Hierarchical Mesoporous SSZ-13 Chabazite Zeolites for Carbon Dioxide Capture
Abstract
:1. Introduction
2. Results and Discussion
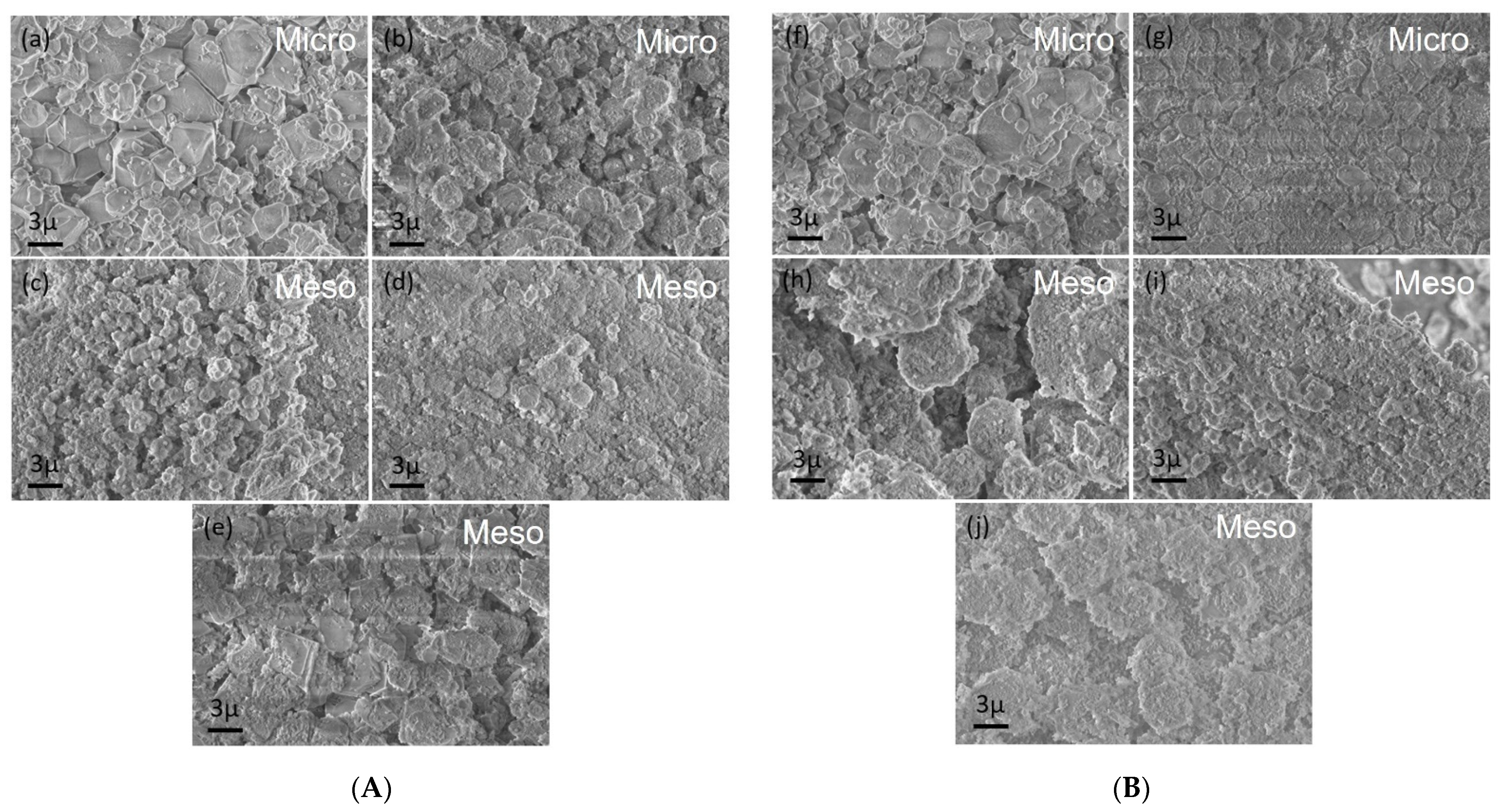
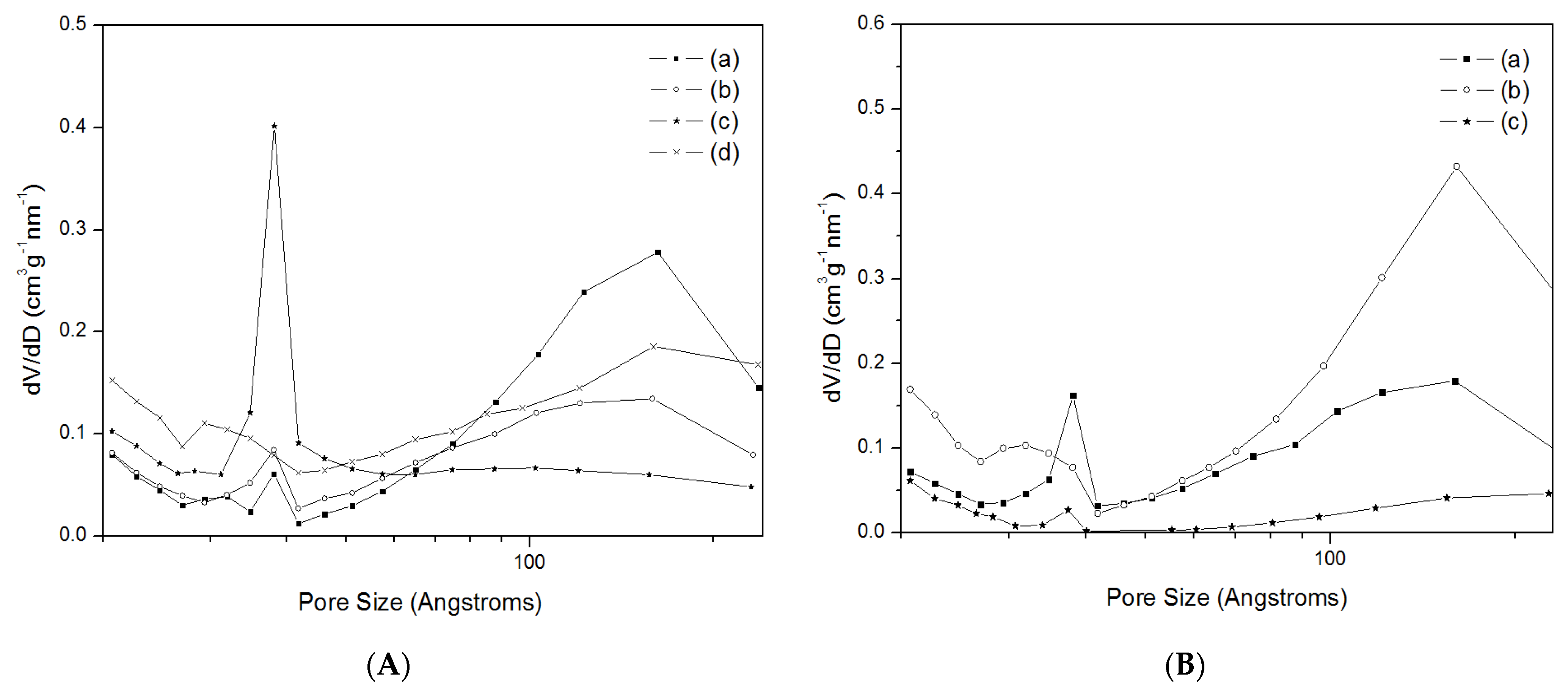
2.1. Characterisation of Materials
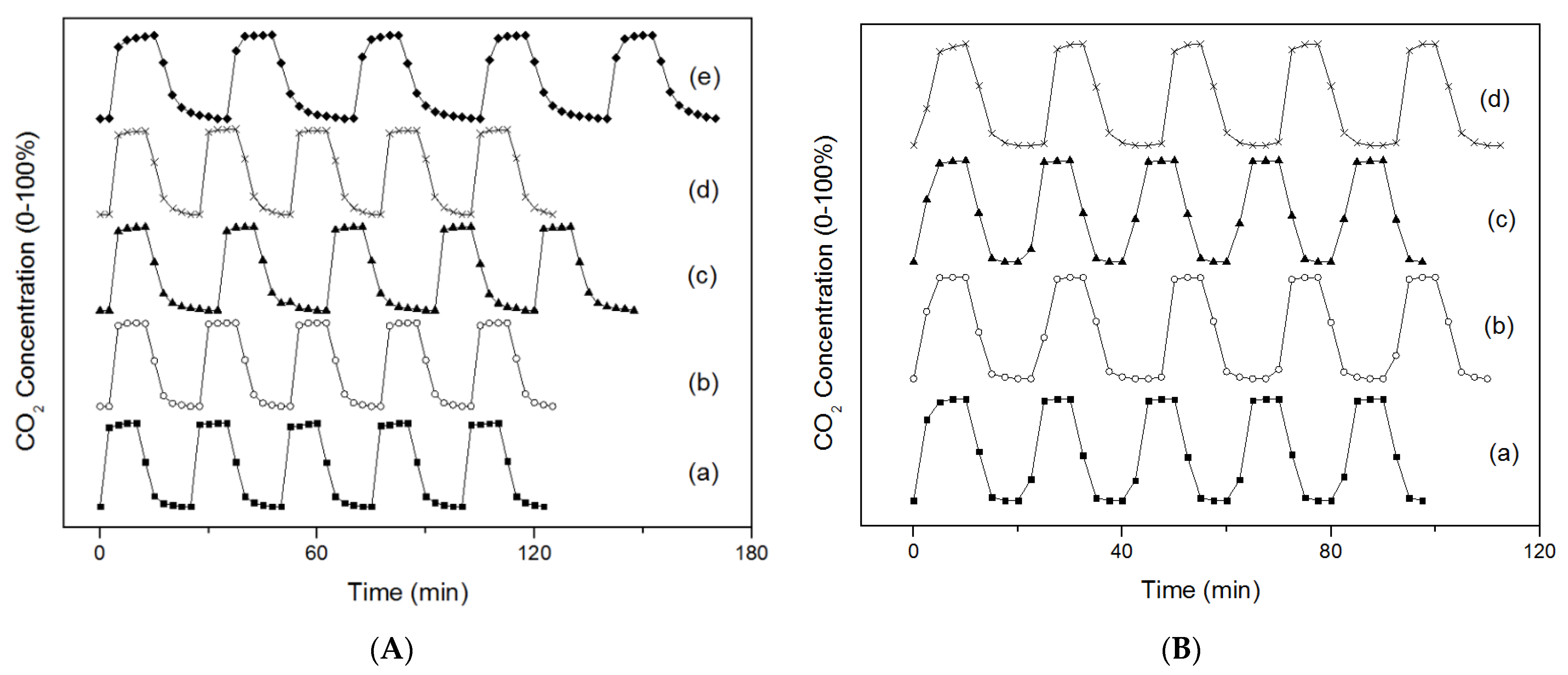
2.2. Breakthrough Curves
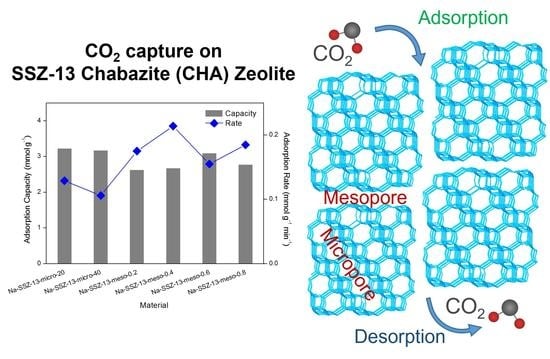
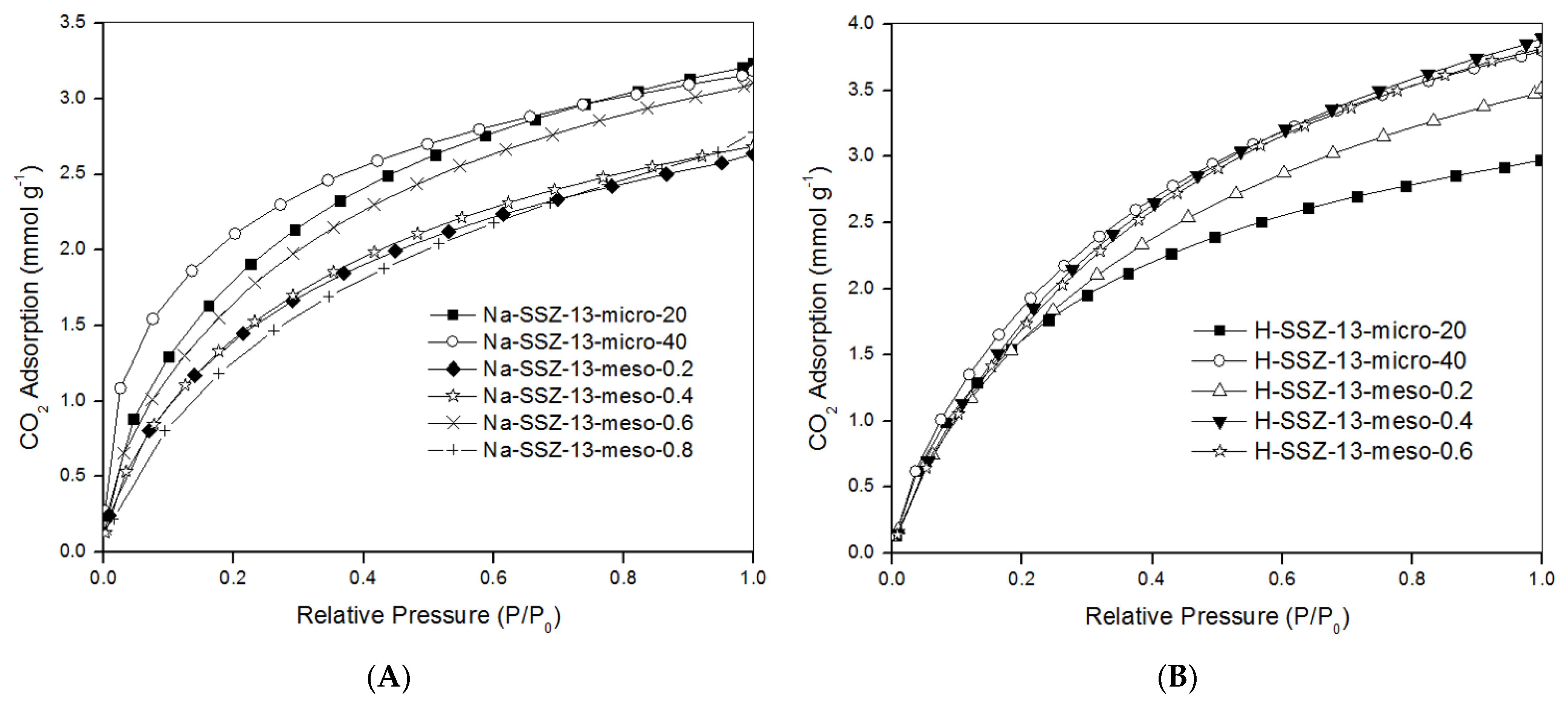
2.3. CO2 Adsorption Isotherms
2.4. Stability of Materials
3. Materials and Methods
3.1. Synthesis of Microporous and Mesoporous SSZ-13
3.2. Characterisation
3.3. Cycling Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef] [Green Version]
- Ram, M.; Child, M.; Aghahosseini, A.; Bogdanov, D.; Lohrmann, A.; Breyer, C. A comparative analysis of electricity generation costs from renewable, fossil fuel and nuclear sources in G20 countries for the period 2015–2030. J. Clean. Prod. 2018, 199, 687–704. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Proced. 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef] [Green Version]
- García, S.; Gil, M.V.; Martín, C.F.; Pis, J.J.; Rubiera, F.; Pevida, C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem. Eng. J. 2011, 171, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pu, Q.; Ning, P.; Lu, S. Activated carbon-based composites for capturing CO2: A review. Greenh. Gases. 2021, 11, 377–393. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal–Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energ. Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuel 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R.; Miccio, F. Fixed bed adsorption as affected by thermodynamics and kinetics: Yellow tuff for CO2 capture. Powder Technol. 2020, 373, 446–458. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on Reactor Configurations for Adsorption-Based CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Dhoke, C.; Cloete, S.; Krishnamurthy, S.; Seo, H.; Luz, I.; Soukri, M.; Park, Y.-k.; Blom, R.; Amini, S.; Zaabout, A. Sorbents screening for post-combustion CO2 capture via combined temperature and pressure swing adsorption. Chem. Eng. J. 2020, 380, 122201. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Q.; Xu, H.; Li, L.; Dong, J.; Li, J. Adsorption of CO2, CH4, and N2 on Gas Diameter Grade Ion-Exchange Small Pore Zeolites. J. Chem. Eng. Data 2012, 57, 3701–3709. [Google Scholar] [CrossRef]
- Merel, J.; Clausse, M.; Meunier, F. Experimental Investigation on CO2 Post-Combustion Capture by Indirect Thermal Swing Adsorption Using 13X and 5A Zeolites. Ind. Eng. Chem. Res. 2008, 47, 209–215. [Google Scholar] [CrossRef]
- Pham, T.D.; Liu, Q.; Lobo, R.F. Carbon dioxide and nitrogen adsorption on cation-exchanged SSZ-13 zeolites. Langmuir 2013, 29, 832–839. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, R.; Webley, P.A. Alkali and alkaline-earth cation exchanged chabazite zeolites for adsorption based CO2 capture. Micropor. Mesopor. Mat. 2008, 111, 478–487. [Google Scholar] [CrossRef]
- Hudson, M.R.; Queen, W.L.; Mason, J.A.; Fickel, D.W.; Lobo, R.F.; Brown, C.M. Unconventional, Highly Selective CO2 Adsorption in Zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; da Silva, F.W.M.; de Moura, P.A.S.; Sousa, A.G.S.; Vieira, R.S.; Rodriguez-Castellon, E.; Azevedo, D.C.S. CO2 adsorption in amine-grafted zeolite 13X. Appl. Surf. Sci. 2014, 314, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, Y.; Shimomura, M.; Endo, A. CO2 adsorption–desorption properties of zeolite beta prepared from OSDA-free synthesis. Micropor. Mesopor. Mat. 2016, 219, 125–133. [Google Scholar] [CrossRef]
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Green Energy 2015, 40, 126–166. [Google Scholar] [CrossRef] [Green Version]
- Hillen, L.; Degirmenci, V. Synthesis Methods for the Production of Hierarchically Mesoporous and Microporous Zeolites. Rev. Adv. Sci. Eng. 2015, 4, 147–162. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Degirmenci, V.; Hensen, E.J.M. Dry gel conversion of organosilane templated mesoporous silica: From amorphous to crystalline catalysts for benzene oxidation. J. Mater. Chem. 2011, 21, 9279–9289. [Google Scholar] [CrossRef]
- Degirmenci, V.; Hensen, E.J.M. Development of a Heterogeneous Catalyst for Lignocellulosic Biomass Conversion: Glucose Dehydration by Metal Chlorides in a Silica-Supported Ionic Liquid Layer. Environ. Prog. Sustain. Energy 2014, 33, 657–662. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Tempelman, C.H.L.; Degirmenci, V.; Guo, M.; Feng, Z.; Li, C.; Hensen, E.J.M. Hierarchical zeolites prepared by organosilane templating: A study of the synthesis mechanism and catalytic activity. Catal. Today 2011, 168, 96–111. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, W.-S. CO2 adsorption on LTA zeolites: Effect of mesoporosity. Appl. Surf. Sci. 2014, 311, 107–109. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Lousberg, N.J.H.G.M.; Hensen, E.J.M. Mesoporous SSZ-13 zeolite prepared by a dual-template method with improved performance in the methanol-to-olefins reaction. J. Catal. 2013, 298, 27–40. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Szyja, B.M.; Hensen, E.J.M. Dual template synthesis of a highly mesoporous SSZ-13 zeolite with improved stability in the methanol-to-olefins reaction. Chem. Commun. 2012, 48, 9492–9494. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Sjostrom, S.; Krutka, H. Evaluation of solid sorbents as a retrofit technology for CO2 capture. Fuel 2010, 89, 1298–1306. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef] [Green Version]
- Zones, S.I.; Hwang, S.-J.; Davis, M.E. Studies of the Synthesis of SSZ-25 Zeolite in a “Mixed-Template” System. Chem. Eur. J. 2001, 7, 1990–2001. [Google Scholar] [CrossRef]
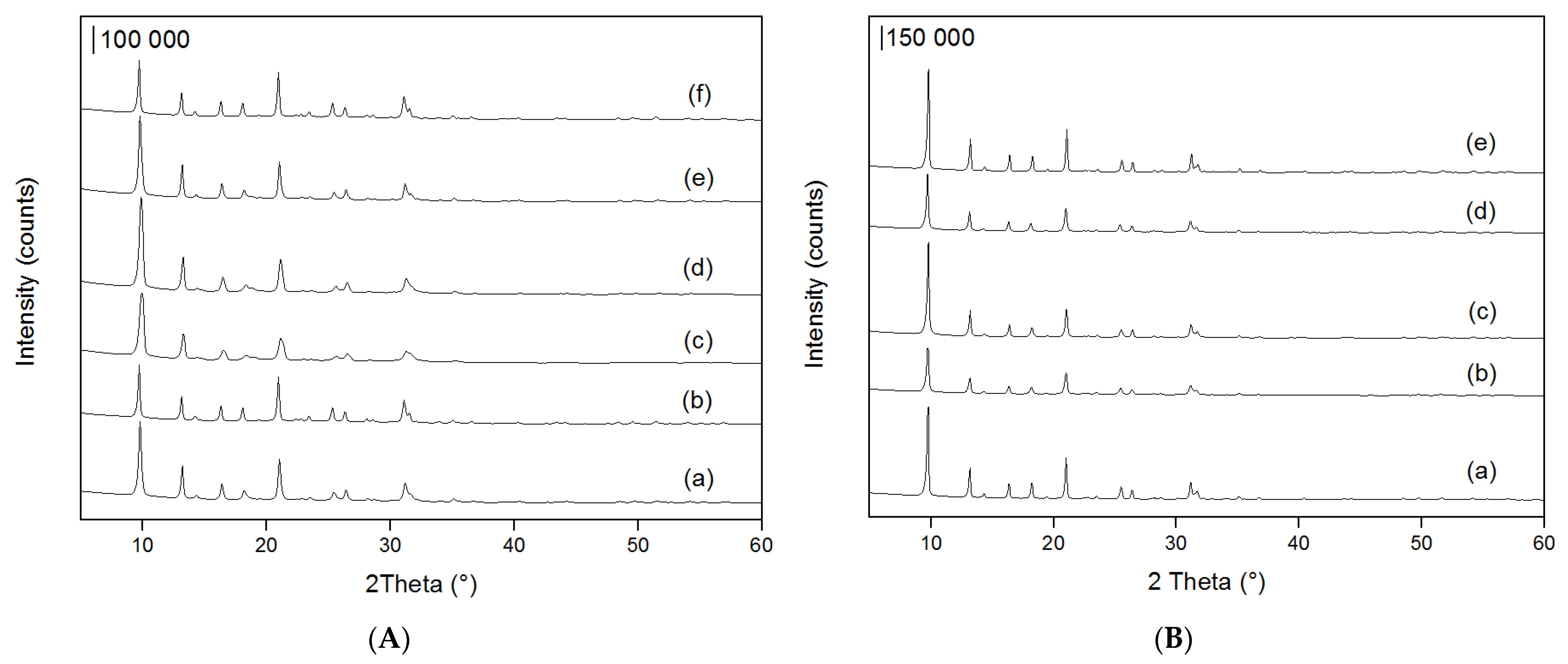



| Material | FWHM 1 | Material | FWHM 1 |
|---|---|---|---|
| Na-SSZ-13-micro-20 | 0.25 | H-SSZ-13-micro-20 | 0.19 |
| Na-SSZ-13-micro-40 | 0.18 | H-SSZ-13-micro-40 | 0.23 |
| Na-SSZ-13-meso-0.2 | 0.42 | H-SSZ-13-meso-0.2 | 0.18 |
| Na-SSZ-13-meso-0.4 | 0.33 | H-SSZ-13-meso-0.4 | 0.20 |
| Na-SSZ-13-meso-0.6 | 0.26 | H-SSZ-13-meso-0.6 | 0.17 |
| Na-SSZ-13-meso-0.8 | 0.19 | - | - |
| Material | BET Surface Area (m2g−1) | Micropore Volume (cm3g−1) | Mesopore Volume (cm3g−1) | Total Pore Volume (cm3g−1) |
|---|---|---|---|---|
| Na-SSZ-13-micro-20 | 594 | 0.246 | - | 0.246 |
| Na-SSZ-13-meso-0.2 | 497 | 0.177 | 0.157 | 0.334 |
| Na-SSZ-13-meso-0.4 | 498 | 0.181 | 0.105 | 0.286 |
| Na-SSZ-13-meso-0.6 | 567 | 0.204 | 0.097 | 0.301 |
| Na-SSZ-13-meso-0.8 | 807 | - | 0.165 | 0.165 |
| H-SSZ-13-micro-40 | 679 | 0.282 | - | 0.230 |
| H-SSZ-13-meso-0.2 | 636 | 0.240 | 0.125 | 0.365 |
| H-SSZ-13-meso-0.4 | 655 | 0.251 | 0.142 | 0.393 |
| H-SSZ-13-meso-0.6 | 735 | 0.298 | 0.035 | 0.333 |
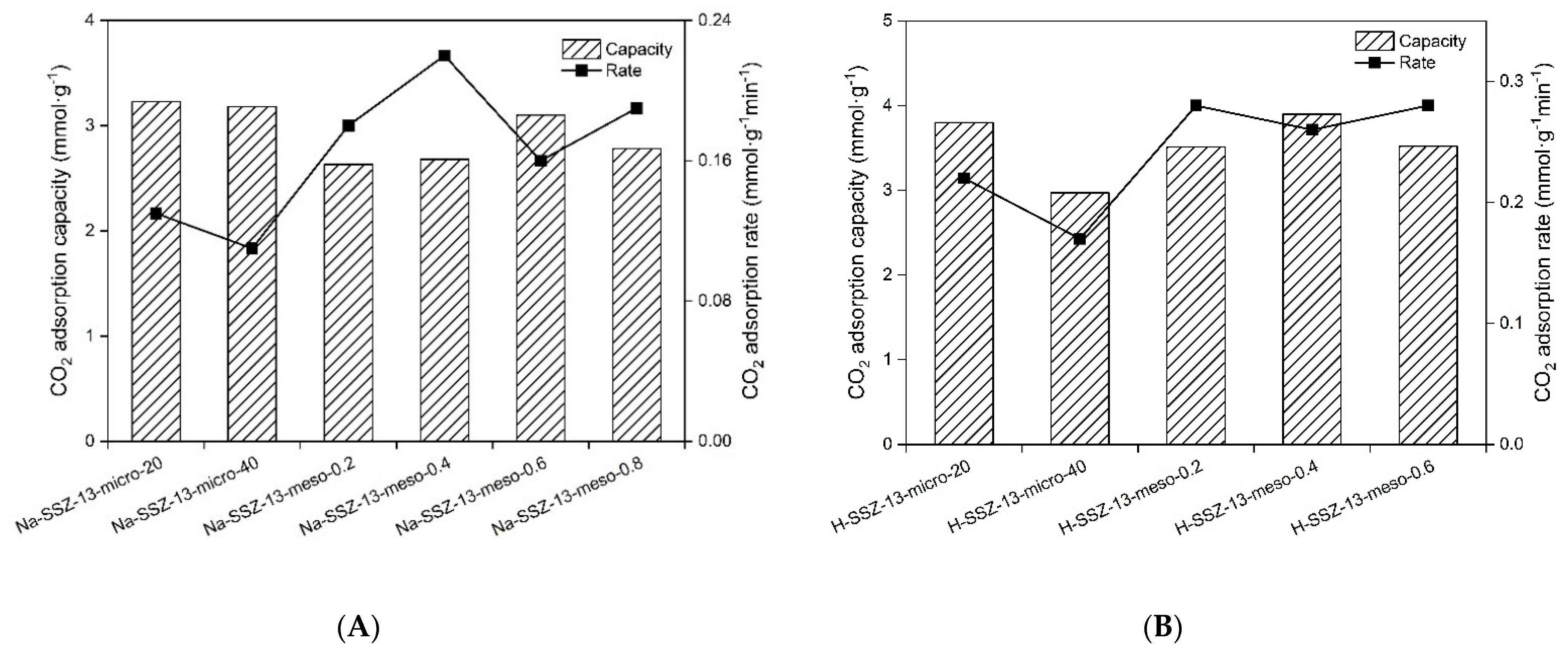
| Material | K | Vmax (cm3g−1) | R2 | CO2 Capacity (mmol·g−1) | CO2 Adsorption Rate (mmol·g−1min−1) |
|---|---|---|---|---|---|
| Na-SSZ-13-micro-20 | 17.95 | 72.56 | 0.9920 | 3.23 | 0.13 |
| Na-SSZ-13-micro-40 | 13.28 | 71.36 | 0.9922 | 3.18 | 0.11 |
| Na-SSZ-13-meso-0.2 | 15.77 | 59.05 | 0.9945 | 2.63 | 0.18 |
| Na-SSZ-13-meso-0.4 | 15.28 | 60.13 | 0.9850 | 2.68 | 0.22 |
| Na-SSZ-13-meso-0.6 | 16.98 | 69.52 | 0.9800 | 3.10 | 0.16 |
| Na-SSZ-13-meso-0.8 | 18.72 | 62.33 | 0.9868 | 2.78 | 0.19 |
| H-SSZ-13-micro-20 | 23.14 | 85.15 | 0.9818 | 3.80 | 0.22 |
| H-SSZ-13-micro-40 | 17.02 | 66.64 | 0.9912 | 2.97 | 0.17 |
| H-SSZ-13-meso-0.2 | 23.40 | 78.65 | 0.9881 | 3.51 | 0.28 |
| H-SSZ-13-meso-0.4 | 26.35 | 87.29 | 0.9857 | 3.90 | 0.26 |
| H-SSZ-13-meso-0.6 | 25.87 | 85.47 | 0.9843 | 3.52 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillen, L.; Degirmenci, V. Hierarchical Mesoporous SSZ-13 Chabazite Zeolites for Carbon Dioxide Capture. Catalysts 2021, 11, 1355. https://doi.org/10.3390/catal11111355
Hillen L, Degirmenci V. Hierarchical Mesoporous SSZ-13 Chabazite Zeolites for Carbon Dioxide Capture. Catalysts. 2021; 11(11):1355. https://doi.org/10.3390/catal11111355
Chicago/Turabian StyleHillen, Lucy, and Volkan Degirmenci. 2021. "Hierarchical Mesoporous SSZ-13 Chabazite Zeolites for Carbon Dioxide Capture" Catalysts 11, no. 11: 1355. https://doi.org/10.3390/catal11111355
APA StyleHillen, L., & Degirmenci, V. (2021). Hierarchical Mesoporous SSZ-13 Chabazite Zeolites for Carbon Dioxide Capture. Catalysts, 11(11), 1355. https://doi.org/10.3390/catal11111355