Production and Purification of Novel Hypocholesterolemic Peptides from Lactic Fermented Spirulina platensis through High Hydrostatic Pressure-Assisted Protease Hydrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymes Extracted under HHP, Hydrolysate Chemical Composition, and HMGR-Inhibiting Capacity Analysis
2.2. Chemical Composition and HMGR-Inhibiting Capacity of the Lactic Fermented S. platensis Hydrolysates
2.3. Effect of HHP-FH-PR6 Hydrolysis with Simulated Gastrointestinal Digestion on HMGR Inhibition
2.4. Separation and Purification of the Primary HMGR-Inhibiting Peptides in the HHP-FH-PR6G Group
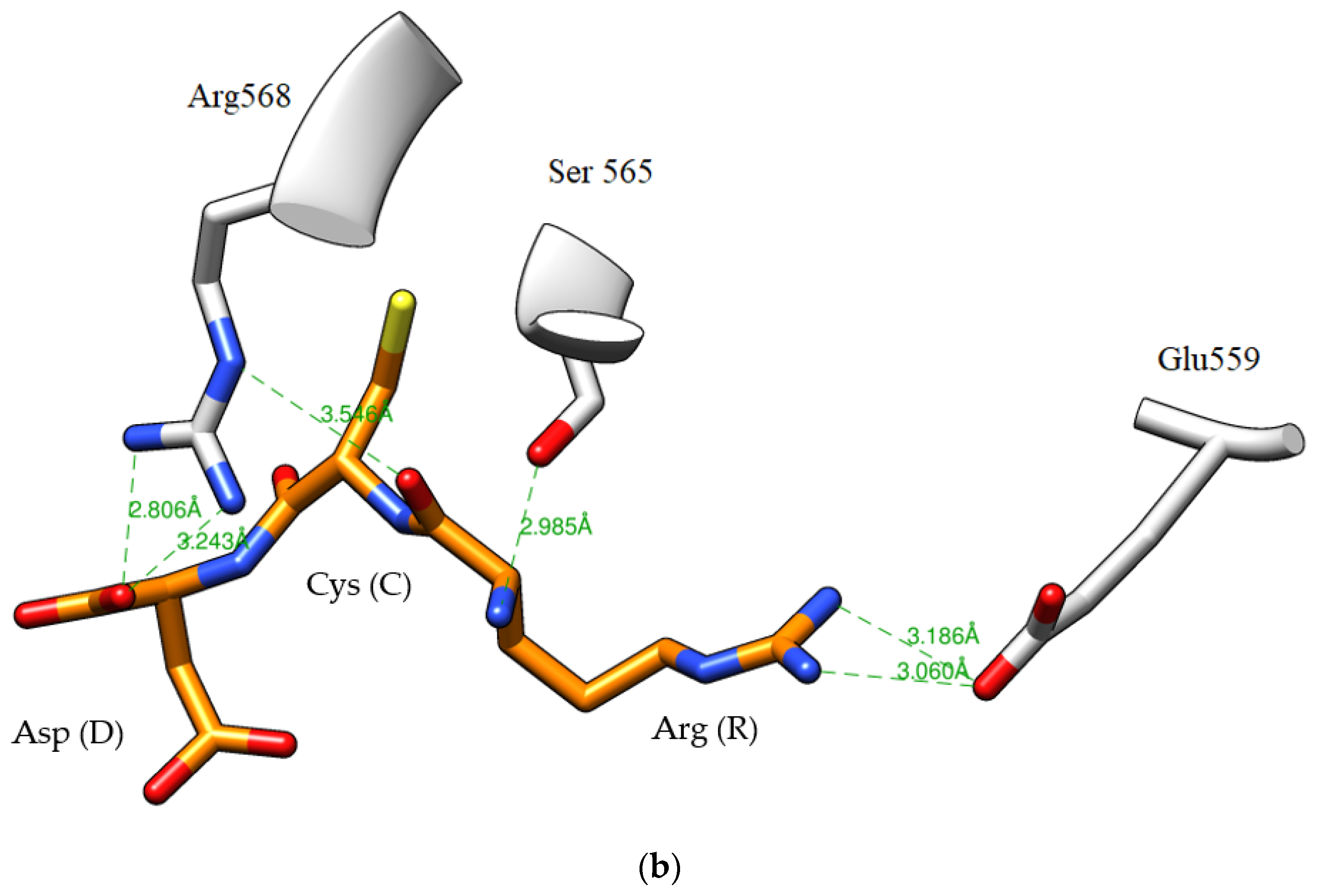
2.5. Peptide Sequence Identification
3. Materials and Methods
3.1. Materials
3.2. Preparation of S. platensis Solution by HHP-Assisted Enzymatic Hydrolysis
3.3. Preparation of Fermented S. platensis through HHP-Assisted Enzymatic Hydrolysis
3.4. Determination of Free Amino Acid Content
3.5. Determination of Soluble Protein Content
3.6. Peptide Content Determination
3.7. Determination of HMGR-Inhibiting Capacity
3.8. In Vitro Gastrointestinal Digestion
3.9. Gel Filtration Chromatography Analysis of S. platensis Protein Hydrolysate
3.10. Purification of the Peptides for HMG-CoA Reductase Inhibitory Activity
3.10.1. Sample Preparation
3.10.2. Chromatographic Conditions Involving Acetonitrile (Concentration: 0–15%)
3.11. ESI/MS/MS and Peptide Data Analyses
3.12. Analysis of HMGR–Peptide Molecular Docking
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A





References
- Laslett, L.; Alagona, P.; Clark, B.A.; Drozda, J.P.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Alla, F. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Opie, L.H.; Frishman, W.H.; Thadani, U. Angiotensin-converting enzyme inhibitors and conventional vasodilators. In Drugs for the Heart, 4th ed.; Opie, L.H., Ed.; Saunders: Philadelphia, PA, USA, 1994; pp. 105–143. [Google Scholar]
- Wenniger, L.M.; Beuers, U. Bile salts and cholestasis. Dig. Liver Dis. 2010, 42, 409–418. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A. Feedback regulation of cholesterol synthesis: Stero-accelerated ubiquitination and degradation of HMG-CoA reductase. Cell Res. 2008, 18, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-associated myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.S.; Kasymova, T.D.; Kwon, D.Y. Isolation and identification of peptides from soy 11S-globulin with hypocholesterolemic activity. Chem. Nat. Compd. 2005, 41, 710–714. [Google Scholar] [CrossRef]
- Marques, M.R.; Fontanari, G.G.; Pimenta, D.C.; Soares-Freitas, R.M.; Arêas, J.A.G. Proteolytic hydrolysis of cowpea proteins is able to release peptides with hypocholesterolemic activity. Food Res. Int. 2015, 77, 43–48. [Google Scholar] [CrossRef]
- Ashraf, J.; Liu, L.; Awais, M.; Xiao, T.; Wang, L.; Zhou, X.; Tong, L.T.; Zhou, S. Effect of thermosonication pre-treatment on mung bean (Vigna radiate) and white kidney bean (Phaseolus vulgaris) proteins: Enzymatic hydrolysis, cholesterol lowering activity and structural characterization. Ultrason. Sonochem. 2020, 66, 105121. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.A.M.; Mendonça, S.; De Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef] [Green Version]
- Nieto, I.M.P.; del Moral, M.P.; Garcia, M.C. Evaluation of the relationship between the peptide profile and the lipid-lowering properties of olive seeds hydrolysates as a tool for tuning hypocholesterolemic functionality. Food Funct. 2020, 11, 4973–4981. [Google Scholar]
- Nopianti, R.; Oktaviani, S. Fractionation of anticholesterol bioactive compounds from bekasam (Indonesian fermented fish product). Pertanika J. Trop. Agric. Sci. 2017, 40, 195–204. [Google Scholar]
- Kwon, D.Y.; Oh, S.W.; Lee, J.S.; Yang, H.J.; Lee, S.H.; Lee, J.H.; Lee, Y.B.; Sohn, H.S. Amino acid substitution of hypocholesterolemic peptide originated from glycinin hydrolyzate. Food Sci. Biotechnol. 2002, 11, 55–61. [Google Scholar]
- Chen, G.W.; Tsai, J.S.; Sun, P.B. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Chen, G.W.; Tsai, J.S.; Sun, P.B. Cardiovascular effects of whey from prozyme 6-facilitated lactic acid bacteria fermentation of milk. J. Food Biochem. 2007, 31, 639–655. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, T.J.; Pan, B.S.; Gong, S.D.; Chung, M.Y. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Chen, G.W.; Liao, K.J. Method of Combining Pressure and Enzyme to Extract Hydrolysate of Animal-Derived Composition. Taiwan Patent No. TWI600379B, 1 October 2017. [Google Scholar]
- Masson, P.; Tonello, C.; Balny, C. High-pressure biotechnology in medicine and pharmaceutical science. J. Biomed. Sci. 2001, 1, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A Review. Enzyme Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Knudsen, J.C.; Otte, J.; Olsen, K.; Skibsted, L.H. Effect of high hydrostatic pressure on the conformation of β-lactoglobulin A as assessed by proteolytic peptide profiling. Int. Dairy J. 2002, 12, 791–803. [Google Scholar] [CrossRef]
- Quirós, A.; Chichón, R.; Recio, I.; López-Fandiño, R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chem. 2007, 104, 1734–1739. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Miao, M.; Mu, W.M.; Li, Y.H. Combined effects of high-pressure and enzymatic treatments on the hydrolysis of chickpea protein isolates and antioxidant activity of the hydrolysates. Food Chem. 2012, 135, 904–912. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Zieli’nski, H.; Wiczkowski, W.; Zieli’nska, D.; Martínez-Villaluenga, C. High-pressure-assisted enzymatic release of peptides and phenolics increases angiotensin converting enzyme I inhibitory and antioxidant activities of Pinto bean hydrolysates. J. Agric. Food Chem. 2016, 64, 1730–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.W.; Lin, H.T.V.; Huang, L.W.; Lin, C.H.; Lin, Y.H. Purification and identification of cholesterol micelle formation inhibitory peptides of hydrolysate from high hydrostatic pressure-assisted protease hydrolysis of fermented seabass byproduct. Int. J. Mol. Sci. 2021, 22, 5295. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Li, C.H.; Lin, C.H.; Yan, Y.Z.; Chuang, Y.Y.; Chen, G.W. Application and development status of an ultra-high pressure processing technique in aquatic food. Fish. Ext. Rep. 2017, 47, 17–32. [Google Scholar]
- Chen, G.W.; Liao, K.J. Method of Combining Pressure and Enzyme to Extract Hydrolysate of Cubilose. Taiwan Patent No. TWI516212B, 11 January 2016. [Google Scholar]
- Seo, Y.C.; Choi, W.S.; Park, J.H.; Park, J.O.; Jung, K.H.; Lee, H.Y. Stable isolation of phycocyanin from Spirulina platensis associated with high-pressure extraction process. Int. J. Mol. Sci. 2013, 14, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. 2018. Available online: https://www.un.org/youthenvoy/2013/09/fao-food-and-agriculture-organization-of-the-united-nations/ (accessed on 1 June 2021).
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. BioMed Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Y.; Lin, L.; Pan, Q.; Yang, X.; Cheng, X. Preventive effect of phycocyanin from Spirulina platensis on alloxan-injured mice. Environ. Toxicol. Pharmacol. 2012, 34, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Joventino, I.P.; Alves, H.G.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.A.; Neves, S.A.; Ferreira, F.V.; Brito, G.A.C.; Viana, G.B. The microalga Spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J. Complementary Integr. Med. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Virginia, A.; Rachmawati, H.; Riani, C.; Retnoningrum, D.S. Study of HMG-CoA reductase inhibition activity of the hydrolyzed product of snakehead fish (Channa striata) skin collagen with 50 kDa collagenase from Bacillus licheniformis F11. 4. Sci. Pharm. 2016, 84, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, M.; Kulozik, U. Study of chymosin hydrolysis of casein micelles under ultra high pressure: Effect on re-association upon pressure release. Int. Dairy J. 2011, 21, 664–669. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Van Loey, A.; Indrawati; Smout, C.; Hendrickx, M. Effects of combined pressure and temperature on enzymes related to quality of fruits and vegetables: From kinetic information to process engineering aspects. Crit. Rev. Food Sci. Nutr. 2003, 43, 527–586. [Google Scholar] [CrossRef] [PubMed]
- Rinto, D.R.; Yasni, S.; Suhartono, M.T. Novel HMG-CoA reductase inhibitor peptide from Lactobacillus acidophilus isolated from Indonesian fermented food bekasam. IJPCBS 2017, 5, 195–204. [Google Scholar]
- Lin, Y.H.; Chen, G.W.; Yeh, C.H.; Song, H.; Tsai, J.S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides and the antihypertensive effect of Chlorella sorokiniana protein hydrolysates. Nutrients 2018, 10, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, V.V.; Koo, M.; Kim, M.J.; Yang, H.J.; Yun, L.; Kwon, D.Y. Modeling an active conformation for linear peptides and design of a competitive inhibitor for HMG-CoA reductase. J. Mol. Recognit. 2008, 21, 224–232. [Google Scholar] [CrossRef] [PubMed]
- UniProt Database. Available online: http://www.uniprot.org (accessed on 1 January 2021).
- E Silva, M.B.D.C.; da Cruz Souza, C.A.; Philadelpho, B.O.; da Cunha, M.M.N.; Batista, F.P.R.; da Silva, J.R.; Ferreira, E.S. In vitro and in silico studies of 3-hydroxy-3-methyl-glutaryl coenzyme a reductase inhibitory activity of the cowpea Gln-Asp-Phe peptide. Food Chem. 2018, 259, 270–277. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef]
- Da Costa, R.F.; Freire, V.N.; Bezerra, E.M.; Cavada, B.S.; Caetano, E.W.S.; Filho, J.L.L.; Albuquerque, E.L. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations. Phys. Chem. Chem. Phys. 2012, 14, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Doi, E.; Shibata, D.; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, C.A.; Tsai, J.S.; Chen, G.W. Preparation and identification of novel antihypertensive peptides from the in vitro gastrointestinal digestion of marine cobia skin hydrolysates. Nutrients 2019, 11, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, T.G. Spectrophotometry. In The Tools of Biochemistry, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 1977; pp. 53–55. [Google Scholar]
- Lowry, O.H.; Resebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, H.D.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Ram, H.; Jaipal, N.; Charan, J.; Kashyap, P.; Kumar, S.; Tripathi, R.; Singh, B.P.; Siddaiah, C.N.; Hashem, A.; Allah, E.F.A. Phytoconstituents of an ethanolic pod extract of Prosopis cineraria triggers the inhibition of HMG-CoA reductase and the regression of atherosclerotic plaque in hypercholesterolemic rabbits. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koning, A.J.; Roberts, C.J.; Wright, R.L. Different subcellular localization of Saccharomyces cerevisiae HMG-CoA reductase isozymes at elevated levels corresponds to distinct endoplasmic reticulum membrane proliferations. Mol. Biol. Cell. 1996, 7, 769–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, J.S.; Chen, G.W. Purification and identification of hypocholesterolemic peptides from freshwater clam hydrolysate with in vitro gastrointestinal digestion. J. Food Biochem. 2017, 41, e12385. [Google Scholar] [CrossRef]
- Pairwise Sequence Alignment Software. Available online: https://www.ebi.ac.uk/Tools/psa/ (accessed on 1 January 2021).
- Hafidz, K.A.; Puspitasari, N.; Azminah, A.Y.; Artha, Y.; Mun’im, A. HMG-CoA Reductase inhibitory activity of Gnetum gnemon seed extract and identification of potential inhibitors for lowering cholesterol level. J. Young Pharm. 2017, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Institute Inc. SAS®9.4 TS1M5 User’s Guide; SAS Institute Press: Cary, NC, USA, 2019; p. 35. [Google Scholar]


| Pressure (MPa) | Enzymes † | Soluble Protein (mg/g) | Peptide Content (mg/g) | Free Amino Acid (mg/g) | Inhibition ‡ (%) |
|---|---|---|---|---|---|
| - | Pravastatin (positive control) | - § | - | - | 89.6 ± 2.8 a |
| 0.1 | Umamizyme G | 483.9 ± 1.4 b | 487.1 ± 0.7 d | 158.9 ± 0.2 e | 20.8 ± 7.6 d |
| Protease A | 283.5 ± 2.9 e | 338.7 ± 0.9 f | 140.5 ± 0.8 f | 41.5 ± 1.9 c | |
| Peptidase R | 416.7 ± 3.3 c | 585.8 ± 1.8 b | 237.0 ± 0.3 b | 40.6 ± 2.8 c | |
| 100 | Umamizyme G | 514.9 ± 3.2 a | 519.9 ± 0.8 c | 165.3 ± 1.0 d | 24.5 ± 5.7 d |
| Protease A | 337.5 ± 1.9 d | 459.1± 1.9 e | 198.8 ± 0.1 c | 51.9 ± 1.0 c | |
| Peptidase R | 414.4 ± 0.7 c | 699.1 ± 3.8 a | 269.2 ± 1.8 a | 67.0 ± 2.8 b |
| Sample | Hydrolysis Time (h) | Soluble Protein (mg/g) | Peptide Content (mg/g) | Free Amino Acid (mg/g) | Inhibition (%) |
|---|---|---|---|---|---|
| - | Pravastatin (positive control) | - § | - | - | 89.5 ± 2.3 a |
| HHP-F † | 0 | 242.3 ± 4.3 c | 96.8 ± 0.6 c | 24.0 ± 0.1 c | 57.9 ± 3.5 d |
| 3 | 242.2 ± 5.9 c | 102.9 ± 2.9 c | 25.9 ± 0.2 c | 62.0 ± 0.6 d | |
| 6 | 244.8 ± 4.2 c | 103.6 ± 1.7 d | 26.7 ± 0.3 c | 63.2 ± 1.8 d | |
| HHP-FH-PR ‡ | 3 | 291.1 ± 3.4 b | 261.5 ± 5.0 b | 78.5 ± 0.2 b | 70.8 ± 2.3 c |
| - | 6 | 328.1 ± 1.4 a | 339.8 ± 7.6 a | 115.2 ± 7.4 a | 78.4 ± 0.6 b |
| Sample | Peptide Content (mg/g) | Inhibition † (%) | IC50 Value ‡ (μg Peptide/mL) |
|---|---|---|---|
| HHP-FH-PR6 | 318.7 ± 0.4 a | 78.4 ± 0.6 a | 2.9 ± 0.1 a |
| Pepsin § | 225.5 ± 3.8 c | 65.6 ± 1.1 a,b | 3.0 ± 0.1 a |
| Pepsin + Pancreatin § | 270.7 ± 0.1 b | 58.9 ± 5.5 b | 3.5 ± 0.2 a |
| Fraction | Molecular Weight (Da) | Peptide Concentration (mg/mL) | Inhibition † (%) | ||
|---|---|---|---|---|---|
| A | 810 | - | 600 | 32.0 ± 0.3 | 74.4 ± 1.1 b |
| B | 560 | - | 420 | 9.1 ± 0.1 | 24.4 ± 0.0 d |
| C | 420 | - | 370 | 4.0 ± 0.0 | 25.6 ± 5.6 d |
| D | 370 | - | 240 | 3.1 ± 0.0 | 93.3 ± 0.0 a |
| E | 230 | - | 190 | 0.2 ± 0.0 | 47.8 ± 1.1 c |
| F | 180 | - | 100 | 0.2 ± 0.0 | 73.3 ± 2.2 b |
| G | 100 | - | 80 | 0.3 ± 0.0 | 65.6 ± 3.3 b |
| Peaks | Sequences | Inhibition (%) | IC50 † (μM) | Origin ‡ |
|---|---|---|---|---|
| D3 | Arg-Cys-Asp | 74.5 ± 2.1 | 6.9 | Mod (f212–214) |
| D4 | Ser-Asn-Val | 29.8 ± 0.0 | 20.1 | Adenine deaminase (f245–247) Methyltransferase (f282–284) PIN (f73–75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-W.; Yang, M.-H. Production and Purification of Novel Hypocholesterolemic Peptides from Lactic Fermented Spirulina platensis through High Hydrostatic Pressure-Assisted Protease Hydrolysis. Catalysts 2021, 11, 873. https://doi.org/10.3390/catal11080873
Chen G-W, Yang M-H. Production and Purification of Novel Hypocholesterolemic Peptides from Lactic Fermented Spirulina platensis through High Hydrostatic Pressure-Assisted Protease Hydrolysis. Catalysts. 2021; 11(8):873. https://doi.org/10.3390/catal11080873
Chicago/Turabian StyleChen, Guan-Wen, and Meng-Hsuan Yang. 2021. "Production and Purification of Novel Hypocholesterolemic Peptides from Lactic Fermented Spirulina platensis through High Hydrostatic Pressure-Assisted Protease Hydrolysis" Catalysts 11, no. 8: 873. https://doi.org/10.3390/catal11080873






