In Situ Growth of ZIF-8 Nanocrystals on the Pore Walls of 3D Ordered Macroporous TiO2 for a One-Pot Cascade Reaction
Abstract
:1. Introduction
2. Results and Discussion
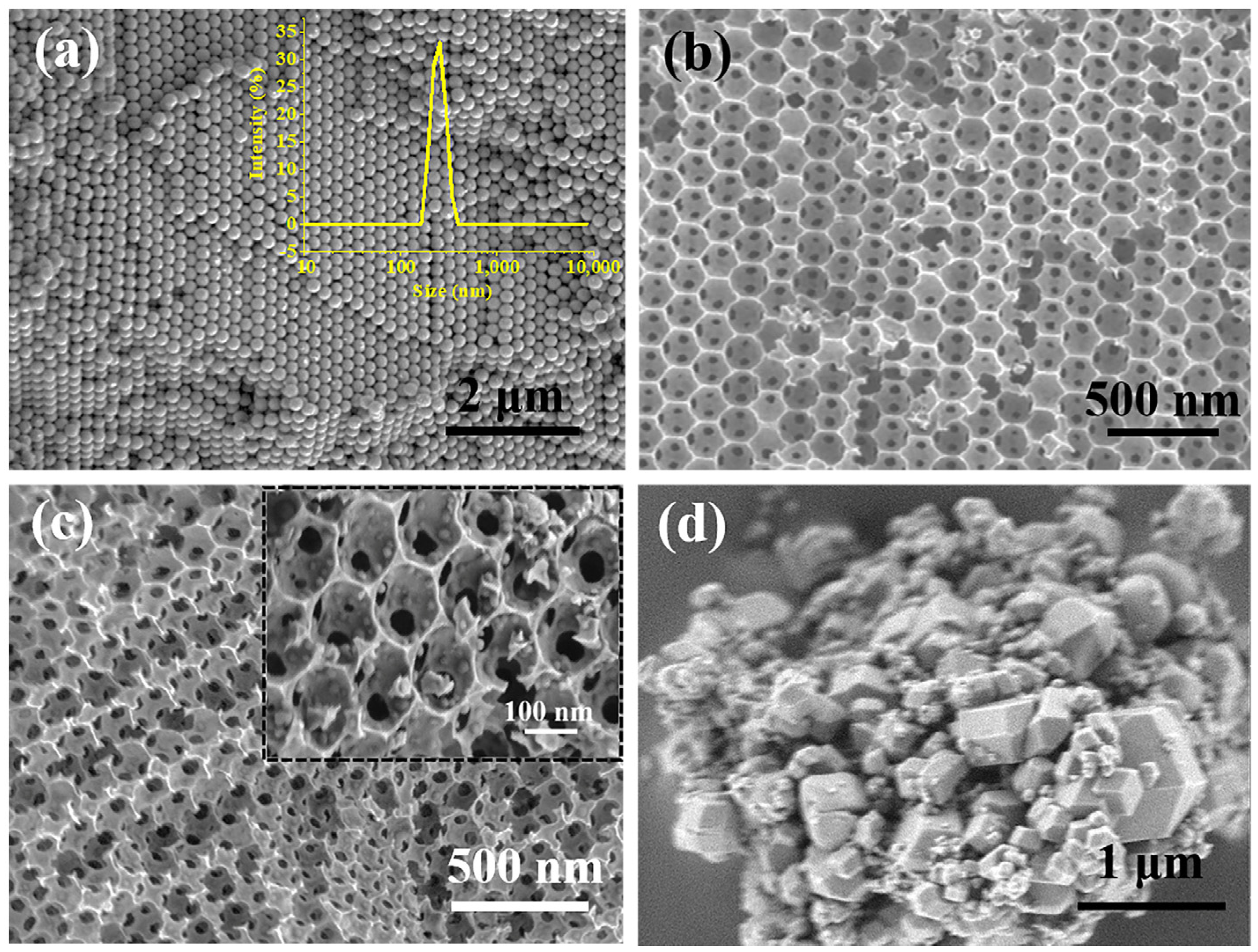
2.1. Characterization of the Microscopic Morphology of Catalyst
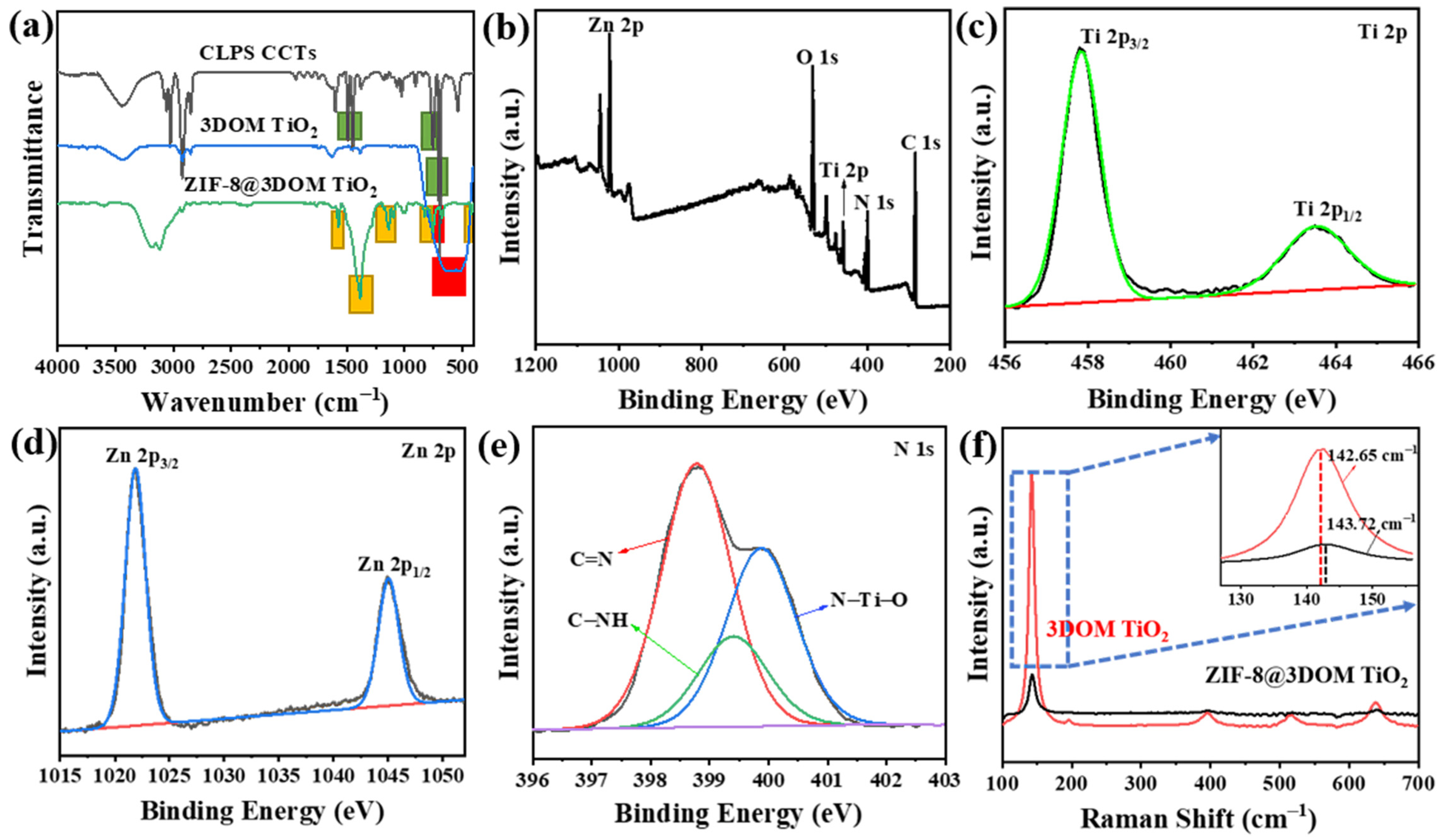
2.2. Characterization of the Catalyst Composition
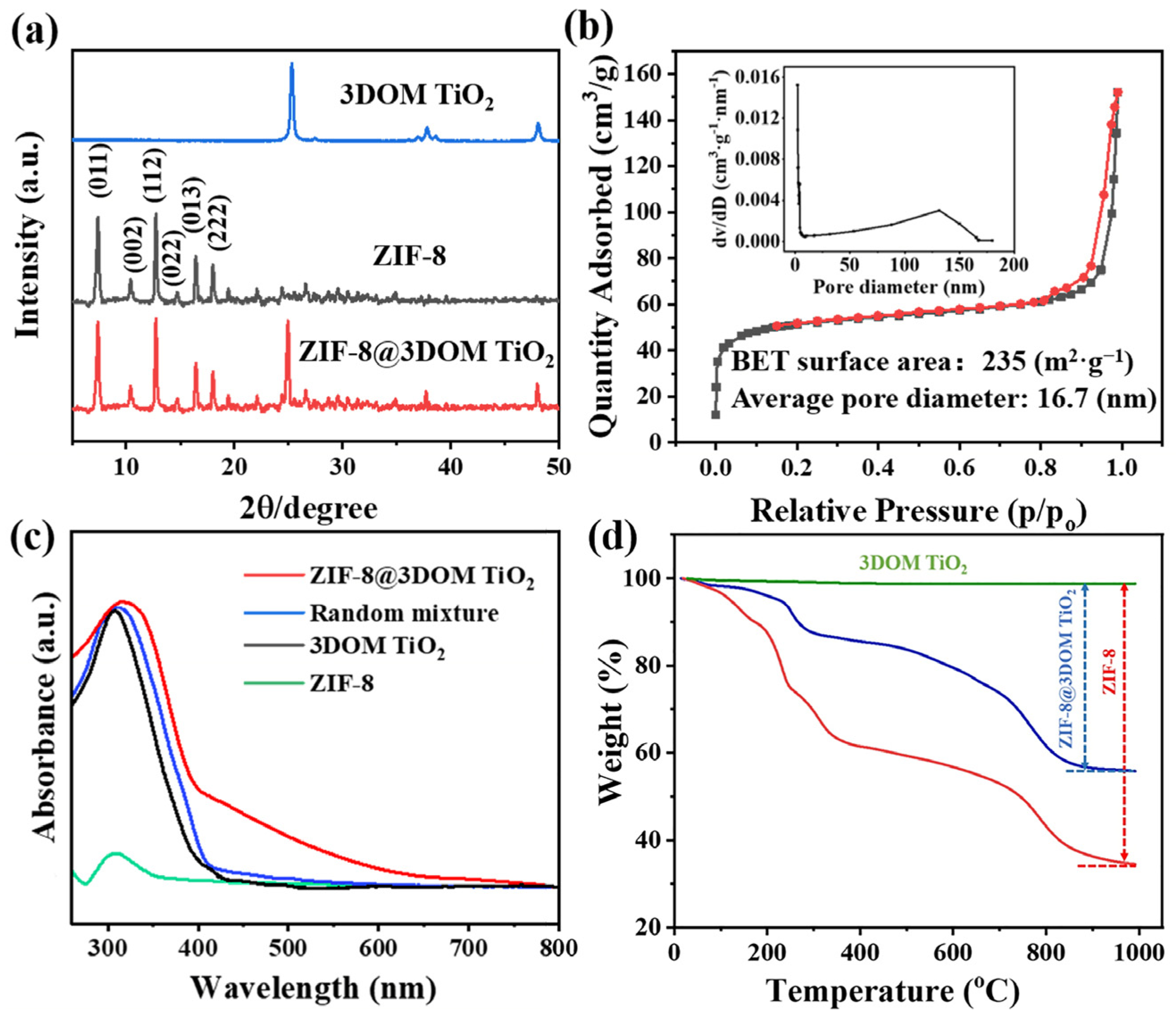
2.3. Characterization of Catalyst Crystal Phase, Nanoporous Structure, Optical Properties, and Thermal Stability
2.4. Analysis of Catalytic Performance
2.5. Catalyst Stability Test and Explanation of Catalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of 3DOM TiO2
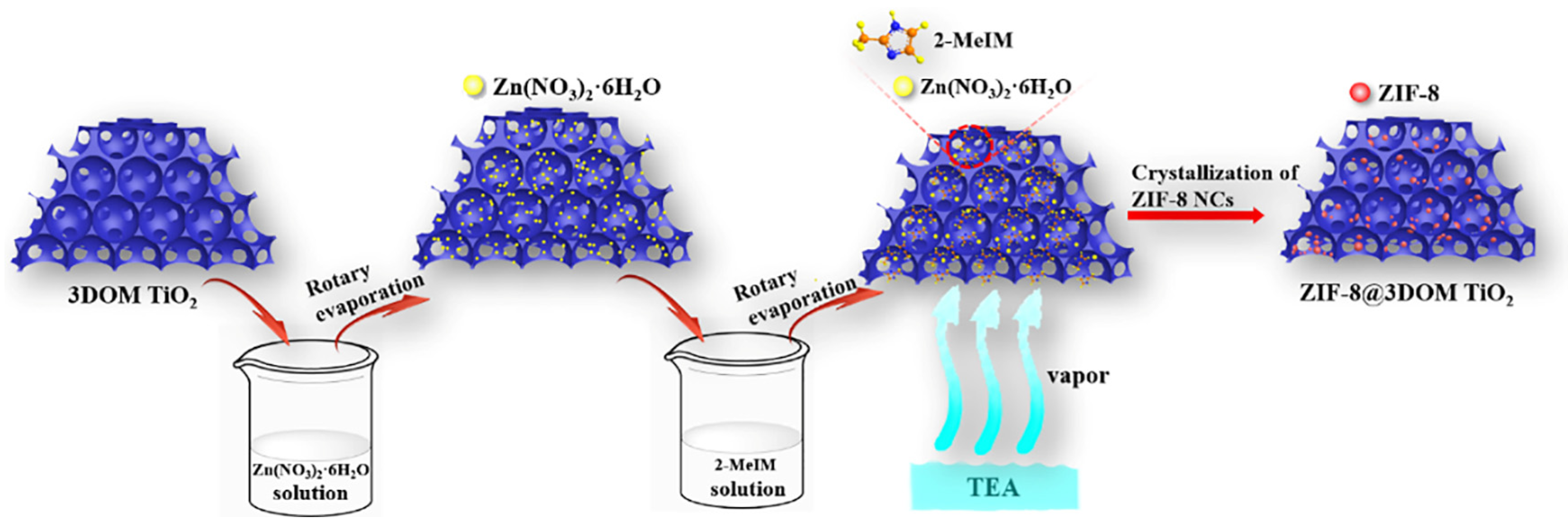
3.3. Preparation of ZIF-8@3DOM TiO2
3.4. Catalytic Applications in a Cascade Reaction
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Chen, Z.J.; Liu, X.Y. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Ejsmont, A.; Freund, R. Controlling the morphology of metal-organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.T.; Huang, Y.; Peng, Y.W.; Huang, Z.Q.; Ma, Q.L. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Gimferrer, M.; Poater, A. Covalent and ionic capacity of MOFs to sorb small gas molecules. Inorg. Chem. 2018, 57, 6981–6990. [Google Scholar] [CrossRef]
- Meng, J.S.; Liu, X.; Niu, C.J.; Pang, Q. Advances in metal-organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef] [PubMed]
- Kokcam-Demir, U.; Goldman, A.; Esrafili, L.; Gharib, M.; Morsali, A. Coordinatively unsaturated metal sites (open metal sites) in metal-organic frameworks: Design and applications. Chem. Soc. Rev. 2020, 49, 2751–2798. [Google Scholar] [CrossRef]
- Troyano, J.; Carne-Sanchez, A.; Avci, C.; Imaz, I. Colloidal metal-organic framework particles: The pioneering case of ZIF-8. Chem. Soc. Rev. 2019, 48, 5534–5546. [Google Scholar] [CrossRef]
- Cao, X.H.; Tan, C.L.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal-organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef]
- Bui, T.T.; Cuong, N.D.; Kim, Y.S. In situ growth of microporous ZIF-8 nanocrystals on a macroporous phyllosilicate mineral. Mater. Lett. 2018, 212, 69–72. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, S.; Xue, H. Metal-organic framework composites and their electrochemical applications. J. Mater. Chem. A 2019, 7, 7301–7327. [Google Scholar] [CrossRef]
- Guo, Y.C.; Feng, L.; Wu, C.; Zhang, X. Synthesis of 3D ordered macro/microporous yolk-shelled nanoreactor with spatially separated functionalities for cascade reaction. ACS Appl. Mater. Interfaces 2019, 11, 33978–33986. [Google Scholar] [CrossRef]
- Li, S.; Huo, F. Metal-organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar]
- Huang, H.M.; Garduno-castro, M.H.; Morrill, C. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626–4638. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, H.Y.; Li, X.Y.; Wu, X.T.; Li, H.X. Amine-functionalized GO as an active and reusable acid-base bifunctional catalyst for one-pot cascade reactions. ACS Catal. 2014, 4, 394–401. [Google Scholar] [CrossRef]
- Pologarzon, F.; Wu, Z.L. Acid-base catalysis over perovskites: A review. J. Mater. Chem. 2018, 6, 2877–2894. [Google Scholar] [CrossRef]
- Liu, H.; Xi, F.G.; Sun, W.; Yang, N.N.; Gao, E.Q. Amino and sulfo-bifunctionalized metal-organic frameworks: One-pot tandem catalysis and the catalytic sites. Inorg. Chem. 2016, 55, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Lu, J.; Weck, M.; Jones, C.W. Acid-base bifunctional shell cross-linked micelle nanoreactor for one-pot tandem reaction. ACS Catal. 2016, 6, 784–787. [Google Scholar] [CrossRef]
- Choluj, A.; Krzesinski, P.; Ruszczynska, A.; Bulska, E.; Kajetanowicz, A. Noncovalent immobilization of cationic ruthenium complex in a metal-organic framework by ion exchange leading to a heterogeneous olefin metathesis catalyst for use in green solvents. Organometallics 2019, 38, 3397–3405. [Google Scholar] [CrossRef]
- Valeroromero, M.J.; Santaclara, J.G.; Oararteta, L.; Vankoppen, L.; Osadchii, D.Y.; Gascon, J.; Kapteijn, F. Photocatalytic properties of TiO2 and Fe-doped TiO2 prepared by metal organic framework-mediated synthesis. Chem. Eng. J. 2019, 360, 75–88. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Zou, R.Q. Metal-organic framework-based catalysts with single metal sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Wang, J.S.; Jin, F.Z.; Ma, H.C.; Li, X.B.; Liu, M.Y.; Kan, J.L.; Chen, G.J.; Dong, Y.B. Au@Cu(II)-MOF: Highly efficient bifunctional heterogeneous catalyst for successive oxidation-condensation reactions. Inorg. Chem. 2016, 55, 6685–6691. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, B.B.; Xu, M.; Mao, G.B. Integration of nanosized ZIF-8 particles onto mesoporous TiO2 nanobeads for enhanced photocatalytic activity. RSC Adv. 2017, 7, 8004–8010. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Huang, L.Q.; Wang, C.N.; Wang, J.S.; Li, J.N.; Luo, X.T. Sonocrystallization of ZIF-8 on electrostatic spinning TiO2 nanofibers surface with enhanced photocatalysis property through synergistic effect. ACS. Appl. Mater. Interfaces 2016, 8, 20274–20282. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B-Environ. 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Jia, A. A novel bifunctional Pd-ZIF-8/rGO catalyst with spatially separated active sites for the tandem Knoevenagel condensation-reduction reaction. Catal. Sci. Technol. 2017, 7, 5572–5584. [Google Scholar]
- Bian, Z.F.; Zhu, J.; Wang, S.H.; Cao, Y.; Qian, X.F.; Li, H.X. Self-assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase. J. Phys. Chem. C 2008, 112, 6258–6262. [Google Scholar] [CrossRef]
- Pi, H.T.; Zhang, D.T.; Zhang, X.M.; Jin, Z.; Zhang, L.; Cui, X.Q.; Zheng, W.T. Passivation of the surface imperfection of TiO2 by using ZIF-8 for efficient carrier separation/transfer. Dalton Trans. 2018, 47, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; He, M.Y.; Ge, J.P. Controlled synthesis of Fe3O4/ZIF-8 nanoparticles for magnetically separable nanocatalysts. Chemisrty 2015, 21, 6879–6887. [Google Scholar] [CrossRef]
- Jian, M.P.; Liu, B.; Zhang, G.S.; Liu, R.P.; Zhang, X.W. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS. Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Yu, H.J.; Zhao, Y.F.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.H.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Zalfani, M.; Der Schueren, B.V.; Hu, Z.; Rooke, J.C.; Bourguiga, R.; Wu, M.; Li, Y.; Van Tendeloo, G.; Su, B. Novel 3DOM BiVO4/TiO2 nanocomposites for highly enhanced photocatalytic activity. J. Mater. Chem. 2015, 3, 21244–21256. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y.H. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Liu, Q.; Wang, K.; Chen, F.Y.; Wang, H.T. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Micropor. Mesopor. Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Elinson, M.N.; Feducovich, S.K.; Stepanov, N.O.; Vereshchagin, A.N.; Nikishin, G. A new strategy of the chemical route to the cyclopropane structure: Direct transformation of benzylidenemalononitriles and malononitrile into 1,1,2,2-tetracyanocyclopropanes. Tetrahedron 2008, 64, 708–713. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, Z.; Lang, X.J. Merging visible light photocatalysis of dye-sensitized TiO2 with TEMPO: The selective aerobic oxidation of alcohols. Catal. Sci. Technol. 2017, 7, 4955–4963. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Higashimoto, S.; Okada, K.; Azuma, M. Characteristics of the charge transfer surface complex on titanium (iv) dioxide for the visible light induced chemo-selective oxidation of benzyl alcohol. RSC Adv. 2012, 2, 669–676. [Google Scholar] [CrossRef]
- Yan, P.Y.; Zhang, X.C.; Wang, X.M.; Zhang, X. Controllable preparation of monodisperse mesoporous silica from microspheres to microcapsules and catalytic loading of Au nanoparticles. Langmuir 2020, 36, 5271–5279. [Google Scholar] [CrossRef] [PubMed]


| Sample | Element * (%) | ||
|---|---|---|---|
| N | C | H | |
| ZIF-8@3DOM TiO2 | 17.14 | 27.57 | 3.82 |
| ZIF-8 | 28.33 | 45.52 | 6.19 |
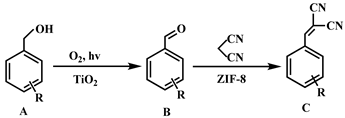
| Entry | Substrates A | Products C | ZIF-8@3DOM TiO2 [a] | Random Mixtures [b] | 3DOM TiO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conv. of A (%) | Yield of B (%) | Yield of C (%) | Conv. of A (%) | Yield of B (%) | Yield of C (%) | Conv. of A (%) | Yield of B (%) | Yield of C (%) | |||
| 1 | 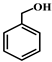 | 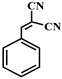 | 98.0 | trace | 98.0 | 90.0 | 2.0 | 88.0 | 58.0 | 58.0 | 0 |
| 2 | 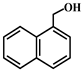 |  | 80.1 | 9.0 | 71.1 | 74.0 | 23.0 | 51.0 | 50.1 | 50.1 | 0 |
| 3 | 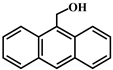 | 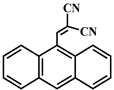 | 73.0 | 13.0 | 60.0 | 64.8 | 30.0 | 34.8 | 40.0 | 40.0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Guo, Y.; Kang, T.; Liu, X.; Wang, X.; Zhang, X. In Situ Growth of ZIF-8 Nanocrystals on the Pore Walls of 3D Ordered Macroporous TiO2 for a One-Pot Cascade Reaction. Catalysts 2021, 11, 533. https://doi.org/10.3390/catal11050533
Chen J, Guo Y, Kang T, Liu X, Wang X, Zhang X. In Situ Growth of ZIF-8 Nanocrystals on the Pore Walls of 3D Ordered Macroporous TiO2 for a One-Pot Cascade Reaction. Catalysts. 2021; 11(5):533. https://doi.org/10.3390/catal11050533
Chicago/Turabian StyleChen, Jing, Yingchun Guo, Tengteng Kang, Xingchi Liu, Xiaomei Wang, and Xu Zhang. 2021. "In Situ Growth of ZIF-8 Nanocrystals on the Pore Walls of 3D Ordered Macroporous TiO2 for a One-Pot Cascade Reaction" Catalysts 11, no. 5: 533. https://doi.org/10.3390/catal11050533








