Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
3.2. HDB Procedures
3.2.1. HDB of Brominated Phenols Using Studied Al Alloys, Electropositive Metals or Cu-Based Catalysts and NaBH4
3.2.2. HDB of Brominated Phenols Using In Situ Generated Cu/Cu2O
3.2.3. HDB Kinetic Experiments
3.3. Evaluation of Kinetics of the HDB of BRX)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, W.; Liu, Y. C–H bond halogenation catalyzed or mediated by copper: An overview. Beilstein J. Org. Chem. 2015, 11, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- Yasamut, K.; Jongcharoenkamol, J.; Ruchirawat, S.; Ploypradith, P. A modified Cu(0)-Cu(I)-mediated Caryl-Caryl Ullmann coupling for the synthesis of biaryls. Tetrahedron 2016, 72, 5994–6000. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Arai, Y.; Fu, X.; Li, X.; Huang, W. Kinetics and mechanisms of debromination of tetrabromobisphenol A by Cu coated nano zerovalent iron. Chem. Eng. J. 2019, 373, 95–103. [Google Scholar] [CrossRef]
- Weidlich, T. The influence of copper on halogenation/dehalogenation reactions of aromatic compounds and its role in the destruction of polyhalogenated aromatic contaminants. Catalysts 2021, 11, 378. [Google Scholar] [CrossRef]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Müller, F.; Applebyki, A.P. Weed Control, 2. Individual Herbicides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-WCH: Weinheim, Germany, 2012; Volume 39, pp. 209–385. [Google Scholar]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef]
- Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; Marques, D.C.; et al. Peer review of the pesticide risk assessment of the active substance bromoxynil (variant evaluated bromoxynil octanoate). J. EFSA 2017, 15, 24. [Google Scholar]
- Holtze, M.S.; Sørensen, S.R.; Sørensen, J.; Aamand, J. Microbial degradation of benzonitrile herbicides dichlobenil, bromoxynil and ioxynil in soil and subsurface environments–insights into degradation pathways, persistent metabolites and involved degrader organisms. Environ. Pollut. 2008, 154, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Knossow, N.; Siebner, H.; Bernstein, A. Isotope analysis method for the herbicide bromoxynil and its application to study photo-degradation processes. J. Hazard. Mater. 2020, 388, 122036. [Google Scholar] [CrossRef]
- Palma, P.; Kuster, M.; Alvarenga, P.; Palma, V.L.; Fernandes, R.M.; Soares, A.M.V.M.; de Alda, M.J.L.; Barcelo, D.; Barbosa, I.R. Risk assessment of representative and priority pesticides, in surface water of the Alqueva reservoir (South of Portugal) using on-line solid phase extraction–liquid chromatography–tandem mass spectrometry. Environ. Int. 2009, 35, 545–551. [Google Scholar] [CrossRef]
- Maddila, S.; Lavanya, P.; Jonnalagadda, S.B. Degradation, mineralization of bromoxynil pesticide by heterogeneous photocatalytic ozonation. J. Ind. Eng. Chem. 2015, 24, 333–341. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, D.; Qiao, J.; Ju, Y.; Chen, Y.; Dionysiou, D.D. New insight into the cosolvent effect on the degradation of tetrabromobisphenol A (TBBPA) over millimeter-scale palladised sponge iron (Pd-s-Fe0) particles. Chem. Eng. J. 2019, 361, 1423–1436. [Google Scholar] [CrossRef]
- Iordache, I.; Wilson, S.; Lundanes, E.; Iordache, M.; Pavel, V.L.; Aelenei, N. The Fenton and sono-Fenton processes applied for pesticide degradation. Environ. Eng. Manag. J. 2010, 9, 519–525. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Degradation of bromoxynil and trifluralin in natural water by direct photolysis and UV plus H2O2 advanced oxidation process. Water Res. 2010, 44, 2221–2228. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Kinetics and mechanism of the degradation of two pesticides in aqueous solutions by ozonation. Chemosphere 2010, 78, 557–562. [Google Scholar] [CrossRef]
- Bououden, Z.; Halladja, S.; Sleiman, M.; Leremboure, M.; Richard, C. Mechanism of bromoxynil phototransformation: Effect of medium and surfactant. J. Photochem. Photobiol. A Chem. 2018, 365, 151–156. [Google Scholar] [CrossRef]
- Horikoshi, S.; Miura, T.; Kajitani, M.; Horikoshi, N.; Serpone, N. Photodegradation of tetrahalobisphenol-A (X=Cl, Br) flame retardants and delineation of factors affecting the process. Appl. Catal. B 2008, 84, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, L.; Senthilkumar, K. Reaction mechanism and kinetics of the degradation of bromoxynil initiated by OH radical. RSC Adv. 2014, 4, 7749. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Shen, J.; Wang, M.; Yang, Q.; Zheng, M. Environmental characteristics and formations of polybrominated dibenzo-p-dioxins and dibenzofurans. Environ. Int. 2021, 152, 106450. [Google Scholar] [CrossRef] [PubMed]
- Schouttete, K.V.K.M.; Hennebel, T.; Dheere, E.; Bertelkamp, C.; De Ridder, D.J.; Maes, S.; Chys, M.; Van Hulle, S.W.H.; Vanden Bussche, J.; Vanhaecke, L.; et al. Effect of oxidation and catalytic reduction of trace organic contaminants on their activated carbon adsorption. Chemosphere 2016, 165, 191–201. [Google Scholar] [CrossRef]
- Ma, X.X.; Liu, Y.; Li, X.Q.; Xu, J.G.; Gu, G.D.; Xia, C.H. Water: The most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst. Appl. Catal. B 2015, 165, 351–359. [Google Scholar] [CrossRef]
- Weidlich, T.; Prokes, L.; Pospisilova, D. Debromination of 2,4,6-tribromophenol coupled with biodegradation. Centr. Eur. J. Chem. 2013, 11, 979–987. [Google Scholar] [CrossRef]
- Weidlich, T.; Krejčová, A.; Prokeš, L. Hydrodebromination of 2,4,6-tribromophenol in aqueous solution using Devarda’s alloy. Monatsh. Chem. 2013, 144, 155–162. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, R.; Huang, K.; Luo, W.; Tao, X.; Dang, Z.; Yin, H.; Guo, C.; Lu, G. Debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by synthetic Pd/Fe0 and Cu/Fe0 in different protic solvents. Chemosphere 2018, 212, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, T.; Lu, G.; Zheng, Z.; Huang, K.; Li, H.; Tao, X.; Yin, H.; Shi, Z.; Lin, Z.; et al. Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci. Total Environ. 2019, 661, 18–26. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Xu, L.; Li, J.; Huang, L.-Z.; Li, F. Enhanced debromination of tetrabromobisphenol a by zero-valent copper nanoparticle-modified green rusts. Environ. Sci. Nano 2019, 6, 970–980. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Todesco, F.; Gennaro, A. Electrocatalytic Activation of Aromatic Carbon-Bromine Bonds toward Carboxylation at Silver and Copper Cathodes. J. Electrochem. Soc. 2013, 160, G3073–G3079. [Google Scholar] [CrossRef]
- Mouselmani, R.; Hachem, A.; Alaaeddine, A.; Métay, E.; Lemaire, M. Reduction of aromatic nitriles into aldehydes using calcium hypophosphite and a nickel precursor. Org. Biomol. Chem. 2018, 16, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
- Lichtenecker, R.J. Synthesis of aromatic 13C/2H-α-ketoacid precursors to be used in selective phenylalanine and tyrosine protein labelling. Org. Biomol. Chem. 2014, 12, 7551–7560. [Google Scholar] [CrossRef] [Green Version]
- Weidlich, T.; Kamenická, B. Recycling of spent hydrodehalogenation catalysts—Problems dealing with separation of aluminium. Inz. Mineral. J. Pol. Mineral. Eng. Soc. 2019, 1, 177–182. [Google Scholar] [CrossRef]
- Bruker AXS GmbH. Diffracplus Basic Evaluating Package. In EVA, 19th ed.; Bruker AXS GmbH: Karlsruhe, Germany, 2013. [Google Scholar]
- Joint Committee on Powder Diffraction Standards. PDF-4+ Database; International Centre of Diffraction Data: Swarthmore, PA, USA.
- Weidlich, T.; Kamenicka, B.; Melanova, K.; Cicmancova, V.; Komersova, A.; Cermak, J. Hydrodechlorination of different chloroaromatic compounds at room temperature and ambient pressure—Differences in reactivity of Cu- and Ni-based Al alloys in an alkaline aqueous solution. Catalysts 2020, 10, 994. [Google Scholar] [CrossRef]
- Raut, S.S.; Kamble, S.P.; Kulkarni, P.S. Efficacy of zero-valent copper (Cu0) nanoparticles and reducing agent for dechlorination of mono chloroaromatics. Chemosphere 2016, 159, 359–366. [Google Scholar] [CrossRef] [PubMed]
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. Available online: https://sdbs.db.aist.go.jp (accessed on 14 April 2021).
- Pouchert, C.J.; Behnke, J. The Aldrich Library of 13C and 1H FT NMR Spectra, 1st ed.; Aldrich Chemical Company, Inc.: St. Louis, MO, USA, 1993; Volume 1–3. [Google Scholar]
- Reich, H.J. Online NMR Database Accessible Free of Charge. Available online: http://www.chem.wisc.edu/areas/organic/index-chem.htm (accessed on 14 April 2021).

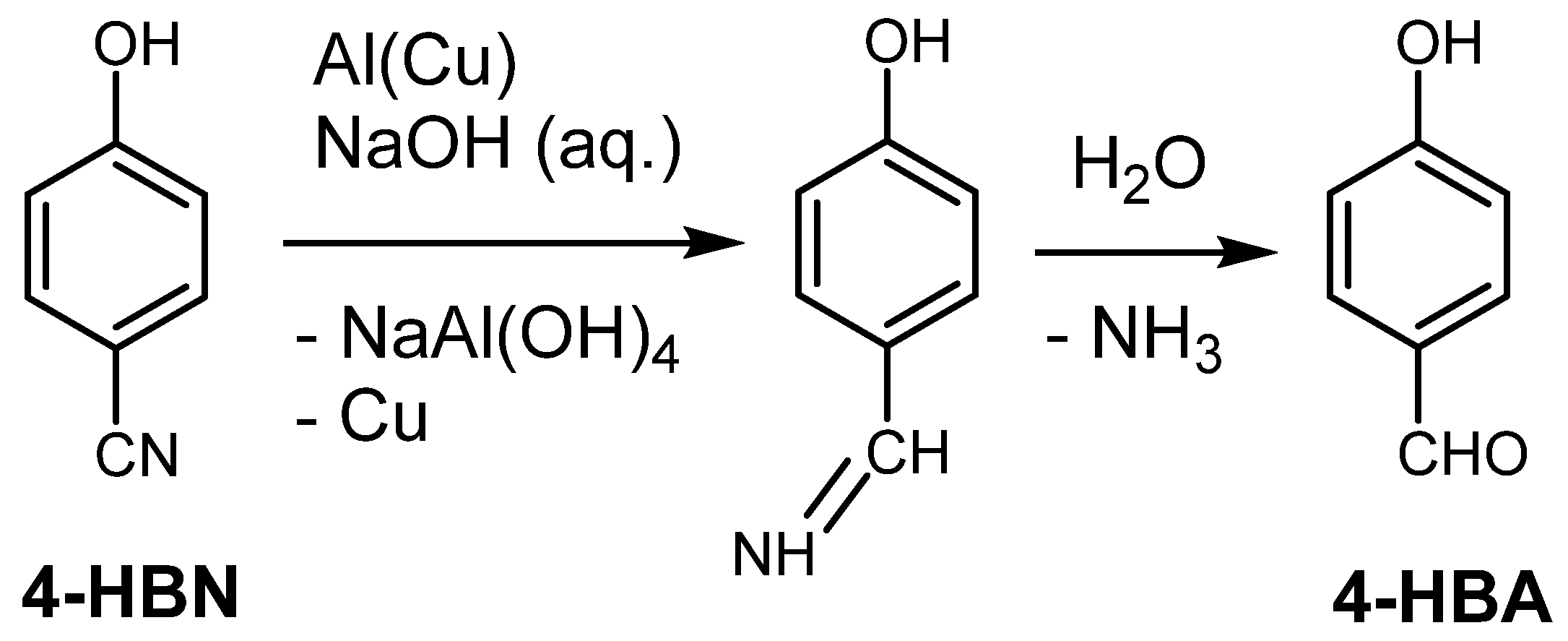

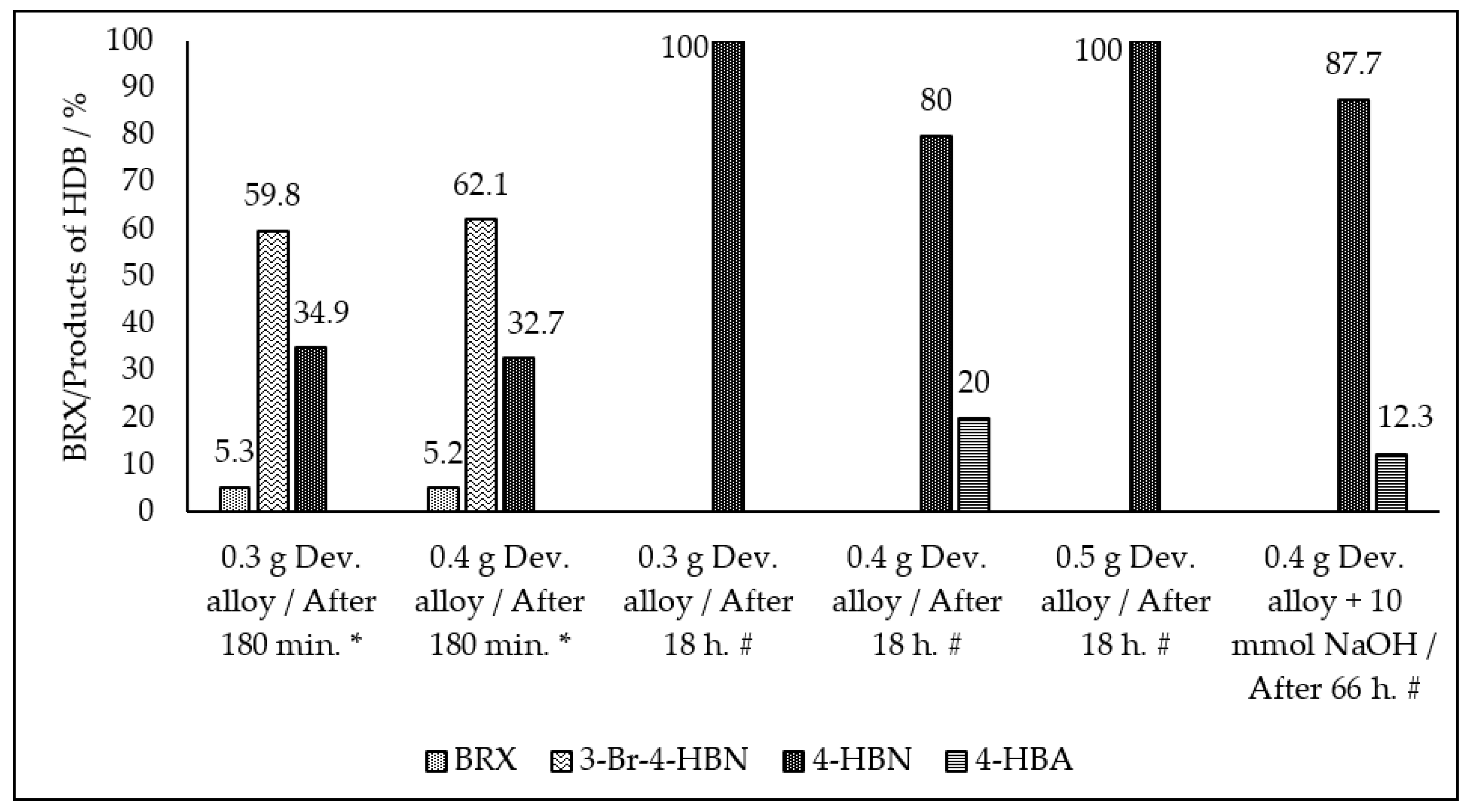
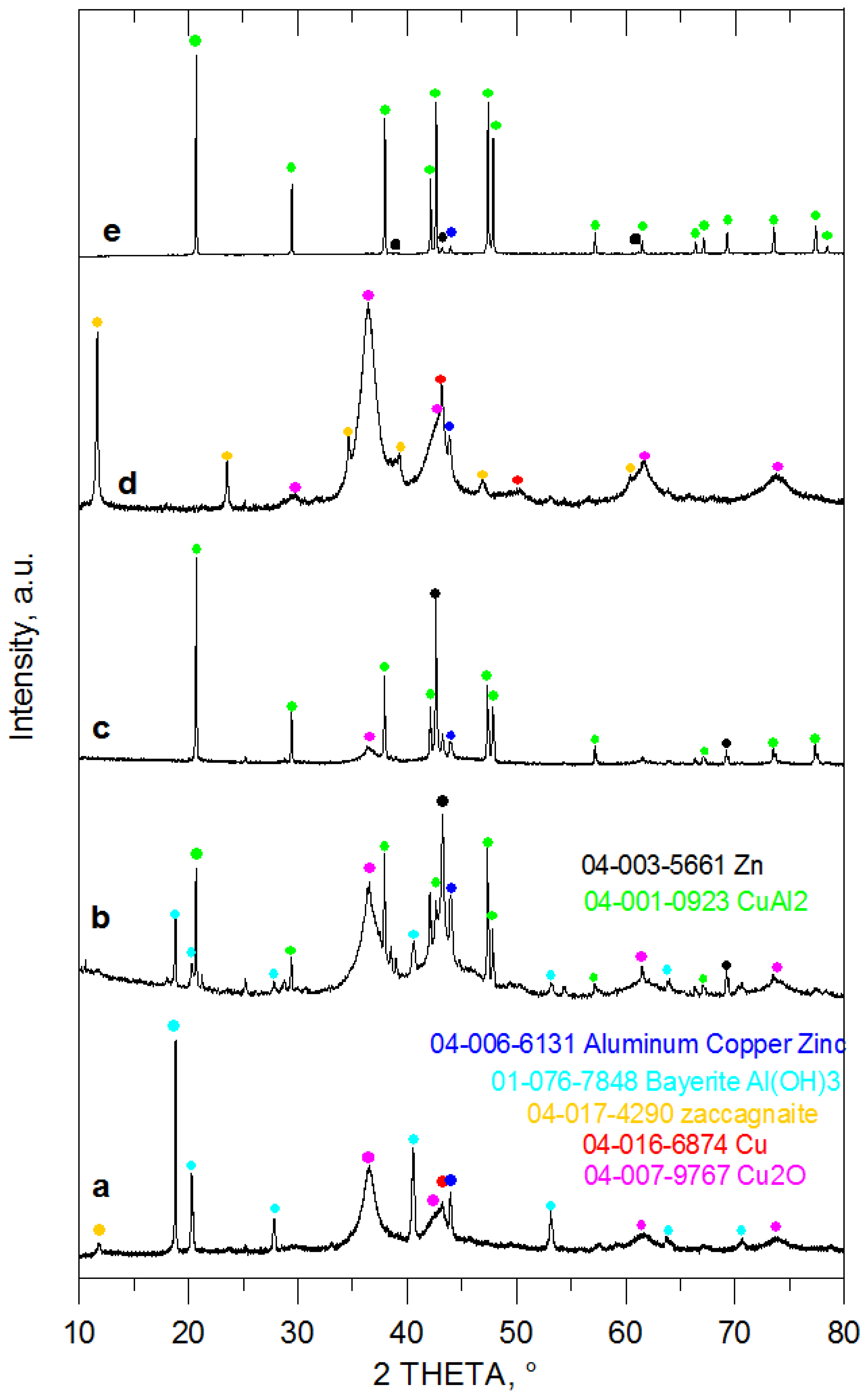
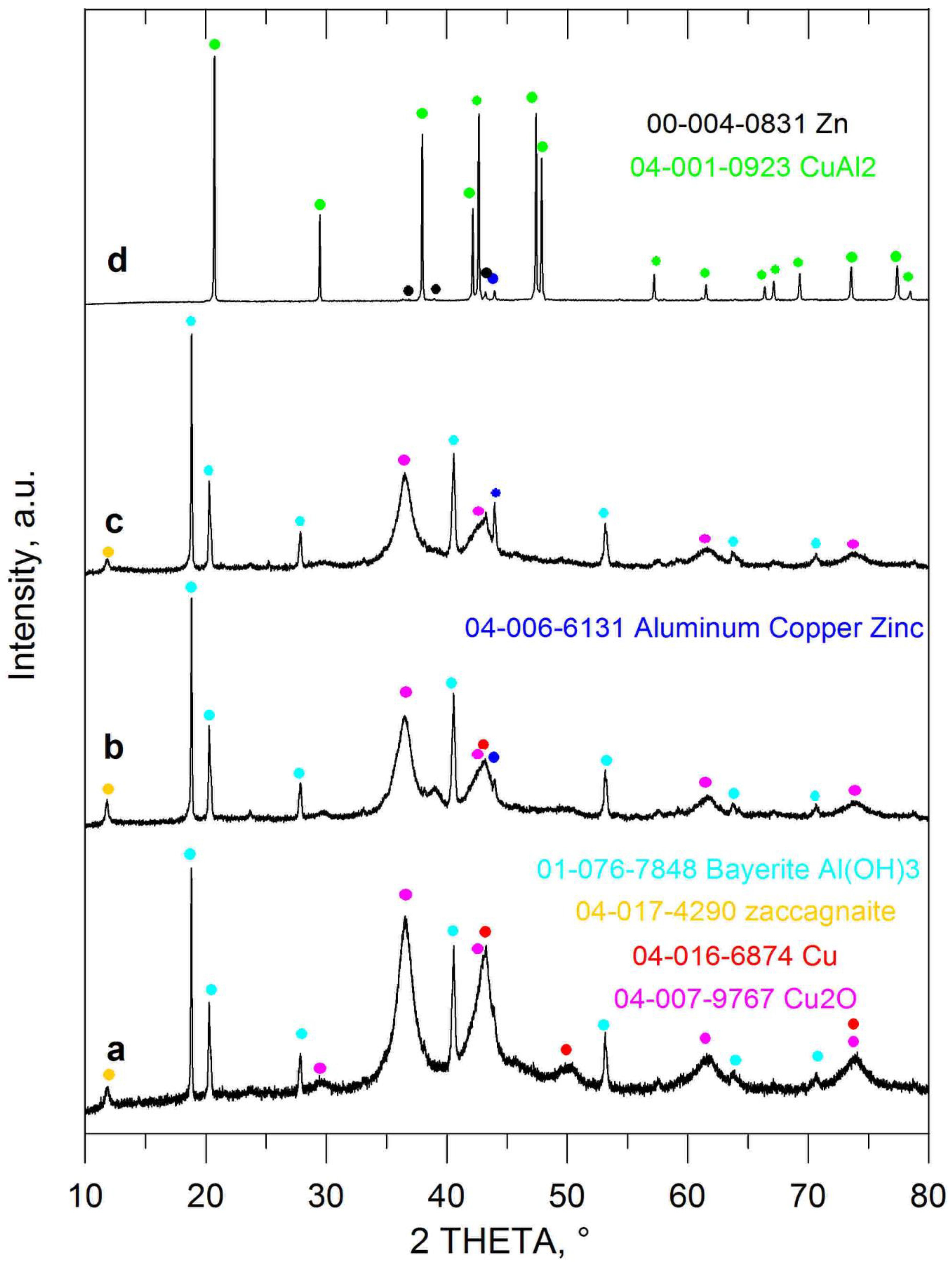
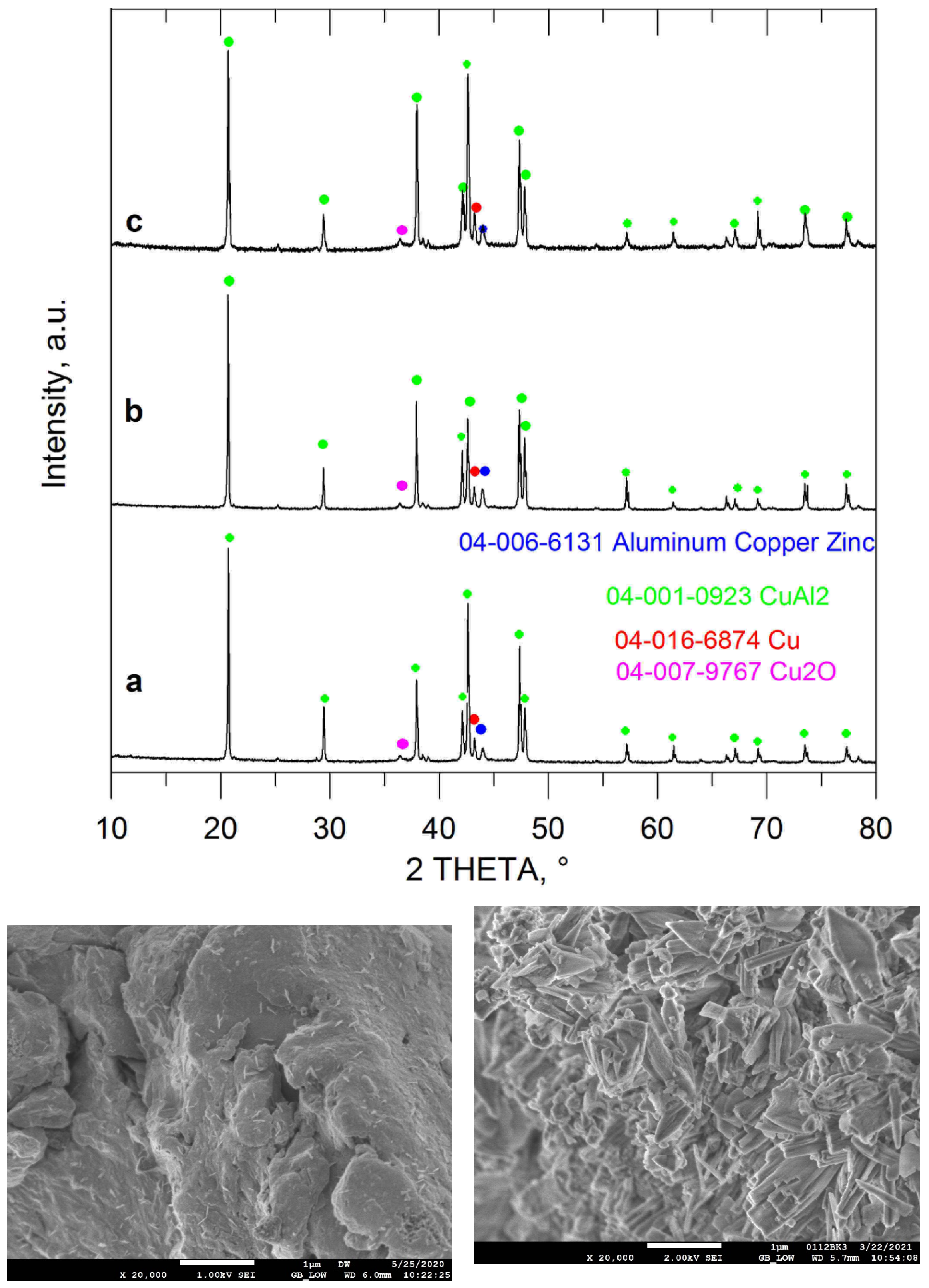
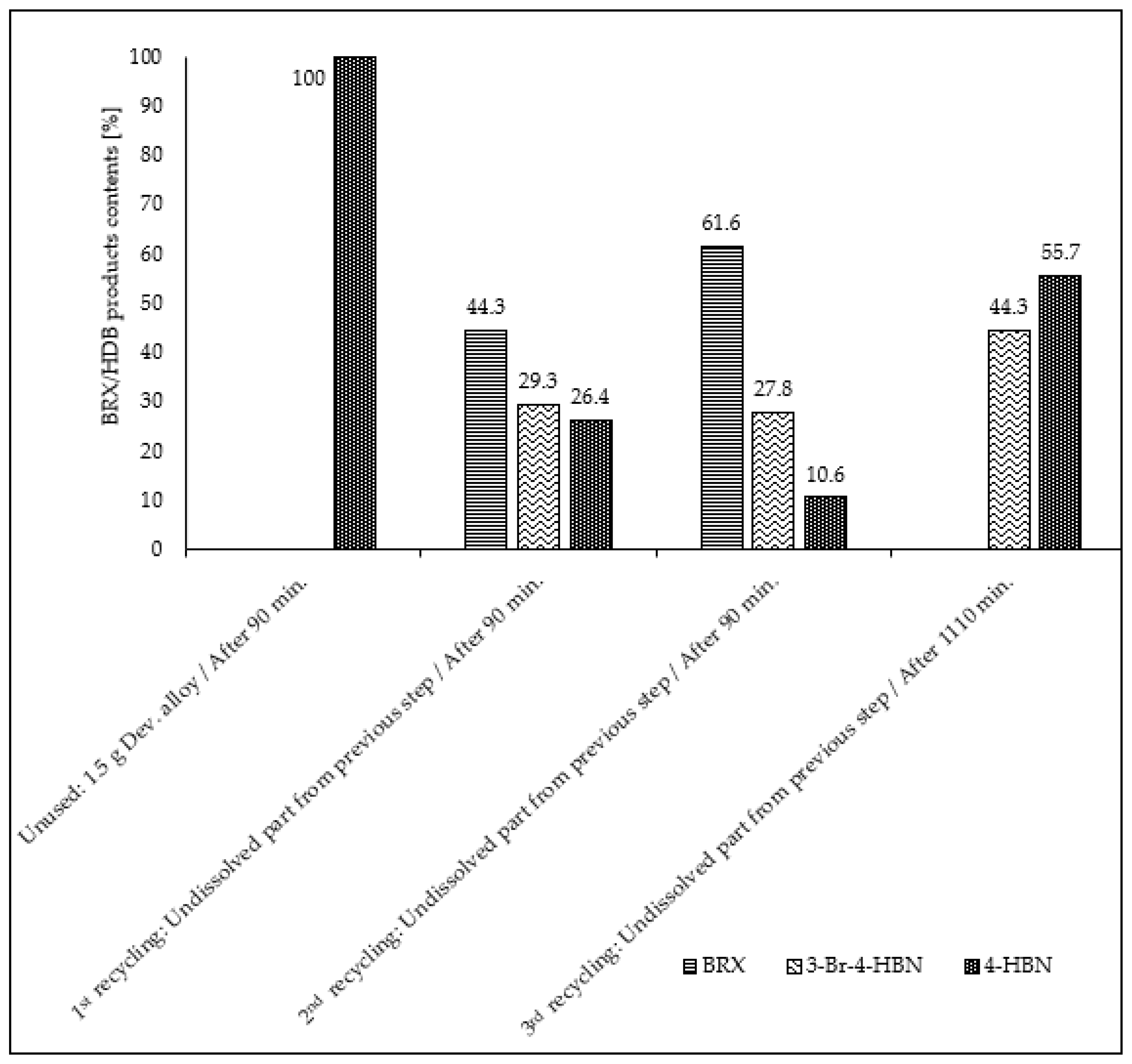


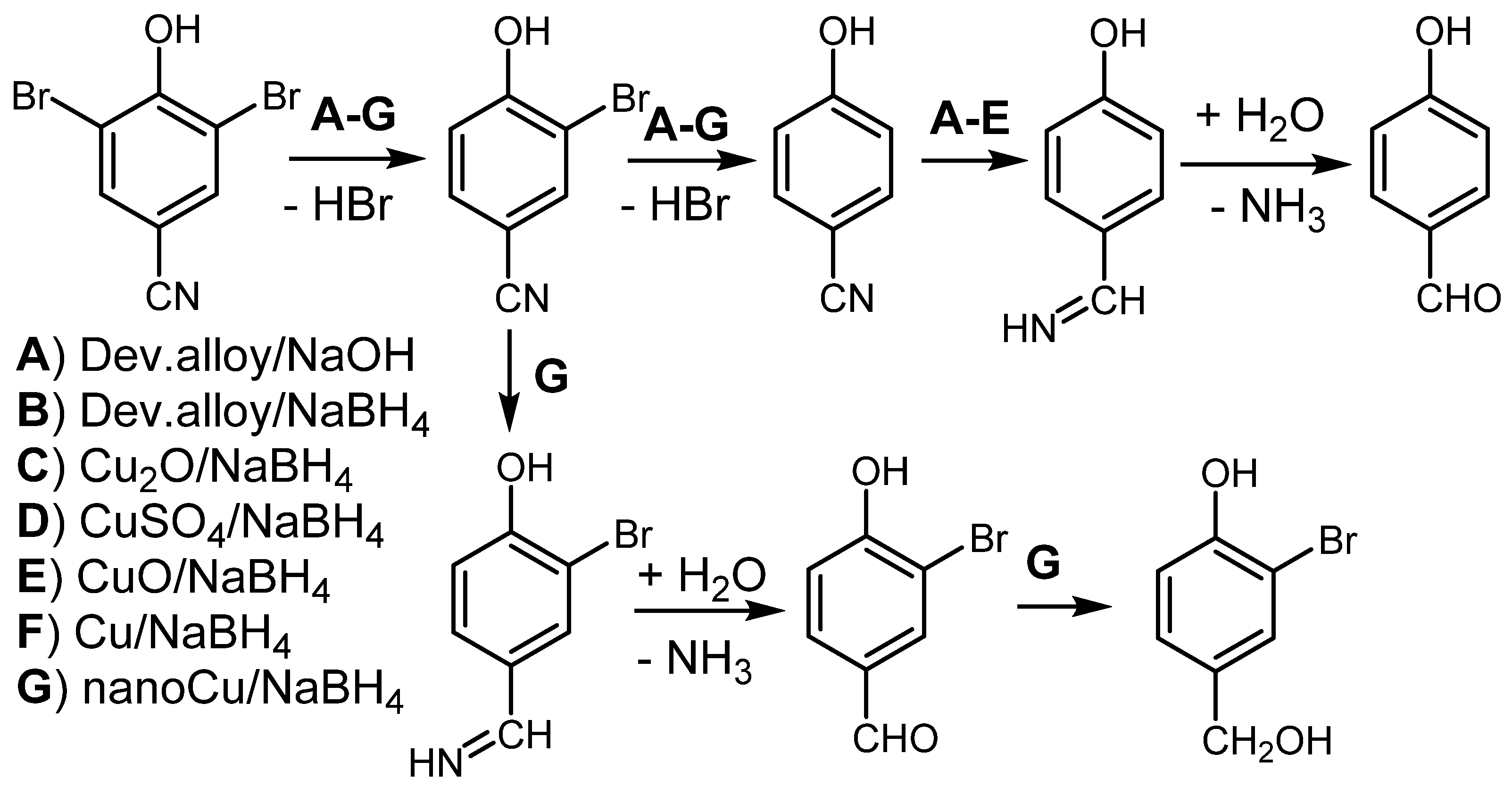
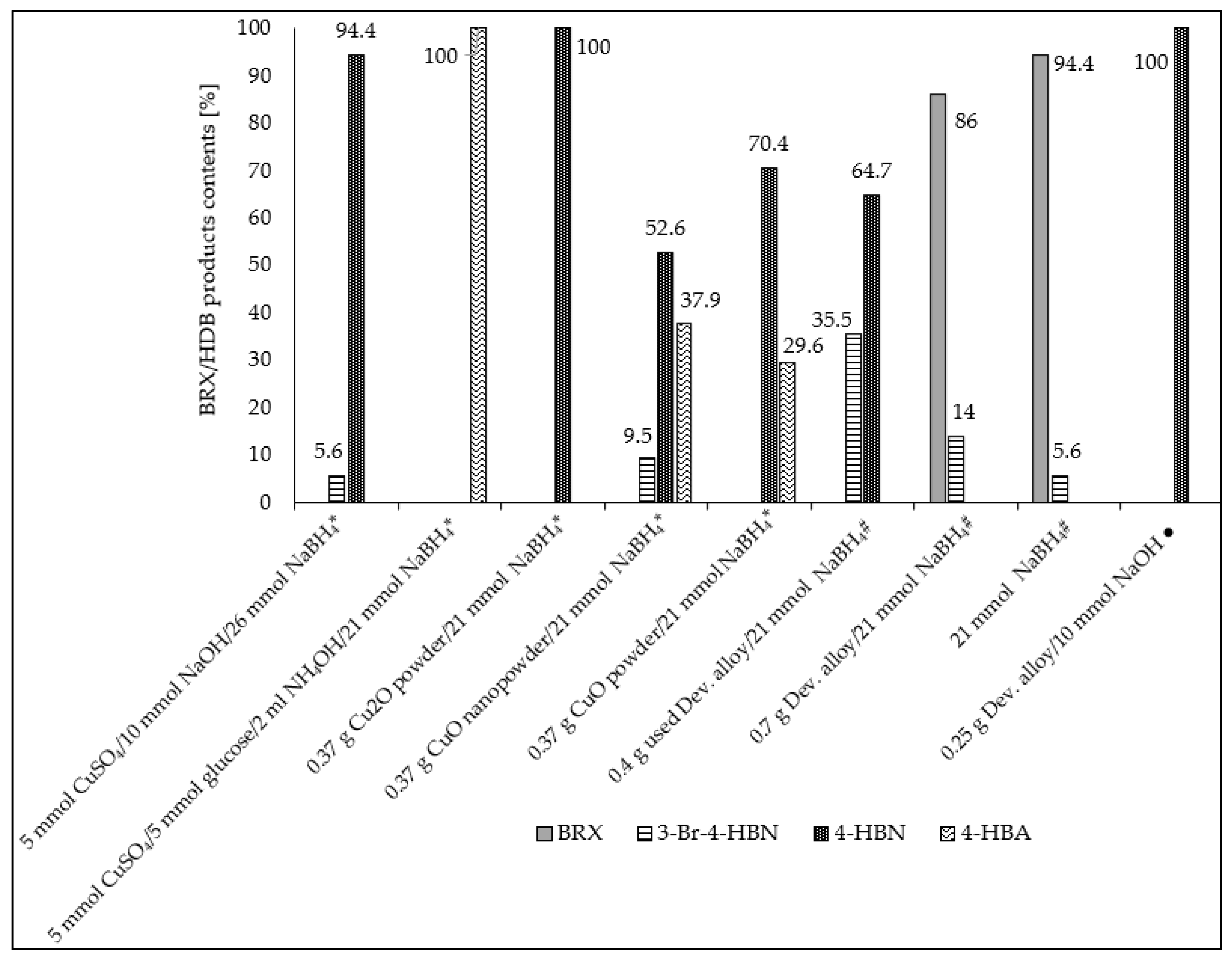

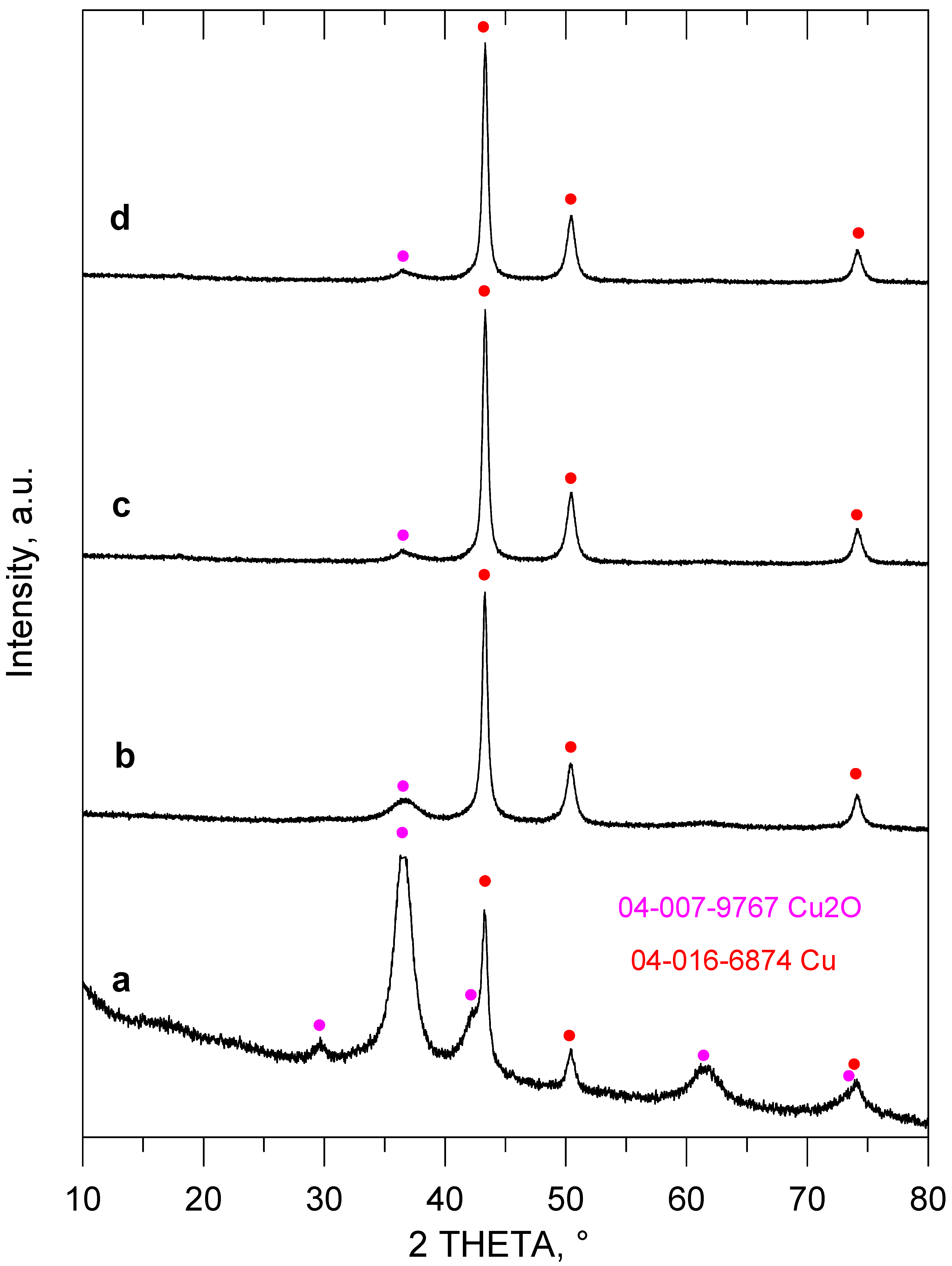


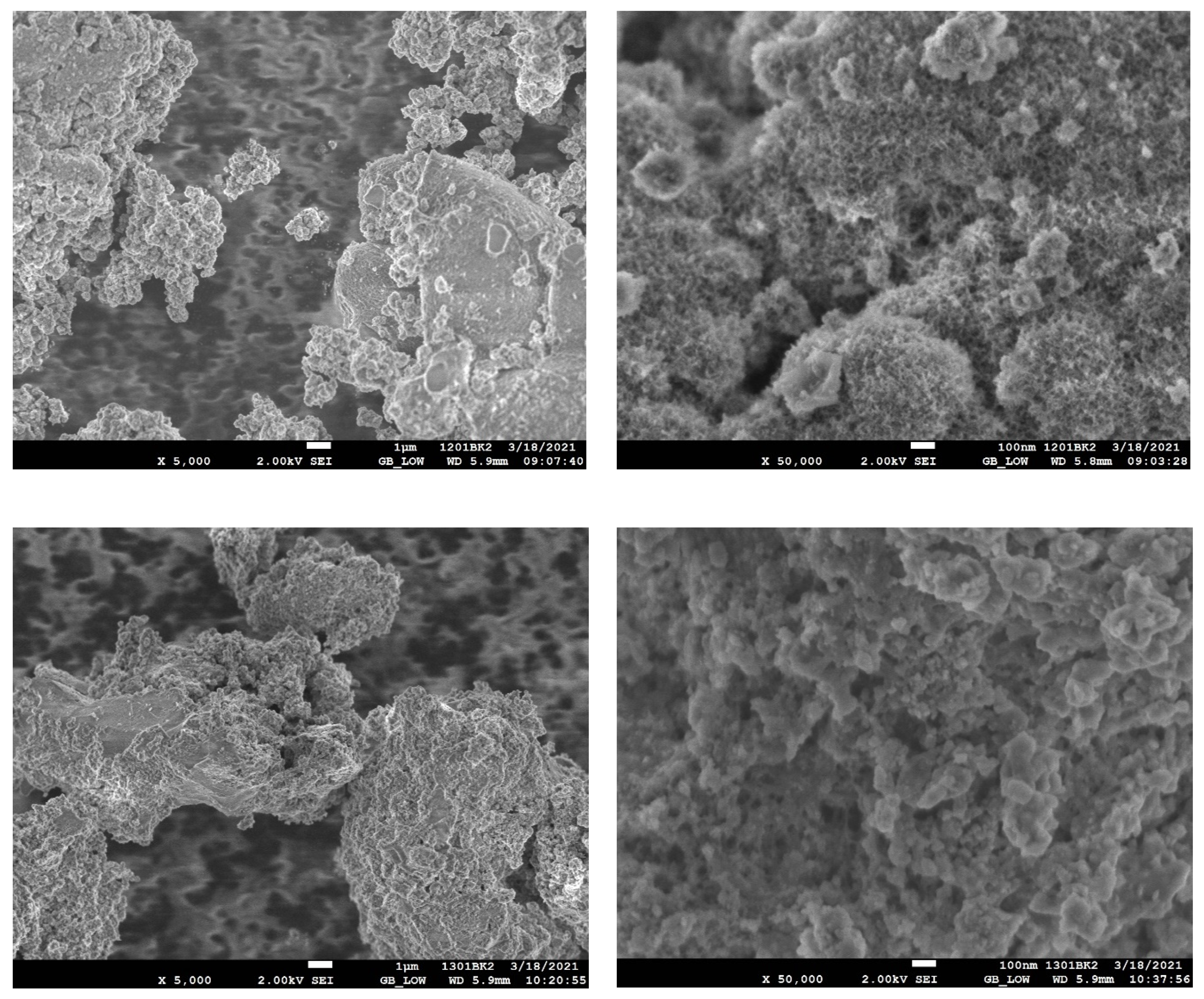
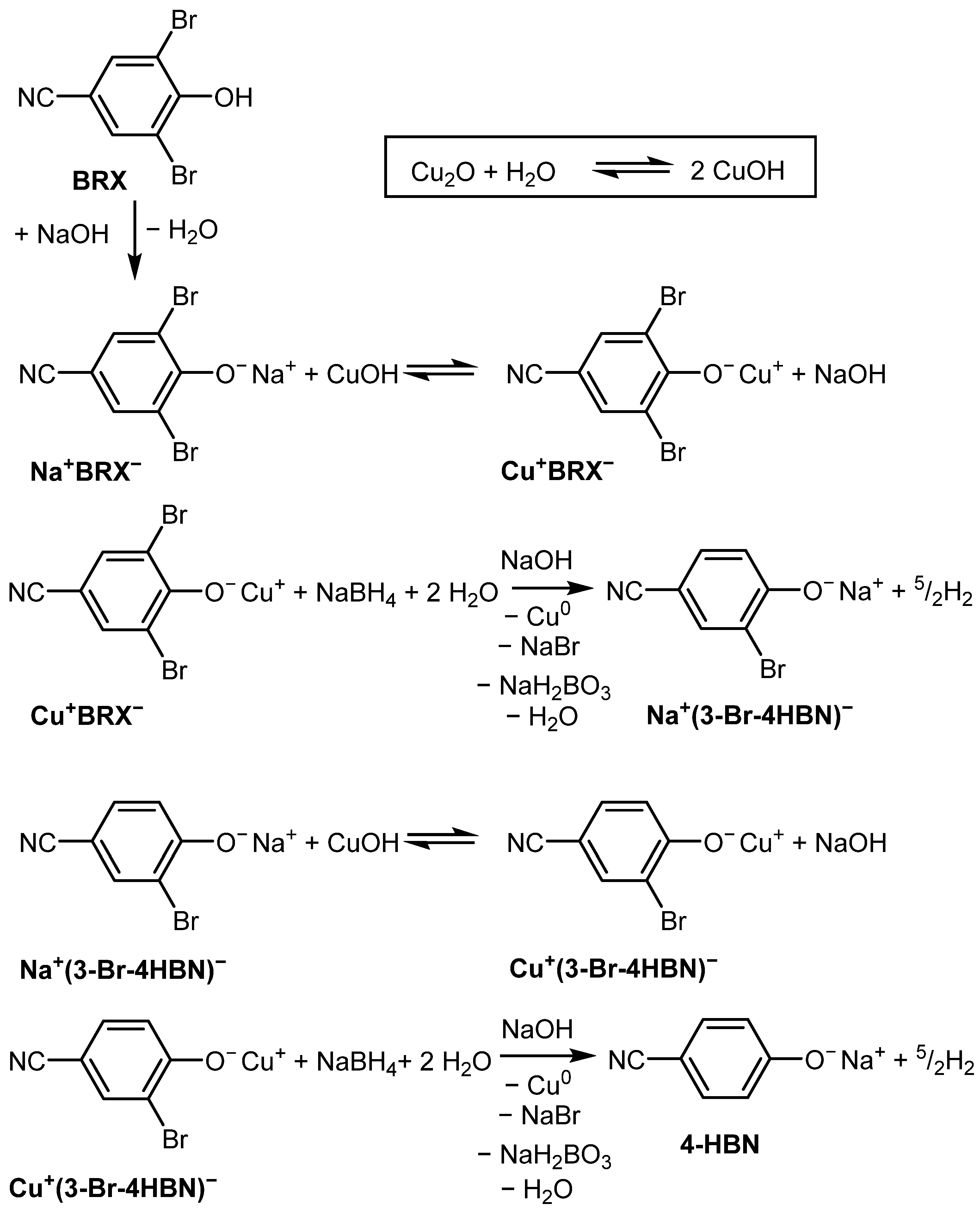

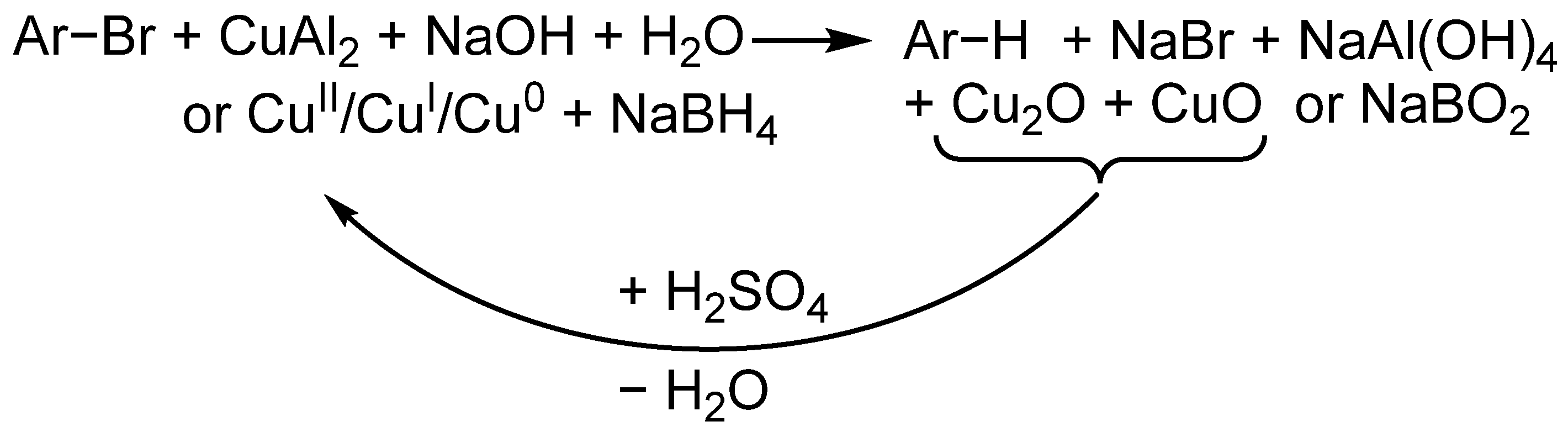
| Entry No. | Reduction Agent Used in aq. Sol of BRX 1 | NaOH Conc. (mmol) | Temperature | Reaction Time | Content of Phenolic Compounds (%) | Content of Dissolved Al (g/L) | Insoluble Matter (g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BRX | 4-HBN | 4-HBA | 3-Br-4-HBN | |||||||
| 1 | 0.6 g Dev. alloy | 10 | R.T. | overnight | - | 57.9 | 42.1 | - | 1.565 | 0.3 |
| 2 | 0.6 g Dev. alloy | 10 | R.T. | 66 h | - | 64.5 | 35.5 | - | 1.103 | 0.36 |
| 3 | 0.4 g Dev. alloy | 10 | R.T. | 66 h | - | 80 | 20 | - | 1.525 | 0.14 |
| 4 | 0.4 g Dev. alloy | 20 | R.T. | overnight | - | 87.7 | 12.3 | - | 1.561 | 0.21 |
| 5 | 0.5 g Dev. alloy | 10 | R.T. | overnight | - | 100 | - | - | 0.671 | 0.41 |
| 6 | 0.3 g Dev. alloy | 10 | R.T. | 150 min. | - | 100 | - | - | 0.794 | 0.22 |
| 7 | 0.25 g Dev. alloy | 10 | R.T. | overnight | - | 100 | - | - | n.d. | n.d. |
| 8 | 0.3 g Dev. alloy | 2 | R.T. | 180 min. | 5.3 | 34.9 | - | 59.8 | n.d. | n.d. |
| 9 | 1 g Cu-Zn alloy | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 10 | 0.91 g silver brazing alloy Ag:Cu:Zn | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 11 | 0.7 g Cu-Sn alloy | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 12 | 1 g Fe3Al alloy | 10 | R.T. | overnight | 100 | - | - | - | n.d. | n.d. |
| 13 | 0.3 g Al | 10 | R.T. | overnight | 100 | - | - | - | n.d. | 0 |
| 14 | 0.36 g Al foil | 10 | R.T. | overnight | 100 | - | - | - | n.d. | 0 |
| 15 | 0.5 g Zn | 10 | R.T. | overnight | 100 | - | - | - | - | n.d. |
| 16 | 0.8 g NaBH4 | 10 | R.T. | 96 h | 100 | - | - | - | - | 0 |
| 17 | 0.4 g NaBH4 | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | - | 6.4 | - | 93.6 | - | 0 |
| Cu/NaBH4 | Cu2O/NaBH4 | Dev. Alloy/NaBH4 | Dev. Alloy/NaOH | |
|---|---|---|---|---|
| k1 ± SD (min−1) calculated based on the time dependence of BRX | (8.8 ± 0.4) × 10−4 | 0.0183 ± 0.0018 | 0.07277 ± 0.0116 | 0.150 ± 0.0082 |
| R2 | 0.9960 | 0.9729 | 0.9620 | 0.9942 |
| k1 ± SD (min−1) calculated based on the time dependence of 3-Br-4-HBN | (7.4 ± 0.2) × 10−4 | 0.0192 ± 0.0026 | * 0.06864 ± 0.0241 | 0.1630 ± 0.0343 |
| k2 ± SD (min−1) calculated based on the time dependence of 3-Br-4-HBN | (7.3 ± 0.3) × 10−5 | 0.00225 ± 0.0030 | * 0.03874 ± 0.0163 | 0.0341 ± 0.0047 |
| R2 | 0.9981 | 0.9565 | 0.9842 | 0.9719 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidlich, T.; Kamenická, B.; Beneš, L.; Čičmancová, V.; Komersová, A.; Čermák, J.; Švec, P. Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts 2021, 11, 699. https://doi.org/10.3390/catal11060699
Weidlich T, Kamenická B, Beneš L, Čičmancová V, Komersová A, Čermák J, Švec P. Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts. 2021; 11(6):699. https://doi.org/10.3390/catal11060699
Chicago/Turabian StyleWeidlich, Tomáš, Barbora Kamenická, Ludvík Beneš, Veronika Čičmancová, Alena Komersová, Jiří Čermák, and Petr Švec. 2021. "Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution" Catalysts 11, no. 6: 699. https://doi.org/10.3390/catal11060699
APA StyleWeidlich, T., Kamenická, B., Beneš, L., Čičmancová, V., Komersová, A., Čermák, J., & Švec, P. (2021). Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts, 11(6), 699. https://doi.org/10.3390/catal11060699







