A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors
Abstract
1. Introduction
2. Overview of Multi-Scale Models
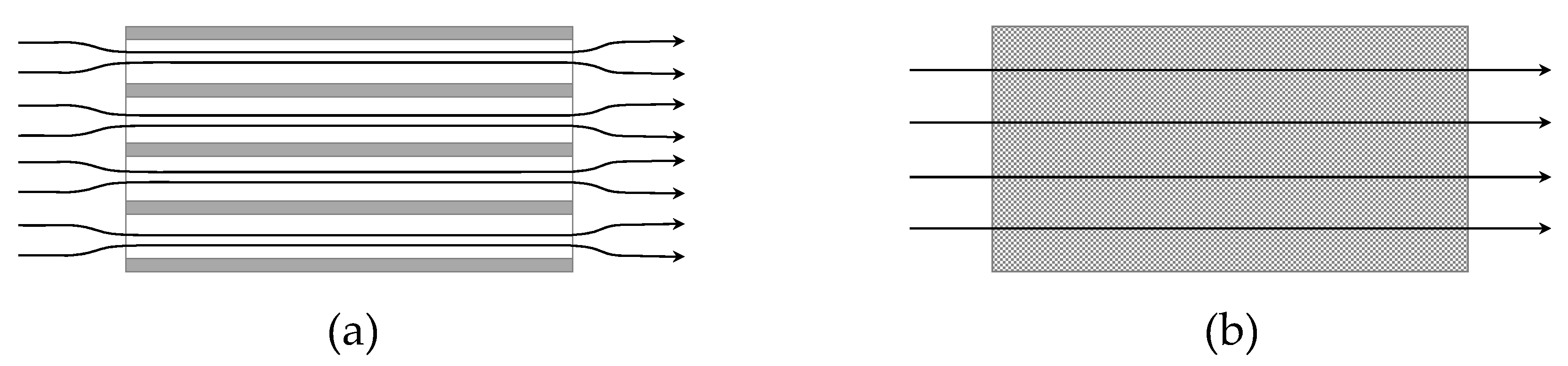
2.1. Bridge Models and Multi-Scale Consistency
- Flow regime
- Pressure drop
- Heat and mass transfer
- Conversion of chemical species
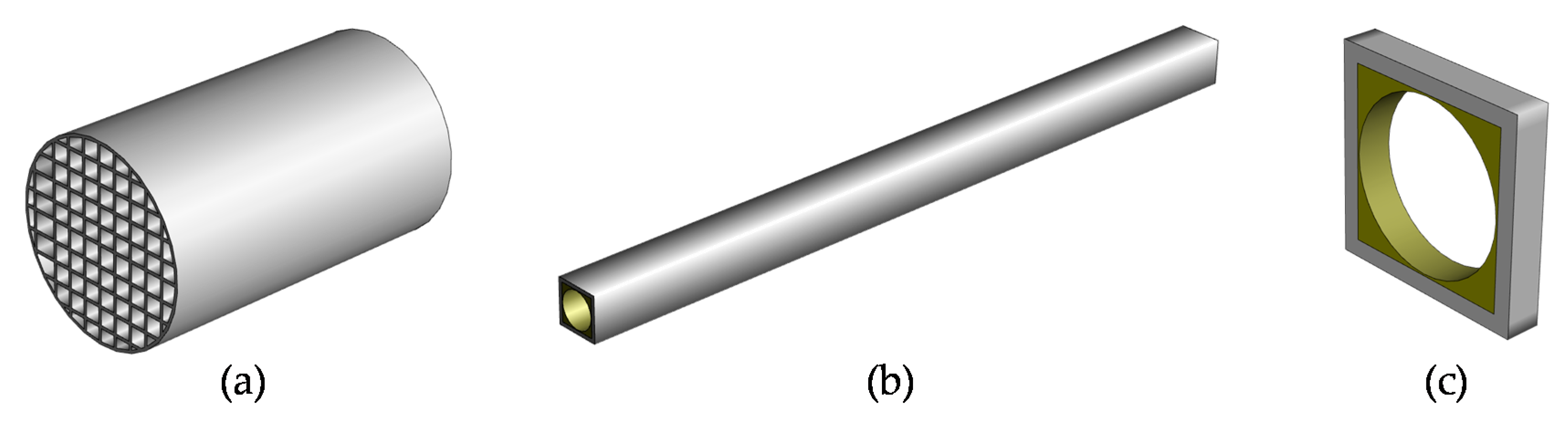
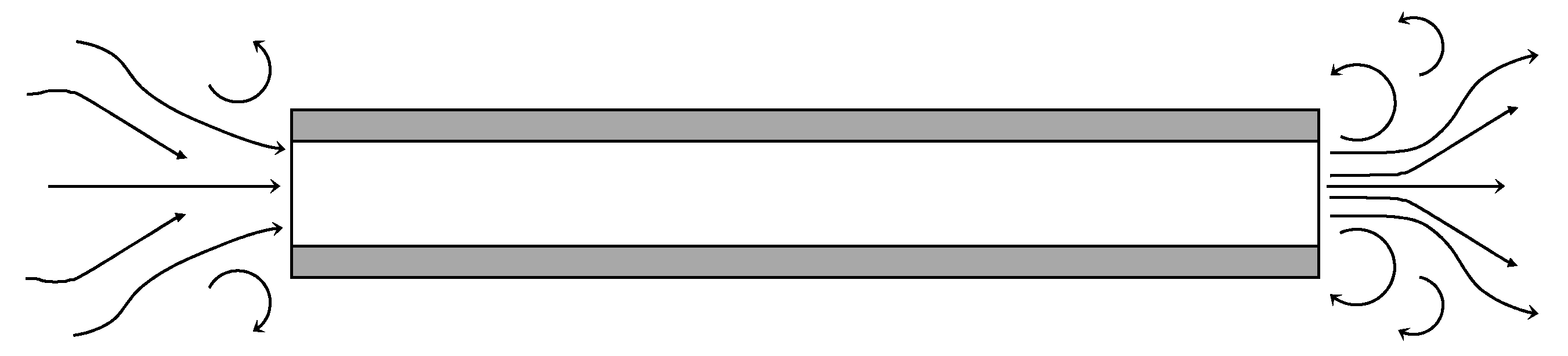
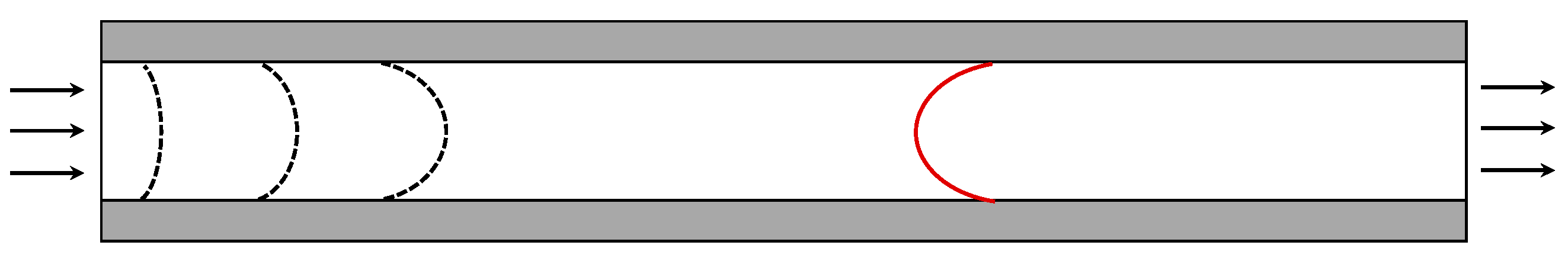
2.2. Flow Regime
2.3. Pressure Drop
2.4. Heat and Mass Transfer
2.5. Conversion of Chemical Species: Accounting for Washcoat Diffusion
2.6. Conversion of Chemical Species: Modeling the Reaction Rate
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Inertial resistance coefficient | |
| Heat capacity, J/kg-k | |
| Channel diameter, m | |
| Mass diffusivity, m2/s | |
| Darcy’s friction factor | |
| Thermal conductivity, W/m-K | |
| Turbulence kinetic energy, m2/s2 | |
| Pressure, Pa | |
| Solid-fluid heat flux, W/m2 | |
| Velocity, m/s | |
| Permeability, m2 | |
| Molecular viscosity, Pa-s | |
| Turbulence viscosity, Pa-s | |
| Density, kg/m3 | |
| Specific turbulence dissipation rate, 1/s |
References
- Hayes, R.E.; Mmbaga, J. Introduction to Chemical Reactor Analysis; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Heck, R.; Farrauto, R.; Gulati, S. Catalytic Air Pollution Control: Commercial Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kapteijn, F.; de Deugd, R.; Moulijn, J. Fischer–Tropsch synthesis using monolithic catalysts. Catal. Today 2005, 105, 350–356. [Google Scholar] [CrossRef]
- Merino, D.; Sanz, O.; Montes, M. Effect of the thermal conductivity and catalyst layer thickness on the Fischer-Tropsch synthesis selectivity using structured catalysts. Chem. Eng. J. 2017, 327, 1033–1042. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Sanz, O.; Montes, M.; Specchia, S. Insights into the effect of catalyst loading on methane steam reforming and controlling regime for metallic catalytic monoliths. Int. J. Hydrog. Energy 2018, 43, 11778–11792. [Google Scholar] [CrossRef]
- Laguna, O.H.; Castaño, M.G.; Centeno, M.A.; Odriozola, J.A. Effect of the catalytic layer thickness on CuOx/CeO2-coated microchannel reactors for the PROX reaction. Chem. Eng. J. 2015, 275, 45–52. [Google Scholar] [CrossRef]
- Chaparro-Garnica, C.Y.; Jordá-Faus, P.; Bailón-García, E.; Ocampo-Pérez, R.; Aguilar-Madera, C.G.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Customizable heterogeneous catalysts: Nonchanneled advanced monolithic supports manufactured by 3D-printing for improved active phase coating performance. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.F.; Soares, O.S.; Figueiredo, J.L.; Sanz, O.; Montes, M.; Pereira, M.F.R. Preparation of ceramic and metallic monoliths coated with cryptomelane as catalysts for VOC abatement. Chem. Eng. J. 2020, 382, 122923. [Google Scholar] [CrossRef]
- Hayes, R.E. Catalytic solutions for fugitive methane emissions in the oil and gas sector. Chem. Eng. Sci. 2004, 59, 4073–4080. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S. Optimization of compact multitubular fixed-bed reactors for the methanol synthesis loaded with highly conductive structured catalysts. Chem. Eng. J. 2014, 255, 257–265. [Google Scholar] [CrossRef]
- Graaf, G.H.; Sijtsema, P.J.J.M.; Stamhuis, E.J.; Joosten, G.E.H. Chemical equilibria in methanol synthesis. Chem. Eng. Sci. 1986, 41, 2883–2890. [Google Scholar] [CrossRef]
- Bertrand, F.; Devals, C.; Vidal, D.; de Préval, C.S.; Hayes, R.E. Towards the simulation of the catalytic monolith converter using discrete channel-scale models. Catal. Today 2012, 188, 80–86. [Google Scholar] [CrossRef]
- Plachá, M.; Kočí, P.; Isoz, M.; Svoboda, M.; Price, E.; Thompsett, D.; Kallis, K.; Tsolakis, A. Pore-scale filtration model for coated catalytic filters in automotive exhaust gas aftertreatment. Chem. Eng. Sci. 2020, 115854. [Google Scholar] [CrossRef]
- Belot, I.; Vidal, D.; Greiner, R.; Votsmeier, M.; Hayes, R.E.; Bertrand, F. Impact of washcoat distribution on the catalytic performance of gasoline particulate filters as predicted by lattice Boltzmann simulations. Chem. Eng. J. 2020, 406, 127040. [Google Scholar] [CrossRef]
- Ekström, F.; Andersson, B. Pressure Drop of Monolithic Catalytic Converters Experiments and Modeling. SAE Transactions. 2002, 111, 425–433. [Google Scholar]
- White, F. Fluid Mechanics; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Turbulence decay inside the channels of an automotive catalytic converter monolith. Emiss. Control Sci. Technol. 2017, 3, 302–309. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Multiscale RANS-based modeling of the turbulence decay inside of an automotive catalytic converter. Chem. Eng. Sci. 2018, 175, 377–386. [Google Scholar] [CrossRef]
- Achenbach, E. Distribution of local pressure and skin friction around a circular cylinder in cross-flow up to Re = 5 × 106. J. Fluid Mech. 1968, 34, 625–639. [Google Scholar] [CrossRef]
- Achenbach, E. Influence of surface roughness on the cross-flow around a circular cylinder. J. Fluid Mech. 1971, 46, 321–335. [Google Scholar] [CrossRef]
- Quinn, W.R.; Militzer, J. Experimental and numerical study of a turbulent free square jet. Phys. Fluids 1988, 31, 1017–1025. [Google Scholar] [CrossRef]
- Brinkerhoff, J.R.; Yaras, M.I. Direct numerical simulation of a square jet ejected transversely into an accelerating, laminar main flow. Flow Turbul. Combust. 2012, 89, 519–546. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Turbulence generation after a monolith in automotive catalytic converters. Chem. Eng. Sci. 2018, 187, 107–116. [Google Scholar] [CrossRef]
- Hettel, M.; Daymo, E.; Schmidt, T.; Deutschmann, O. CFD-Modeling of fluid domains with embedded monoliths with emphasis on automotive converters. Chem. Eng. Process 2020, 147, 107728. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Effect of substrate geometry and flow condition on the turbulence generation after a monolith. Can. J. Chem. Eng. 2020, 98, 947–956. [Google Scholar] [CrossRef]
- Cornejo, I.; Hayes, R.E.; Nikrityuk, P. A new approach for the modeling of turbulent flows in automotive catalytic converters. Chem. Eng. Res. Des. 2018, 140, 308–319. [Google Scholar] [CrossRef]
- Ergun, S.; Orning, A.A. Fluid flow through randomly packed columns and fluidized beds. Ind. Eng. Chem. 1949, 41, 1179–1184. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Okafor, O.; Sans, V.; Zhang, X.; Ou, X.; Da Fonte, C.P.; Fan, X.; Maestri, M.; Groppi, G.; et al. Investigation of pressure drop in 3D replicated open-cell foams: Coupling CFD with experimental data on additively manufactured foams. Chem. Eng. J. 2019, 377, 120123. [Google Scholar] [CrossRef]
- Shah, R.K. A correlation for laminar hydrodynamic entry length solutions for circular and noncircular ducts. J. Fluids Eng. 1978, 100, 177–179. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Pressure correction for automotive catalytic converters: A multi-zone permeability approach. Chem. Eng. Res. Des. 2019, 147, 232–243. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. The influence of channel geometry on the pressure drop in automotive catalytic converters: Model development and validation. Chem. Eng. Sci. 2020, 212, 115317. [Google Scholar] [CrossRef]
- Batchelor, G. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 1967. [Google Scholar]
- Mesquida, I.M.V.; Cornejo, I.; Nikrityuk, P.; Greiner, R.; Votsmeier, M.; Hayes, R.E. Towards a fully predictive multi-scale pressure drop model for a wall-flow filter. Chem. Eng. Res. Des. 2020, 164, 261–280. [Google Scholar] [CrossRef]
- Quadri, S. The Effect of Oblique Entry into an Automotive Catalyst on the Flow Distribution within the Monolith. Ph.D. Thesis, Coventry University, Coventry, UK, 2008. [Google Scholar]
- Benjamin, S.F.; Haimad, N.; Roberts, C.A.; Wollin, J. Modelling the flow distribution through automotive catalytic converters. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2001, 215, 379–383. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Lange, C.; Hayes, R.E. Influence of upstream turbulence on the pressure drop inside a monolith. Chem. Eng. Process. 2020, 147, 107735. [Google Scholar] [CrossRef]
- Arab, S.; Commenge, J.M.; Portha, J.F.; Falk, L. Methanol synthesis from CO2 and H2 in multi-tubular fixed-bed reactor and multi-tubular reactor filled with monoliths. Chem. Eng. Res. Des. 2014, 92, 2598–2608. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chen, C.; Jia, L.; Hou, B.; Li, D. Effects of macropores on reducing internal diffusion limitations in Fischer–Tropsch synthesis using a hierarchical cobalt catalyst. RSC Adv. 2017, 7, 9436–9445. [Google Scholar] [CrossRef]
- Eckert, E.R.G.; Sakamoto, H.; Simon, T.W. The heat/mass transfer analogy factor, Nu/Sh, for boundary layers on turbine blade profiles. Int. J. Heat Mass Transf. 2001, 44, 1223–1233. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.; Incropera, F.P.; Dewitt, D.P. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Graetz, L. Uber Die Warmeleitungsfahigheit Von Flussingkeiten—Part 1. Ann. Phys. Chem. 1883, 18, 79–94. [Google Scholar]
- Kays, W.; Crawford, M. Convective Heat and Mass Transfer; McGraw-Hill Higher Education: New York, NY, USA, 2004. [Google Scholar]
- Hayes, R.E.; Donoso-Bravo, A.; Mmbaga, J.P. Entry length effects for momentum, heat and mass transfer in circular ducts with laminar flow. Can. J. Chem. Eng. 2015, 93, 863–869. [Google Scholar] [CrossRef]
- Cornejo, I.; Cornejo, G.; Nikrityuk, P.; Hayes, R.E. Entry length convective heat transfer in a monolith: The effect of upstream turbulence. Int. J. Therm. Sci. 2019, 138, 235–246. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Improved Nu number correlations for gas flow in monolith reactors using temperature-dependent fluid properties. Int. J. Therm. Sci. 2020, 155, 106419. [Google Scholar] [CrossRef]
- Bräuer, H.W.; Fetting, F. Stofftransport bei wandreaktion im einlaufgebiet eines strömungsrohres. Chemie Ingenieur Technik 1966, 38, 30–35. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Heat and mass transfer inside of a monolith honeycomb: From channel to full size reactor scale. Catal. Today 2020. [Google Scholar] [CrossRef]
- Hayes, R.E.; Rojas, A.; Mmbaga, J. The effective thermal conductivity of monolith honeycomb structures. Catal. Today 2009, 147, S113–S119. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Continuous vs. discrete models of nonadiabatic monolith catalysts. AIChE J. 1996, 42, 2382–2387. [Google Scholar] [CrossRef]
- Sanz, O.; Velasco, I.; Reyero, I.; Legorburu, I.; Arzamendi, G.; Gandía, L.M.; Montes, M. Effect of the thermal conductivity of metallic monoliths on methanol steam reforming. Catal. Today 2016, 273, 131–139. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Ferreira, C.; Kohler, S. Enabling small-scale methanol synthesis reactors through the adoption of highly conductive structured catalysts. Catal. Today 2013, 215, 176–185. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Mass and heat transfer effects in catalytic monolith reactors. Chem. Eng. Sci. 1994, 49, 3587–3599. [Google Scholar] [CrossRef]
- Leung, D.; Hayes, R.E.; Kolaczkowski, S.T. Diffusion limitation in the washcoat of a catalytic monolith reactor. Can. J. Chem. Eng. 1996, 74, 94–103. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T.; Thomas, W.J.; Titiloye, J. Intraphase diffusion and interphase mass transfer effects during the catalytic oxidation of CO in a tube wall reactor. Proc. Roy. Soc. Lond. 1995, A448, 321–334. [Google Scholar]
- Hayes, R.E.; Kolaczkowski, S.T.; Li, P.K.; Awdry, S. The palladium catalysed oxidation of methane: Reaction kinetics and the effect of diffusion barriers. Chem. Eng. Sci. 2001, 56, 4815–4835. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T.; Li, P.K.; Awdry, S. Evaluating the effective diffusivity of methane in the washcoat of a honeycomb monolith. Appl. Catal. B Environ. 2000, 25, 93–104. [Google Scholar] [CrossRef]
- Zhang, F.; Hayes, R.E.; Kolaczkowski, S.T. A new technique to measure the effective diffusivity in the washcoat of a monolith reactor. Chem. Eng. Res. Des. 2004, 82, 481–489. [Google Scholar] [CrossRef]
- Aris, R. On shape factors for irregular geometries—I. Chem. Eng. Sci. 1957, 6, 262–268. [Google Scholar] [CrossRef]
- Papadias, D.; Edsberg, L.; Björnbom, P. Simplified method for effectiveness factor calculations in irregular geometries of washcoats. Chem. Eng. Sci. 2000, 55, 1447–1459. [Google Scholar] [CrossRef]
- Hayes, R.E.; Liu, B.; Votsmeier, M. Calculating effectiveness factors in non-uniform washcoat shapes. Chem. Eng. Sci. 2005, 60, 2037–2050. [Google Scholar] [CrossRef]
- Nien, T.; Mmbaga, J.P.; Hayes, R.E.; Votsmeier, M. Hierarchical multi-scale model reduction in the simulation of catalytic converters. Chem. Eng. Sci. 2013, 93, 362–375. [Google Scholar] [CrossRef]
- Votsmeier, M. Efficient implementation of detailed surface chemistry into reactor models using mapped rate data. Chem. Eng. Sci. 2009, 64, 1384–1389. [Google Scholar] [CrossRef]
- Votsmeier, M.; Scheuer, A.; Drochner, A.; Vogel, H.; Gieshoff, J. Simulation of automotive NH3 oxidation catalysts based on pre-computed rate data from mechanistic surface kinetics. Catal. Today 2010, 151, 271–277. [Google Scholar] [CrossRef]
- Fadic, A.; Nien, T.; Mmbaga, J.; Hayes, R.; Votsmeier, M. A Case Study in Multi-scale Model Reduction: The Effect of Cell Density on Catalytic Converter Performance. Can. J. Chem. Eng. 2014, 92, 1607–1617. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo, I.; Hayes, R.E. A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors. Catalysts 2021, 11, 89. https://doi.org/10.3390/catal11010089
Cornejo I, Hayes RE. A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors. Catalysts. 2021; 11(1):89. https://doi.org/10.3390/catal11010089
Chicago/Turabian StyleCornejo, Ivan, and Robert E. Hayes. 2021. "A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors" Catalysts 11, no. 1: 89. https://doi.org/10.3390/catal11010089
APA StyleCornejo, I., & Hayes, R. E. (2021). A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors. Catalysts, 11(1), 89. https://doi.org/10.3390/catal11010089





