Comparative Analysis of Universal Protein Extraction Methodologies for Screening of Lipase Activity from Agricultural Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparative Analysis of Protein Extraction Efficiency
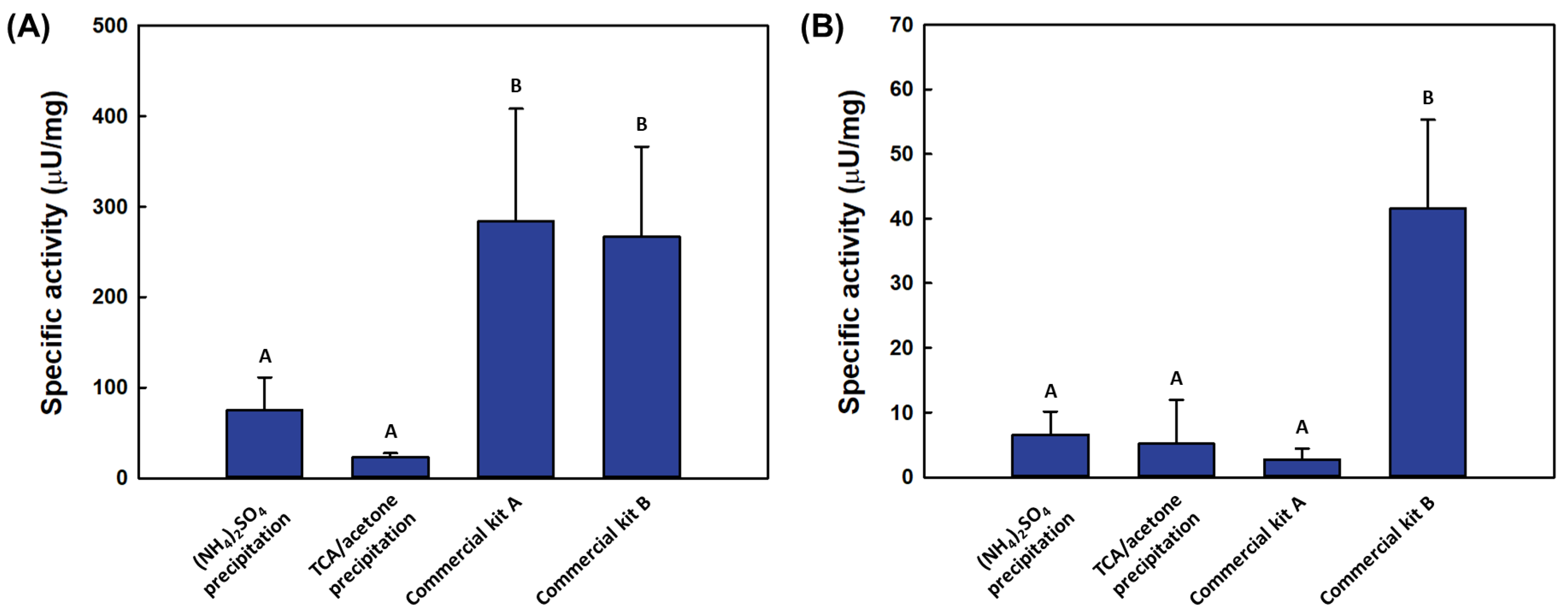
2.2. Comparative Analysis of Catalytic Activity by Spectrophotometric Assay
2.3. Comparative Analysis of Catalytic Activity by Fluorometric Assay
3. Materials and Methods
3.1. Materials
3.2. Protein Extraction from Agricultural Products
3.2.1. Ammonium Sulfate [(NH4)2SO4] Precipitation
3.2.2. TCA/Acetone Precipitation
3.2.3. Protein Extraction Using Plant Total Protein Extraction Kit
3.2.4. Protein Extraction Using Pierce™ Plant Total Protein Extraction Kit
3.3. Spectrophotometric Assay for Measuring Lipase Activity
3.4. Fluorometric Assay for Measuring Lipase Activity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.-Y.; Park, K.-M.; Yoo, Y.; Yu, H.; Lee, C.J.; Jung, H.-S.; Kim, K.; Chang, P.-S. Catalytic characteristics of a sn-1(3) regioselective lipase from Cordyceps militaris. Biotechnol. Prog. 2019, 35, e2744. [Google Scholar] [CrossRef]
- Choi, Y.; Park, J.-Y.; Chang, P.-S. Integral stereoselectivity of lipase based on the chromatographic resolution of enantiomeric/regioisomeric diacylglycerols. J. Agric. Food Chem. 2021, 69, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Seitz, E.W. Industrial application of microbial lipases: A review. J. Am. Oil Chem. Soc. 1974, 51, 12–16. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Phukon, L.C.; Chourasia, R.; Kumari, M.; Godan, T.K.; Sahoo, D.; Parameswaran, B.; Rai, A.K. Production and characterisation of lipase for application in detergent industry from a novel Pseudomonas helmanticensis HS6. Bioresour. Technol. 2020, 309, 123352. [Google Scholar] [CrossRef]
- Malekabadi, S.; Badoei-dalfard, A.; Karami, Z. Biochemical characterization of a novel cold-active, halophilic and organic solvent-tolerant lipase from B. licheniformis KM12 with potential application for biodiesel production. Int. J. Biol. Macromol. 2018, 109, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tai, F.; Chen, S. Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J. Sep. Sci. 2008, 31, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, X.; Ku, L.; Chen, Y.; Wang, W. Evaluation of three protein-extraction methods for proteome analysis of maize leaf midrib, a compound tissue rich in sclerenchyma cells. Front. Plant Sci. 2016, 7, 856. [Google Scholar] [CrossRef]
- Lee, C.-H. A simple outline of methods for protein isolation and purification. Endocrinol. Metab. 2017, 32, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, H.; Wu, Z.; Wang, Y.; Liu, H.; Wu, X.; Wang, W. Modified TCA/acetone precipitation of plant proteins for proteomic analysis. PLoS ONE 2018, 13, e0202238. [Google Scholar] [CrossRef] [PubMed]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. In Methods in Enzymology; Lorsch, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 541, pp. 85–94. [Google Scholar]
- Gupta, N.; Rathi, P.; Gupta, R. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal. Biochem. 2002, 311, 98–99. [Google Scholar] [CrossRef]
- Jacks, T.J.; Kircher, H.W. Fluorometric assay for the hydrolytic activity of lipase using fatty acyl esters of 4-methylumbelliferone. Anal. Biochem. 1967, 21, 279–285. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, S.-N.; Lee, S.H.; Choe, J.-S.; Choi, Y. Development of 9th revision Korean food composition table and its major changes. Korean J. Community Nutr. 2018, 23, 352–365. [Google Scholar] [CrossRef]
- Méchin, V.; Damerval, C.; Zivy, M. Total protein extraction with TCA-acetone. In Plant Proteomics: Methods and Protocols; Thiellement, H., Zivy, M., Damerval, C., Méchin, V., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 1–8. [Google Scholar]
- Jiang, L.; He, L.; Fountoulakis, M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J. Chromatogr. A 2004, 1023, 317–320. [Google Scholar] [CrossRef]
- Pohl, T. Concentration of proteins and removal of solutes. In Methods in Enzymology; Deutscher, M.P., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 182, pp. 68–83. [Google Scholar]
- Rajalingam, D.; Loftis, C.; Xu, J.J.; Kumar, T.K.S. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci. 2009, 18, 980–993. [Google Scholar] [CrossRef]
- Isaacson, T.; Damasceno, C.M.B.; Saravanan, R.S.; He, Y.; Catalá, C.; Saladié, M.; Rose, J.K.C. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat. Protoc. 2006, 1, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.W.; Zhao, Y.; Niu, L.P.; Jiang, R.; Song, Y.; Feng, H.; Feng, K.; Qi, C. A rapid method for determining the concentration of recombinant protein secreted from Pichia pastoris. J. Phys. Conf. Ser. 2011, 276, 012144. [Google Scholar] [CrossRef]
- Fan, J.-X.; Yang, X.-X.; Song, J.-Z.; Huang, X.-M.; Cheng, Z.-X.; Yao, L.; Juba, O.S.; Liang, Q.; Yang, Q.; Odeph, M.; et al. Heterologous expression of transaldolase gene Tal from Saccharomyces cerevisiae in Fusarium oxysporum for enhanced bioethanol production. Appl. Biochem. Biotechnol. 2011, 164, 1023–1036. [Google Scholar] [CrossRef]
- Hong, J.K.; Choi, H.W.; Hwang, I.S.; Kim, D.S.; Kim, N.H.; Choi, D.S.; Kim, Y.J.; Hwang, B.K. Function of a novel GDSL-type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 2008, 227, 539–558. [Google Scholar] [CrossRef]
- Park, J.H.; Park, K.-M.; Chang, Y.; Park, J.-Y.; Han, J.; Chang, P.-S. Cloning and protein expression of the sn-1(3) regioselective lipase from Cordyceps militaris. Enzym. Microb. Technol. 2018, 119, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Janes, L.E.; Löwendahl, A.C.; Kazlauskas, R.J. Quantitative screening of hydrolase libraries using pH indicators: Identifying active and enantioselective hydrolases. Chem. A Eur. J. 1998, 4, 2324–2331. [Google Scholar] [CrossRef]
- Reichelt, W.N.; Waldschitz, D.; Herwig, C.; Neutsch, L. Bioprocess monitoring: Minimizing sample matrix effects for total protein quantification with bicinchoninic acid assay. J. Ind. Microbiol. Biotechnol. 2016, 43, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Bánfalvi, Z.; Kostyál, Z.; Barta, E. Solanum brevidens possesses a non-sucrose-inducible patatin gene. Mol. Gen. Genet. 1994, 245, 517–522. [Google Scholar] [CrossRef]
- Andrews, D.L.; Beames, B.; Summers, M.D.; Park, W.D. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem. J. 1988, 252, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Fernández-No, I.C.; Barros-Velázquez, J.; Gallardo, J.M.; Cañas, B.; Calo-Mata, P. Comparative analysis of protein extraction methods for the identification of seafood-borne pathogenic and spoilage bacteria by MALDI-TOF mass spectrometry. Anal. Methods 2010, 2, 1941–1947. [Google Scholar] [CrossRef][Green Version]
- Azri, W.; Ennajah, A.; Jing, M. Comparative study of six methods of protein extraction for two-dimensional gel electrophoresis of proteomic profiling in poplar stems. Can. J. Plant Sci. 2013, 93, 895–901. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Park, J.-Y.; Kim, C.S.; Park, K.-M.; Chang, P.-S. Inhibitory characteristics of flavonol-3-O-glycosides from Polygonum aviculare L. (common knotgrass) against porcine pancreatic lipase. Sci. Rep. 2019, 9, 18080. [Google Scholar] [CrossRef]


| Source 1 | (NH4)2SO4 Precipitation | TCA/Acetone Precipitation | Commercial Kit A 2 | Commercial Kit B 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/mL) | Extraction Yield (mg/0.1 g Sample) | Concentration (mg/mL) | Extraction Yield (mg/0.1 g Sample) | Concentration (mg/mL) | Extraction Yield (mg/0.1 g Sample) | Concentration (mg/mL) | Extraction Yield (mg/0.1 g Sample) | |
| 1 | 14.05 | 0.22 | 2.75 | 2.75 | 4.77 | 1.91 | 3.06 | 0.92 |
| 2 | 13.93 | 0.22 | 4.56 | 4.56 | 4.56 | 1.83 | 12.99 | 3.90 |
| 3 | 16.36 | 0.26 | 2.94 | 2.94 | 4.01 | 1.60 | 8.74 | 2.62 |
| 4 | 4.25 | 0.07 | 4.40 | 4.40 | 11.02 | 4.41 | 13.72 | 4.11 |
| 5 | 13.45 | 0.22 | 2.84 | 2.84 | 14.28 | 5.71 | 8.34 | 2.50 |
| 6 | 13.26 | 0.21 | 3.01 | 3.01 | 12.99 | 5.19 | 11.39 | 3.42 |
| 7 | 14.78 | 0.24 | 3.01 | 3.01 | 4.62 | 1.85 | 7.30 | 2.19 |
| 8 | 16.38 | 0.26 | 2.87 | 2.87 | 5.74 | 2.30 | 7.09 | 2.13 |
| 9 | 16.74 | 0.27 | 2.95 | 2.95 | 5.03 | 2.01 | 7.01 | 2.10 |
| 10 | 13.74 | 0.22 | 2.79 | 2.79 | 2.79 | 1.12 | 9.22 | 2.77 |
| 11 | 15.63 | 0.25 | 3.80 | 3.80 | 14.07 | 5.63 | 14.78 | 4.44 |
| 12 | 18.47 | 0.30 | 3.82 | 3.82 | 11.25 | 4.50 | 15.20 | 4.56 |
| 13 | 18.63 | 0.30 | 3.19 | 3.19 | 9.12 | 3.65 | 13.65 | 4.10 |
| 14 | 16.02 | 0.26 | 6.78 | 6.78 | 13.02 | 5.21 | 14.58 | 4.37 |
| 15 | 15.34 | 0.25 | 4.34 | 4.34 | 11.32 | 4.53 | 16.89 | 5.07 |
| 16 | 8.75 | 0.14 | 2.87 | 2.87 | 5.25 | 2.10 | 4.52 | 1.36 |
| 17 | 9.92 | 0.16 | 2.83 | 2.83 | 2.83 | 1.13 | 5.17 | 1.55 |
| 18 | 4.19 | 0.07 | 2.79 | 2.79 | 2.79 | 1.12 | 3.47 | 1.04 |
| 19 | 12.85 | 0.21 | 2.76 | 2.76 | 2.76 | 1.11 | 5.23 | 1.57 |
| 20 | 4.24 | 0.07 | 2.71 | 2.71 | 3.37 | 1.35 | 4.27 | 1.28 |
| 21 | 5.47 | 0.09 | 5.89 | 5.89 | 5.84 | 2.34 | 5.64 | 1.69 |
| 22 | 15.45 | 0.25 | 2.69 | 2.69 | 2.69 | 1.07 | 5.08 | 1.52 |
| 23 | 3.41 | 0.05 | 2.54 | 2.54 | 3.77 | 1.51 | 4.36 | 1.31 |
| 24 | 8.68 | 0.14 | 2.82 | 2.82 | 4.59 | 1.84 | 5.50 | 1.65 |
| Source 1 | (NH4)2SO4 Precipitation | TCA/Acetone Precipitation | Commercial Kit A 2 | Commercial Kit B 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | |
| 1 | 864.55 | 61.55 | 62.12 | 22.56 | 1518.92 | 318.37 | 2318.93 | 757.52 |
| 2 | 923.22 | 66.26 | 57.29 | 12.56 | 445.22 | 97.57 | 2018.32 | 155.39 |
| 3 | 406.91 | 24.88 | 23.12 | 7.87 | 377.57 | 94.20 | 2213.32 | 253.15 |
| 4 | 129.08 | 30.39 | 81.11 | 18.45 | 2607.46 | 236.60 | 2626.09 | 191.46 |
| 5 | 589.83 | 43.87 | 82.83 | 29.18 | 583.61 | 40.87 | 1522.71 | 182.49 |
| 6 | 202.94 | 15.30 | 45.56 | 15.13 | 1284.57 | 98.93 | 963.60 | 84.58 |
| 7 | 168.08 | 11.38 | 47.97 | 15.92 | 670.24 | 145.11 | 607.43 | 83.25 |
| 8 | 311.65 | 19.02 | 43.49 | 15.17 | 698.54 | 121.63 | 608.12 | 85.73 |
| 9 | 708.90 | 42.34 | 21.05 | 7.15 | 594.66 | 118.32 | 1236.60 | 176.33 |
| 10 | 527.70 | 38.41 | 61.78 | 22.13 | 446.25 | 159.87 | 980.17 | 106.29 |
| 11 | 129.08 | 8.26 | 183.95 | 48.39 | 1503.73 | 106.89 | 1348.08 | 91.19 |
| 12 | 1893.73 | 102.52 | 86.97 | 22.77 | 901.82 | 80.18 | 1492.69 | 98.20 |
| 13 | 2400.38 | 128.87 | 62.81 | 19.66 | 427.62 | 46.88 | 1175.17 | 86.09 |
| 14 | 1221.76 | 76.28 | 91.11 | 13.44 | 923.57 | 70.92 | 3819.90 | 262.08 |
| 15 | 164.97 | 10.75 | 81.80 | 18.85 | 1151.35 | 101.73 | 2042.13 | 120.93 |
| 16 | 64.88 | 7.41 | 173.60 | 60.43 | 1168.61 | 222.65 | 3749.84 | 829.36 |
| 17 | 1648.34 | 166.10 | 81.11 | 28.68 | 1752.91 | 619.96 | 3276.66 | 633.42 |
| 18 | 1336.34 | 318.78 | 65.23 | 23.38 | 2224.36 | 797.43 | 1339.45 | 386.08 |
| 19 | 810.02 | 63.03 | 57.98 | 20.99 | 720.63 | 260.86 | 955.66 | 182.80 |
| 20 | 503.20 | 118.67 | 59.71 | 22.05 | 1204.85 | 357.61 | 517.00 | 121.00 |
| 21 | 1757.75 | 321.17 | 155.65 | 26.41 | 5668.41 | 970.45 | 1941.36 | 344.09 |
| 22 | 143.92 | 9.32 | 76.27 | 28.39 | 868.00 | 323.10 | 1653.86 | 325.37 |
| 23 | 272.65 | 79.93 | 67.30 | 26.48 | 1158.95 | 307.72 | 404.84 | 92.87 |
| 24 | 261.26 | 30.11 | 62.12 | 22.05 | 5075.13 | 1106.15 | 4119.47 | 748.99 |
| Source 1 | (NH4)2SO4 Precipitation | TCA/Acetone Precipitation | Commercial Kit A 2 | Commercial Kit B 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | Velocity (nM/min) | Specific Activity (μU/mg) | |
| 1 | 361.51 | 25.74 | 1.50 | 0.54 | 7.17 | 1.50 | 378.94 | 123.79 |
| 2 | 30.01 | 2.15 | 6.34 | 1.39 | 6.34 | 1.39 | 235.61 | 18.14 |
| 3 | 23.07 | 1.41 | 6.90 | 2.35 | 6.81 | 1.70 | 203.06 | 23.23 |
| 4 | 120.32 | 28.33 | 1.46 | 0.33 | 7.73 | 0.70 | 301.71 | 22.00 |
| 5 | 56.34 | 4.19 | 7.83 | 2.76 | 1.05 | 0.07 | 334.82 | 40.13 |
| 6 | 44.51 | 3.36 | 6.04 | 2.01 | 5.69 | 0.44 | 351.70 | 30.87 |
| 7 | 3.36 | 0.23 | 9.11 | 3.02 | 19.48 | 4.22 | 136.49 | 18.71 |
| 8 | 5.03 | 0.31 | 7.02 | 2.45 | 6.10 | 1.06 | 196.31 | 27.67 |
| 9 | 47.76 | 2.85 | 30.09 | 10.21 | 10.61 | 2.11 | 193.99 | 27.66 |
| 10 | 60.78 | 4.42 | 19.29 | 6.91 | 19.29 | 6.91 | 195.80 | 21.23 |
| 11 | 52.10 | 3.33 | 0.88 | 0.23 | 23.25 | 1.65 | 170.50 | 11.53 |
| 12 | 73.24 | 3.96 | 2.15 | 0.56 | 17.09 | 1.52 | 256.05 | 16.85 |
| 13 | 23.29 | 1.25 | 1.55 | 0.49 | 6.39 | 0.70 | 128.78 | 9.43 |
| 14 | 73.38 | 4.58 | 0.37 | 0.05 | 19.19 | 1.47 | 200.25 | 13.74 |
| 15 | 5.96 | 0.39 | 1.71 | 0.39 | 17.37 | 1.53 | 165.99 | 9.83 |
| 16 | 24.29 | 2.77 | 231.52 | 80.59 | 4.25 | 0.81 | 501.09 | 110.83 |
| 17 | 156.46 | 15.77 | 5.19 | 1.84 | 5.19 | 1.84 | 457.28 | 88.40 |
| 18 | 34.87 | 8.32 | 3.74 | 1.34 | 3.74 | 1.34 | 228.32 | 65.81 |
| 19 | 43.64 | 3.40 | 5.82 | 2.11 | 5.82 | 2.11 | 139.47 | 26.68 |
| 20 | 107.74 | 25.41 | 2.69 | 0.99 | 15.56 | 4.62 | 208.58 | 48.82 |
| 21 | 39.61 | 7.24 | 3.14 | 0.53 | 0.57 | 0.10 | 462.33 | 81.95 |
| 22 | 3.60 | 0.23 | 4.40 | 1.64 | 4.40 | 1.64 | 383.47 | 75.44 |
| 23 | 28.23 | 8.28 | 1.80 | 0.71 | 20.81 | 5.52 | 208.42 | 47.81 |
| 24 | 0.17 | 0.02 | 0.97 | 0.34 | 91.75 | 20.00 | 197.34 | 35.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.; Park, J.-Y.; Choi, Y.; Chang, P.-S.; Park, K.-M. Comparative Analysis of Universal Protein Extraction Methodologies for Screening of Lipase Activity from Agricultural Products. Catalysts 2021, 11, 816. https://doi.org/10.3390/catal11070816
Ha J, Park J-Y, Choi Y, Chang P-S, Park K-M. Comparative Analysis of Universal Protein Extraction Methodologies for Screening of Lipase Activity from Agricultural Products. Catalysts. 2021; 11(7):816. https://doi.org/10.3390/catal11070816
Chicago/Turabian StyleHa, Jisu, Jun-Young Park, Yoonseok Choi, Pahn-Shick Chang, and Kyung-Min Park. 2021. "Comparative Analysis of Universal Protein Extraction Methodologies for Screening of Lipase Activity from Agricultural Products" Catalysts 11, no. 7: 816. https://doi.org/10.3390/catal11070816
APA StyleHa, J., Park, J.-Y., Choi, Y., Chang, P.-S., & Park, K.-M. (2021). Comparative Analysis of Universal Protein Extraction Methodologies for Screening of Lipase Activity from Agricultural Products. Catalysts, 11(7), 816. https://doi.org/10.3390/catal11070816








