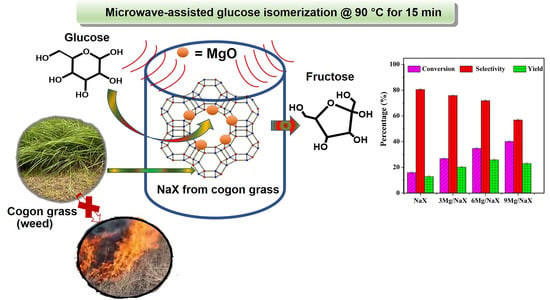
Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of Fructose via Microwave-Assisted Catalytic Glucose Isomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase of Silica from Cogon Grass
2.2. Catalyst Characterization
2.3. Catalytic Performance of Microwave-Assisted Glucose Isomerization into Fructose
2.4. Catalyst Regeneration and Reuse
2.5. Comparison of Catalytic Performances of the Best Catalyst in the Present Work to the Relevant Literature
3. Materials and Methods
3.1. Silica Production from Cogon Grass
3.2. Synthesis of NaX Zeolite
3.3. Catalyst Preparation
3.4. Characterization of Cogon Grass Silica and Catalyst
3.5. Catalytic Testing for Glucose Isomerization into Fructose
3.6. Regeneration and Reuse Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I, Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Graça, I.; Bacariza, M.C.; Chadwick, D. Glucose isomerisation into fructose over Mg-impregnated Na-zeolites: Influence of zeolite structure. Microporous Mesoporous Mater. 2018, 255, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Beltramini, J.N.; Mori, A.; Nakamura, M.; Karim, M.R.; Kim, Y.; Nakamura, M.; Hayami, S. Microwave-assisted catalytic conversion of glucose to 5-hydroxymethylfurfural using “three dimensional” graphene oxide hybrid catalysts. R. Soc. Chem. Adv. 2020, 10, 11727–11736. [Google Scholar] [CrossRef]
- Buchholz, K.; Seibel, J. Industrial carbohydrate biotransformations. Carbohydr. Res. 2008, 343, 1966–1979. [Google Scholar] [CrossRef]
- Graça, I.; Iruretagoyena, D.; Chadwick, D. Glucose isomerisation into fructose over magnesium-impregnated NaY zeolite catalysts. Appl. Catal. B Environ. 2017, 206, 434–443. [Google Scholar] [CrossRef]
- Graça, I.; Bacariza, M.C.; Fernandes, A.; Chadwick, D. Desilicated NaY zeolites impregnated with magnesium as catalysts for glucose isomerisation into fructose. Appl. Catal. B Environ. 2018, 224, 660–670. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Ipsakis, D.K.; Karakoulia, S.A.; Kalogiannis, K.G.; Yiannoulakis, H.; Triantafyllidis, K.S.; Lappas, A.A. Isomerization of glucose into fructose over natural and synthetic MgO catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16459–16470. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Xiao, K.; Xia, H. Isomerization of glucose into fructose by environmentally friendly Fe/β zeolite catalysts. Carbohydr. Res 2017, 446–447, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, R.; Takeuchi, K. Encouragements for the Use of Microwaves in Industrial Chemistry. Chem. Rec. 2019, 19, 51–64. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Gude, V.G.; Patil, P.; Deng, S. Microwave energy potential for large scale biodiesel production. In Proceedings of the World Renewable Energy Forum, WREF 2012, Including World Renewable Energy Congress XII and Colorado Renewable Energy Society (CRES) Annual Conference, Denver, CO, USA, 13–17 May 2012; pp. 751–759. [Google Scholar]
- Iris, K.M.Y.; Xiong, X.; Tsang, D.C.W.; Ng, Y.H.; Clark, J.H.; Fan, J.; Zhang, S.; Hu, C.; Ok, Y.S. Graphite oxide- and graphene oxide-supported catalysts for microwave-assisted glucose isomerisation in water. Green Chem. 2019, 21, 4341–4353. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Effects of microwave dielectric heating on heterogeneous catalysis. Catal. Lett. 2003, 88, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, L.W.; Dong, Y.N.; Zhang, Q.; Weng, W.Z.; Wan, H.L. Glucose isomerization into fructose catalyzed by MgO/NaY catalyst. Chin. J. Chem. Phys. 2018, 31, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Glucose to fructose isomerization in aqueous media over homogeneous and heterogeneous catalysts. ChemCatChem 2016, 8, 1100–1110. [Google Scholar] [CrossRef]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? C. R. Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Durand, R.; Roux, A.; Tichit, D. Isomerization of glucose into fructose in the presence of cation-exchanged zeolites and hydrotalcites. Appl. Catal. A Gen. 2000, 193, 257–264. [Google Scholar] [CrossRef]
- Osakoo, N.; Pansakdanon, C.; Sosa, N.; Deekamwong, K.; Keawkumay, C.; Rongchapo, W.; Chanlek, N.; Jitcharoen, J.; Prayoonpokarach, S.; Wittayakun, J. Characterization and comprehension of zeolite NaY/mesoporous SBA-15 composite as adsorbent for paraquat. Mater. Chem. Phys. 2017, 193, 470–476. [Google Scholar] [CrossRef]
- Sharma, P.; Jeong, S.J.; Han, M.H.; Cho, C.H. Influence of silica precursors on octahedron shaped nano NaY zeolite crystal synthesis. J. Taiwan Inst. Chem. Eng. 2015, 50, 259–265. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Rongchapo, W.; Keawkumay, C.; Chanlek, N.; Prayoonpokarach, S.; Wittayakun, J. Extraction of silica from cogon grass and utilization for synthesis of zeolite NaY by conventional and microwave-assisted hydrothermal methods. J. Taiwan Inst. Chem. Eng. 2018, 83, 152–158. [Google Scholar] [CrossRef]
- Kulawong, S.; Chanlek, N.; Osakoo, N. Facile synthesis of hierarchical structure of NaY zeolite using silica from cogon grass for acid blue 185 removal from water. J. Environ. Chem. Eng. 2020, 8, 104114. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Hattayanone, M.; Cao, J. Effect of agricultural waste burning season on PM2.5-bound polycyclic aromatic hydrocarbon (PAH) levels in Northern Thailand. Atmos. Pollut. Res. 2017, 8, 1069–1080. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Jantarit, N.; Tayraukham, P.; Osakoo, N.; Föttinger, K.; Wittayakun, J. Formation of EMT/FAU intergrowth and nanosized SOD zeolites from synthesis gel of zeolite NaX containing ethanol. Mater. Res. Express 2020, 7, 075011. [Google Scholar] [CrossRef]
- Osakoo, N.; Khemthong, P.; Roessner, F.; Kidkhunthod, P.; Chanlek, N.; Prayoonpokarach, S.; Wittayakun, J. Development and characterization of silica supported cobalt oxides for ethanol oxidation using different preparation methods, Radiat. Phys. Chem. 2020, 171, 108718. [Google Scholar] [CrossRef]
- Oruji, S.; Khoshbin, R.; Karimzadeh, R. Preparation of hierarchical structure of Y zeolite with ultrasonic-assisted alkaline treatment method used in catalytic cracking of middle distillate cut: The effect of irradiation time. Fuel Process. Technol. 2018, 176, 283–295. [Google Scholar] [CrossRef]
- Baranowski, C.J.; Bahmanpour, A.M.; Héroguel, F.; Luterbacher, J.S.; Kröcher, O. Prominent role of mesopore surface area and external acid sites for the synthesis of polyoxymethylene dimethyl ethers (OME) on a hierarchical H-ZSM-5 zeolite. Catal. Sci. Technol. 2019, 9, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Rakmae, S.; Osakoo, N.; Pimsuta, M.; Deekamwong, K.; Keawkumay, C.; Butburee, T.; Faungnawakij, K.; Geantet, C.; Prayoonpokarach, S.; Wittayakun, J.; et al. Defining nickel phosphides supported on sodium mordenite for hydrodeoxygenation of palm oil. Fuel Process. Technol. 2020, 198, 106236. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhu, J.H. A query on the Mg 2p binding energy of MgO. Res. Chem. Intermed. 2019, 45, 947–950. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Dong, D.; Li, D.; Hill, M.R.; Hill, A.J.; Wang, H. Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes. Microporous Mesoporous Mater. 2010, 127, 167–175. [Google Scholar] [CrossRef]
- Manadee, S.; Sophiphun, O.; Osakoo, N.; Supamathanon, N.; Kidkhunthod, P.; Chanlek, N.; Wittayakun, J.; Prayoonpokarach, S. Identification of potassium phase in catalysts supported on zeolite NaX and performance in transesterification of Jatropha seed oil. Fuel Process. Technol. 2017, 156, 62–67. [Google Scholar] [CrossRef]
- Selvamani, T.; Sinhamahapatra, A.; Bhattacharjya, D.; Mukhopadhyay, I. Rectangular MgO microsheets with strong catalytic activity. Mater. Chem. Phys. 2011, 129, 853–861. [Google Scholar] [CrossRef]
- Han, H.; Liu, M.; Ding, F.; Wang, Y.; Guo, X.; Song, C. Effects of cesium ions and cesium oxide in side-chain alkylation of toluene with methanol over cesium-modified zeolite X. Ind. Eng. Chem. Res. 2016, 55, 1849–1858. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, K.; Chen, L.; Kübel, C.; Richards, R. MgO(111) Nanosheets with unusual surface activity. J. Phys. Chem. C 2007, 111, 12038–12044. [Google Scholar] [CrossRef]
- Arishtirova, K.; Kovacheva, P.; Vassilev, S. BaO/NaX zeolite as a basic catalyst for oxidative methylation of toluene with methane. Appl. Catal. A Gen. 2001, 213, 197–202. [Google Scholar] [CrossRef]
- Lecomte, J.; Finiels, A.; Moreau, C. Kinetic study of the isomerization of glucose into fructose in the presence of anion-modified hydrotalcites. Starch Staerke 2002, 54, 75–79. [Google Scholar] [CrossRef]
- Turner, M.D.; Laurence, R.L.; Conner, W.C.; Yngvesson, K.S. Microwave radiation’s influence on sorption and competitive sorption in zeolites. Am. Inst. Chem. Eng. J. 2000, 46, 758–768. [Google Scholar] [CrossRef]
- Xiouras, C.; Radacsi, N.; Sturm, G.; Stefanidis, G.D. Furfural synthesis from D-xylose in the presence of sodium chloride: Microwave versus conventional heating. ChemSusChem 2016, 9, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.S.J.; Verweij, M.D.; Van Gerven, T.; Stankiewicz, A.I.; Stefanidis, G.D. On the effect of resonant microwave fields on temperature distribution in time and space. Int. J. Heat Mass Transf. 2012, 55, 3800–3811. [Google Scholar] [CrossRef]
- Sturm, G.S.J.; Verweij, M.D.; Van Gerven, T.; Stankiewicz, A.I.; Stefanidis, G.D. On the parametric sensitivity of heat generation by resonant microwave fields in process fluids. Int. J. Heat Mass Transf. 2013, 57, 375–388. [Google Scholar] [CrossRef]
- Cherbański, R.; Rudniak, L. Modelling of microwave heating of water in a monomode applicator e Influence of operating conditions. Int. J. Therm. Sci. 2013, 74, 214–229. [Google Scholar] [CrossRef]
- Kumar, S.; Nepak, D.; Kansal, S.K.; Elumalai, S. Expeditious isomerization of glucose to fructose in aqueous media over sodium titanate nanotubes. R. Soc. Chem. Adv. 2018, 8, 30106–30114. [Google Scholar] [CrossRef] [Green Version]
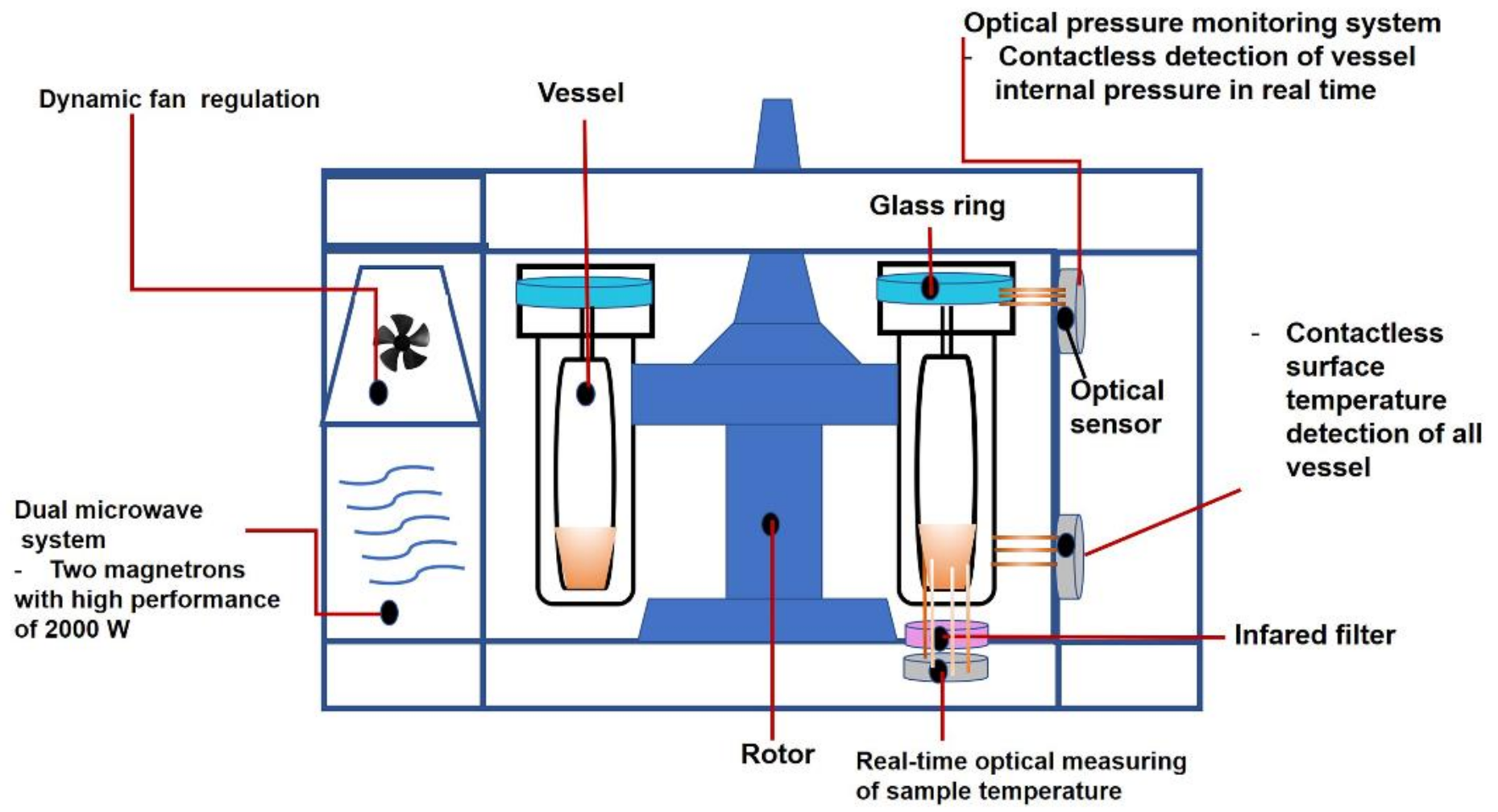
- Manual User, Speedwave Xpert Microwave Digestion System. Available online: https://www.berghof-instruments.com/en/product/speedwave-xpert (accessed on 2 August 2021).
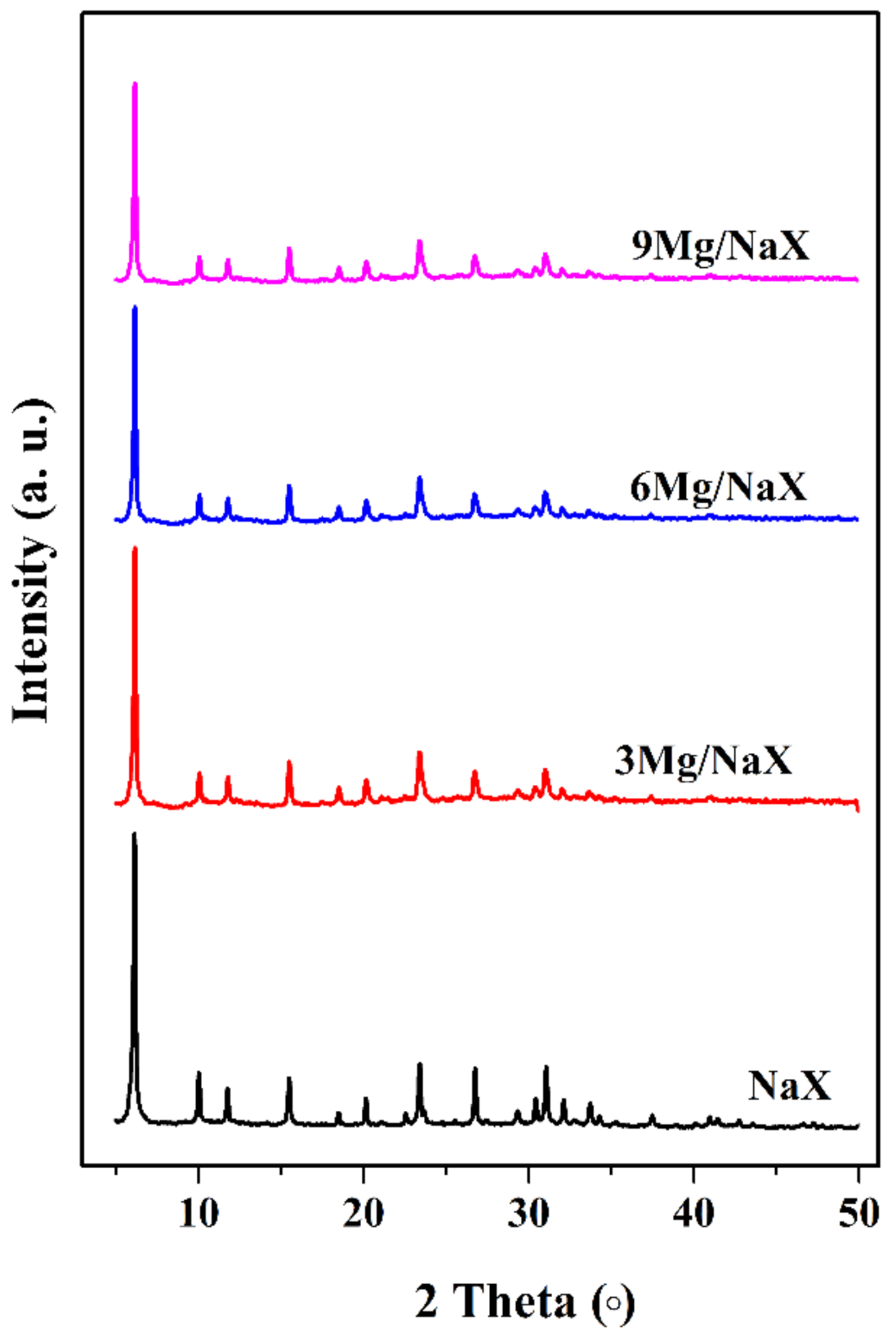

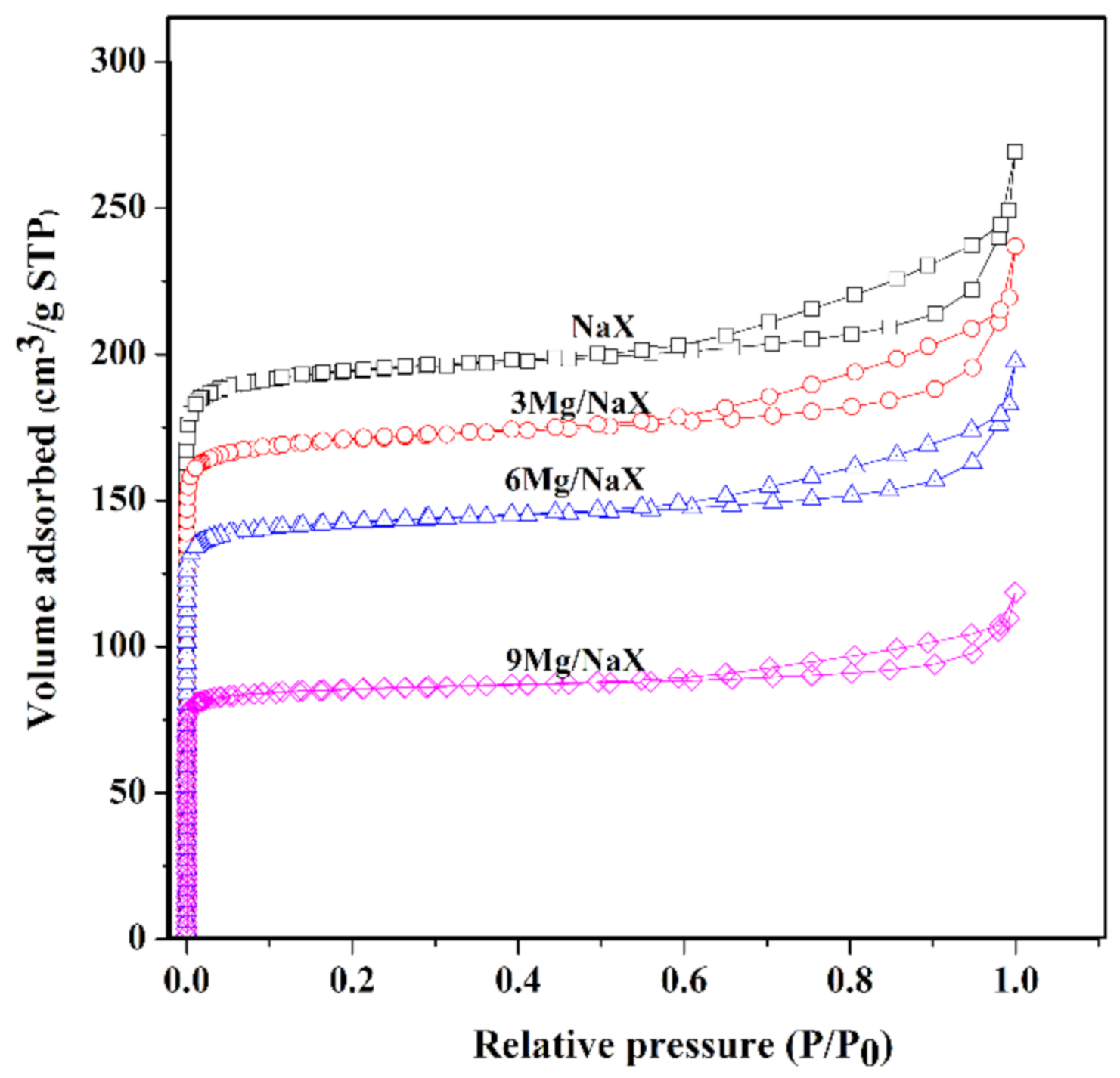




| Sample | a Crystallinity (%) | N2-Sorption | c CO2-TPD (μmol/g) | d Mg Content (wt.%) | |||
|---|---|---|---|---|---|---|---|
| b Surface Area (m2g−1) | b Micropore Volume (cm3g−1) | Weak | Medium | Strong | |||
| NaX | 100 | 630 | 0.27 | - | 241 | - | - |
| 3Mg/NaX | 94.5 | 522 | 0.23 | 18.9 | 41.4 | 207 | 3.10 ± 0.08 |
| 6Mg/NaX | 90.1 | 445 | 0.19 | 27.9 | 48.4 | 273 | 6.07 ± 0.02 |
| 9Mg/NaX | 78.3 | 238 | 0.10 | 20.7 | 43.2 | 367 | 9.05 ± 0.03 |
| Catalyst | Temp. (°C) | Time (h) | Catalyst Amount (g) | Fructose Yield (%) | Fructose Productivity (g fructose/g catalyst/h) | Ref. |
|---|---|---|---|---|---|---|
| NaX | 95 | 1 | 1 | 17 | 0.57 | [20] |
| Hydrotalcite (Mg/Al = 2.5) | 95 | 1 | 1 | 20 | 0.84 | [20] |
| 10% MgNaY | 100 | 0.5 | 0.1 | 31 | 6.20 | [8] |
| Fe/beta-zeolite (0.060) | 150 | 1.5 | 0.1 | 22 | 1.46 | [11] |
| Natural-MgO | 90 | 0.75 | 1 | 33.4 | 3.56 | [10] |
| Sodium titanate nanotubes | 90 | 0.5 | 0.15 | 30.8 | 6.41 | [42] |
| NaX from cogon grass | 90 | 0.25 | 0.15 | 12.5 | 3.22 | This work |
| 6Mg/NaX from cogon grass | 90 | 0.25 | 0.15 | 25.8 | 6.88 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulawong, S.; Youngjan, S.; Khemthong, P.; Chanlek, N.; Wittayakun, J.; Osakoo, N. Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of Fructose via Microwave-Assisted Catalytic Glucose Isomerization. Catalysts 2021, 11, 981. https://doi.org/10.3390/catal11080981
Kulawong S, Youngjan S, Khemthong P, Chanlek N, Wittayakun J, Osakoo N. Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of Fructose via Microwave-Assisted Catalytic Glucose Isomerization. Catalysts. 2021; 11(8):981. https://doi.org/10.3390/catal11080981
Chicago/Turabian StyleKulawong, Sittichai, Saran Youngjan, Pongtanawat Khemthong, Narong Chanlek, Jatuporn Wittayakun, and Nattawut Osakoo. 2021. "Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of Fructose via Microwave-Assisted Catalytic Glucose Isomerization" Catalysts 11, no. 8: 981. https://doi.org/10.3390/catal11080981
APA StyleKulawong, S., Youngjan, S., Khemthong, P., Chanlek, N., Wittayakun, J., & Osakoo, N. (2021). Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of Fructose via Microwave-Assisted Catalytic Glucose Isomerization. Catalysts, 11(8), 981. https://doi.org/10.3390/catal11080981