | WS (mg/mL) | PNEC a (g/L) | Pharmaceutical | Class | Group | WS (mg/mL) | PNEC a (μg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Acebutolol | β-blocker | Cardioselective | 0.259 | n.a. | Amoxicillin | Antibiotic | Penicillin | 3.43 | 489.53 |
| Atenolol | β-blocker | Cardioselective | 13.3 | n.a. | Cephalexin | Antibiotic | Cephalosporin | 10 | 228.73 |
| Cephazolin | Antibiotic | Cephalosporin | 0.21 | 2432.48 | Ciprofloxacin | Antibiotic | Fluoroquinone | 30 | 77.02 |
| Chloramphenicol | Antibiotic | Chloramphenicol | 2.5 | 186.59 | Diclofenac | NSAIDs | Acid acetic derivatives | 0.002 | 11.32 |
| Enoxacin | Antibiotic | Fluoroquinone | 3.43 | 94.93 | Gemfibrozil | NSAIDs | Fibric acid derivatives | 0.011 | 223.09 |
| Gentamycin | Antibiotic | Aminoglycosides | 100 | n.a. | Ibuprofen | NSAIDs | Propionic acid derivatives | 0.021 | 25.43 |
| Levofloxacin | Antibiotic | Fluoroquinone | 40.4 | 57.10 | Metronidazole | Antibiotic | Nitroimidazole | 5.92 | 13.00 |
| Nafcillin | Antibiotic | Penicillin | 0.017 | n.a. | Naproxen | NSAIDs | Propionic acid derivatives | 0.015 | 10.36 |
| Ofloxacin | Antibiotic | Fluoroquinone | 25 | 57.10 | Propranolol | β-blocker | Cardioselective | 0.061 | 13.01 |
| Sulfamethazine | Antibiotic | Sulfonamides | 1.5 | n.a. | Sulfamethizole | Antibiotic | Sulfonamides | 0.10 | n.a. |
| Sulfamethoxazole | Antibiotic | Sulfonamides | 0.61 | 164.58 | Tetracycline | Antibiotic | Tetracyclines | 3.87 | 460.38 |
| Pharmaceutical (Concentration) | Catalyst (Concentration) | Anode/Cathode | Operational Conditions | Removal and TOC Decay | References |
|---|---|---|---|---|---|
| Sulfamethazine (0.2 mM) | Pyrite (2 g/L) | BDD/CF | 0.05 M Na2SO4, pH:3, AFR: 1 L/min, I = 300 mA | 100% removal (40 min) | [40] |
| Tetracycline (0.2 mM) | Pyrite (2 g/L) | BDD/CF | 0.05 M Na2SO4, pH:3, AFR: 1 L/min, I = 300 mA | 100% removal (20 min) 96% TOC decay (480 min) | [27] |
| Tetracycline (0.2 mM) | Chalcopyrite (1 g/L) | BDD/CF | 0.05 M Na2SO4, pH:5.94, AFR: 0.6 L/min, I = 300 mA | 100% removal (7 min) 85% TOC decay (120 min) | [17] |
| Cephalexin (50 mg/L) | Chalcopyrite (1 g/L) | IrO2/air diffusion cathode | 0.05 M Na2SO4, pH:3, AFR: 0.6 L/min, I = 125 mA | 94% removal (15 min) 44% TOC decay (300 min) | [16] |
| Metronidazole (70 mg/L) | Nano–Fe2O3 (1 g/L) | Pt sheet/GF | 0.05 M Na2SO4, pH:3, I = 200 mA | 92.3% removal (30 min) | [41] |
| Amoxicillin (20 mg/L) | Nano–Fe2O3 (1 g/L) | Pt sheet/GF | 0.01 M Na2SO4, pH:3, AFR: 1 L/min, I = 300 mA | 98.2% removal (60 min) | [42] |
| Diclofenac (140 mg/L) | Fe2O3–modified chitosan (20 g/L) | BDD/CF | 0.01 M Na2SO4, pH:6, AFR: 1 L/min, I = 300 mA | 95% removal (120 min) 74.4% TOC decay (8 h) | [43] |
| Sulfamethizole (25 mg/L) | FeCl3–modified perlite (n.a.) | BDD/CF | 0.01 M Na2SO4, pH:6, AFR: 1 L/min, I = 300 mA | 100% removal (15 min) 65% TOC decay (120 min) | [32] |
| Enoxacin (0.25 mM) | Fe2O3–modified kaolin (1.7 g/L) | BDD/CF | 0.05 M Na2SO4, pH:3, AFR: 1 L/min, I = 300 mA | 100% removal (15 min) 98% TOC decay (420 min) | [31] |
| Diclofenac (10 mg/L) | MSWCNTs–FeCl2 (80 mg/L) | Ti–RuO2/GF | 0.05 M Na2SO4, pH:5, AFR: 1 mL/min, d = 20 mA/cm2 | 97.8% removal (120 min) 71% TOC decay (120 min) | [44] |
| Gentamicin (20 mg/L) | Cu–Fe–NLDH (1.25 g/L) | Pt sheet/graphite plate | 0.05 M Na2SO4, pH:6, AFR: 10 L/h, I = 400 mA | 91.3% removal (100 min) | [15] |
| Nafcillin (36 mg/L) | Fe/Cu bimetallic nanoparticles (1 g/L) | BDD/carbon–PTFE air diffusion | 0.05 M Na2SO4, pH:7, d = 5 mA/cm2 | 100% removal (7 min) | [29] |
| Chloramphenicol (80 mg/L) | Fe3O4–GO (0.5 g/L) | Pt gauze/CF | 0.05 M Na2SO4, pH:3, AFR: 10 L/h, I = 300 mA | 100% removal (45 min) 86% TOC decay (300 min) | [45] |
| Metronidazole (80 mg/L) | Fe3O4–GO (0.5 g/L) | Pt gauze/CF | 0.05 M Na2SO4, pH:3, AFR: 10 L/h, I = 300 mA | 100% removal (15 min) 73% TOC decay (300 min) | [45] |
| Propranolol/acebutolol (200 ng/mL each) | Fe–C (119 mg/L) | BDD/air diffusion cathode | 0.05 M Na2SO4, pH:7, d = 75 mA/cm2 | 100% removal (15 min) | [46] |
| Diclofenac (50 mg/L) | Pyrite (8 g/L) | Pt mesh/air diffusion cathode | 0.05 M Na2SO4, pH:7, AFR: 0.4 L/min, d = 31.84 mA/cm2 | 97.8% removal (8 min) 85% TOC decay (180 min) | [47] |
| Gemfibrozil (10 mg/L) | nano-ZVI@C–N (0.2 g/L) | Ti–IrO2/air diffusion cathode | 0.05 M Na2SO4, pH:6, AFR: 1 L/min, I = 300 mA | 95% removal (60 min) | [48] |
| Pharmaceutical (Concentration) | Catalyst (Concentration) | Anode/Cathode | Operational Conditions | Removal and TOC Decay | References |
|---|---|---|---|---|---|
| Ibuprofen (10 mg/L) | Ferric citrate | Ti–RuO2/Cit-Fe–ACFs | 0.05 M Na2SO4, pH: 6.8, AFR: 0.1 L/min, d = 5 mA/cm2 | 97% removal (120 min) | [49] |
| Sulfamethazine (10 mg/L) | Fe/Fe3C@CP (0.05 g/L) | Ti–RuO2/CB–CF | 0.05 M Na2SO4, pH: 3, I = 25 mA | 99% removal (30 min) | [33] |
| Levofloxacin (80 mg/L) | Fe/Fe3C@CP (0.05 g/L) | Ti–RuO2/CB–CF | 0.05 M Na2SO4, pH: 3, I = 25 mA | 97% removal (60 min) | [33] |
| Ibuprofen (4 mg/L) | Fe–NFP | Ti–PbO2/GF | pH: 6.3, d = 15.77 mA/cm2 | 98% removal (38 min) | [35] |
| Naproxen (4 mg/L) | Fe–NFP | Ti–PbO2/GF | pH: 6.24, d = 18.91 mA/cm2 | 93% removal (38 min) | [35] |
| Diclofenac (6.71 mg/L) | MSWCNTs-Fe3O4 (58.33 mg/L) | Ti-RuO2/MSWCNTs-Fe3O4–GF | 0.05 M Na2SO4, pH: 5.56, AFR: 1 mL/min, d = 19.74 mA/cm2 | 98% removal (83 min) | [39] |
| Ciprofloxacin (0.1 mM) | Fe2+(0.1 mM)/MnCo2O4 | Pt plate/MnCo2O4–CF | 0.05 M Na2SO4, pH: 3, AFR: 10 L/h, I = 300 mA | 100% removal (300 min) 75% TOC decay (300 min) | [36] |
| Sulfamethazine (10 mg/L) | MoS2 (0.02 g/L)/Fe0 (0.224 g/L) | Ti–TiO2–RuO2/CB–CF | 0.05 M Na2SO4, pH: 4, AFR: 50 mL/min, I = 50 mA | 100% removal (10 min) 42% TOC decay (60 min) | [14] |
| Tetracycline (20 mg/L) | Cu-doped Fe@Fe2O3 (50% wt. Cu) | Ti–TiO2–RuO2/Cu-doped Fe@Fe2O3–Ni foam | 0.05 M Na2SO4, pH: 3, AFR: 0.1 L/min, d = 40 mA/cm2 | 98.1% removal (120 min) 89% TOC decay (360 min) | [20] |
| Atenolol, propranolol (200 ng/L each) | Cu–B–F (n.a.) | BDD/Cu–B–F-modified graphite | 0.02 M Na2SO4, pH: 7, I = 100 mA | 99.9% removal (10 min) | [38] |
| Ciprofloxacin (0.1 mM) | Meso-NiMn2O4 (n.a.) | Pt plate/meso-NiMn2O4–CF | 0.05 M Na2SO4, pH: 3, AFR: 50 mL/min | 100% removal (90 min) 75.9% removal (360 min) | [50] |
| Cefazolin (20 mg/L) | CUFeNLDH-CNTs (1.25 g/L) | Pt sheet/CUFeNLDH–CNTs-graphite | 0.02 M Na2SO4, pH: 6, AFR: 10 L/h, I = 300 mA | 89.9% removal (100 min) 70.1 COD decay (300 min) | [51] |
| Sulfamethoxazole (0.2 mM) | FeIIFeIIILDH (n.a.) | Ti4O7/FeIIFeIIILDH–CF | 0.05 M Na2SO4, pH: 3, AFR: 1 L/min, d = 7.5 mA/cm2 | 100% removal (40 min) 97% TOC decay (480 min) | [37] |
| Ofloxacin (0.1 mM) | FeIIFeIIILDH (n.a.) | BDD/FeIIFeIIILDH–CF | 0.05 M Na2SO4, pH:7, AFR: 0.75 L/min, d = 12.5 mA/cm2 | 100% removal (30 min) 100% TOC decay (480 min) | [13] |
| Pharmaceutical (Concentration) | Catalyst (Concentration) | Anode/Cathode | Operational Conditions | Light Source | Removal and TOC Decay | References |
|---|---|---|---|---|---|---|
| Cephalexin (50 mg/L) | Chalcopyrite (1 g/L) | IrO2/air diffusion cathode | 0.05 M Na2SO4, pH: 3, AFR: 0.6 L/min, I = 125 mA | 6 W UVA fluorescent | 100% removal (15 min) 92% TOC decay (300 min) | [16] |
| Bezafibrate (10 mg/L) | Fe-MOFs (0.05 g/L) | IrO2/air diffusion cathode | 0.05 M Na2SO4, pH: 5.1, AFR: 1 L/min, I = 100 mA | 150 W Xe lamp (λ > 400 nm) | 92% removal (90 min) 61% TOC decay (240 min) | [48] |
| Thiamphenicol (50 mg/L) | Pyrite (2 g/L) | IrO2/air diffusion cathode | 0.02 M Na2SO4, pH: 3.95, AFR: 1 L/min, I = 100 mA | 6 W UVA fluorescent | 100% removal (60 min) 85% TOC decay (360 min) | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijide, J.; Dunlop, P.S.M.; Pazos, M.; Sanromán, M.A. Heterogeneous Electro-Fenton as “Green” Technology for Pharmaceutical Removal: A Review. Catalysts 2021, 11, 85. https://doi.org/10.3390/catal11010085
Meijide J, Dunlop PSM, Pazos M, Sanromán MA. Heterogeneous Electro-Fenton as “Green” Technology for Pharmaceutical Removal: A Review. Catalysts. 2021; 11(1):85. https://doi.org/10.3390/catal11010085
Chicago/Turabian StyleMeijide, Jessica, Patrick S. M. Dunlop, Marta Pazos, and María Angeles Sanromán. 2021. "Heterogeneous Electro-Fenton as “Green” Technology for Pharmaceutical Removal: A Review" Catalysts 11, no. 1: 85. https://doi.org/10.3390/catal11010085
APA StyleMeijide, J., Dunlop, P. S. M., Pazos, M., & Sanromán, M. A. (2021). Heterogeneous Electro-Fenton as “Green” Technology for Pharmaceutical Removal: A Review. Catalysts, 11(1), 85. https://doi.org/10.3390/catal11010085








