Partial Oxidation of Methane over CaO Decorated TiO2 Nanocatalyst for Syngas Production in a Fixed Bed Reactor
Abstract
:1. Introduction
2. Results and Discussion
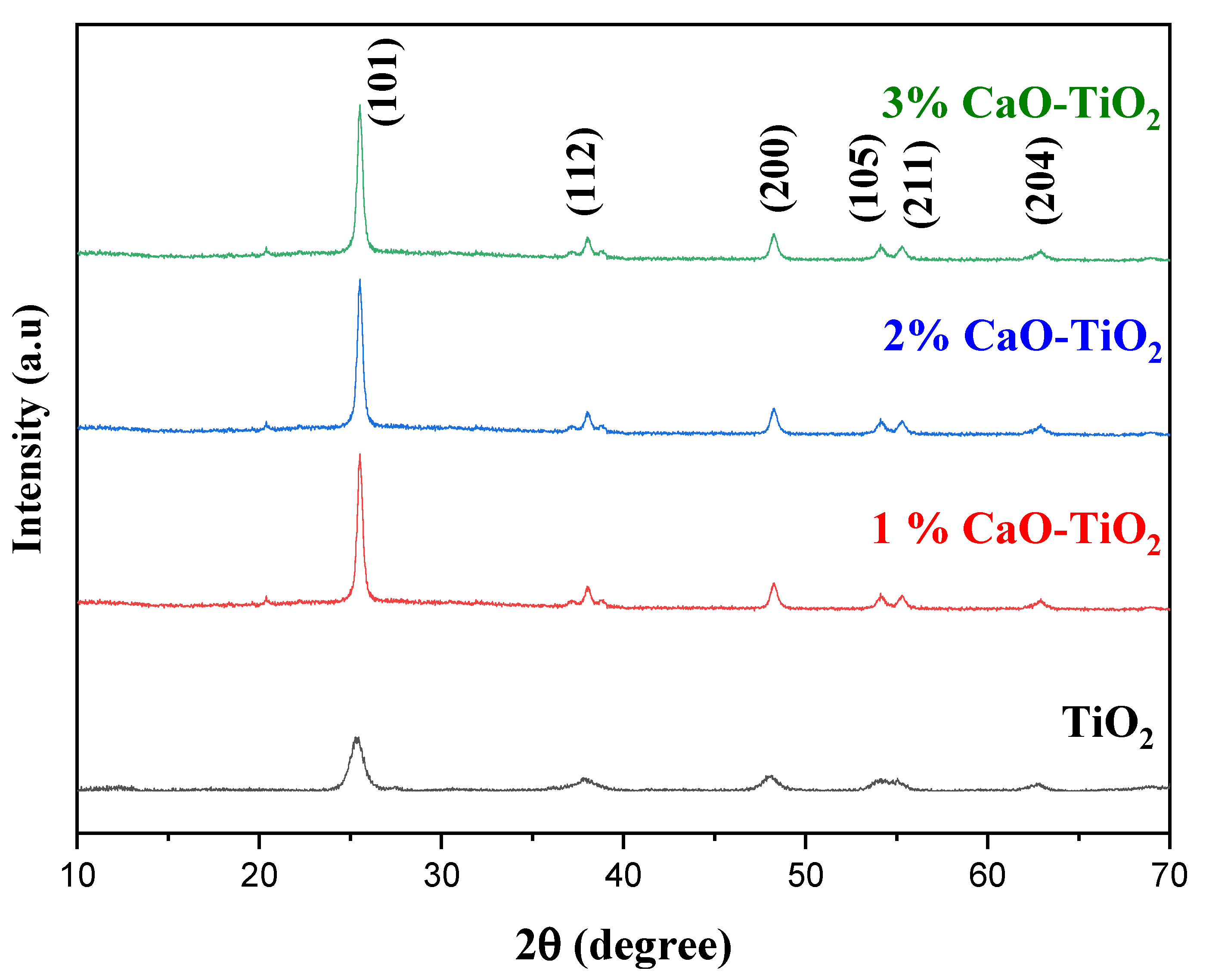
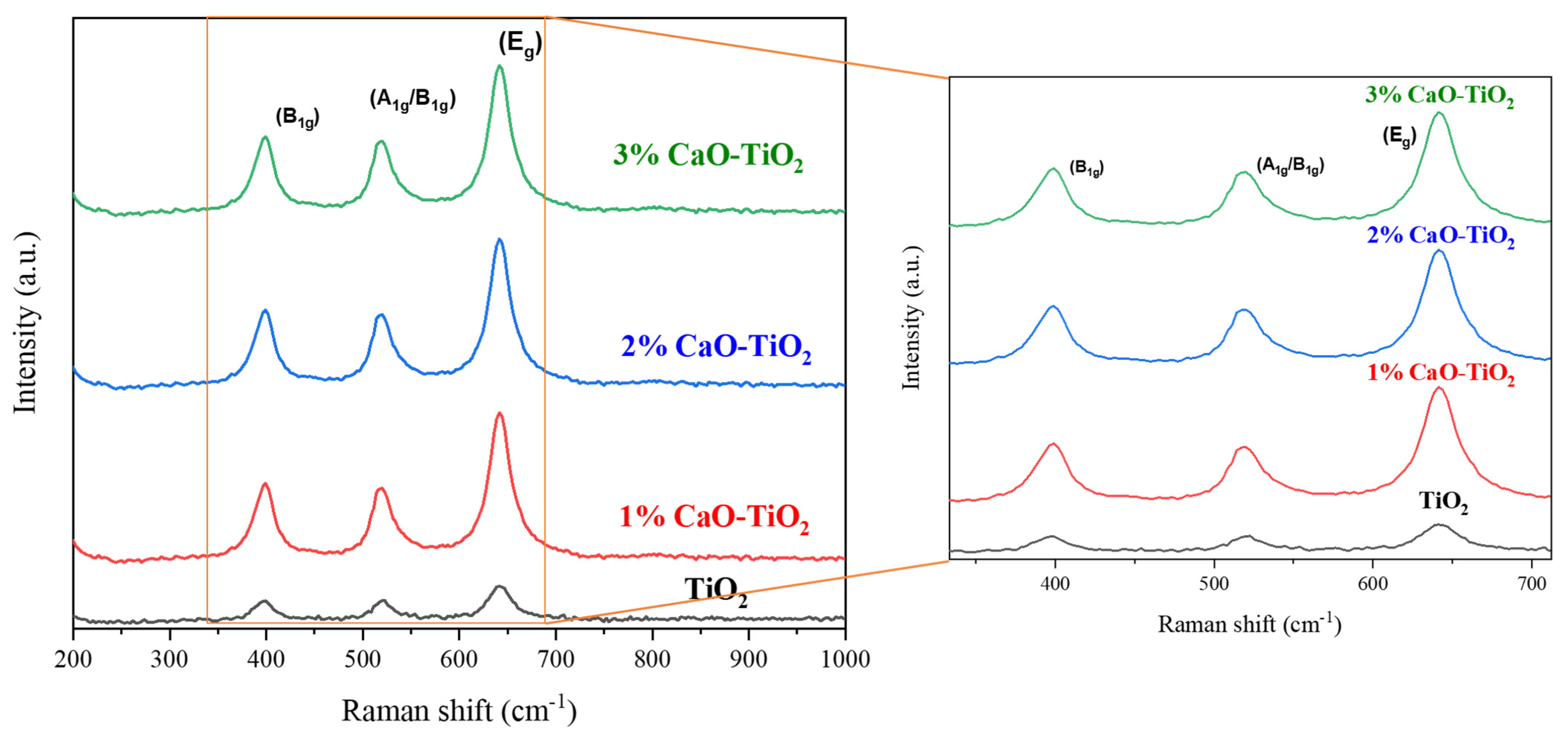
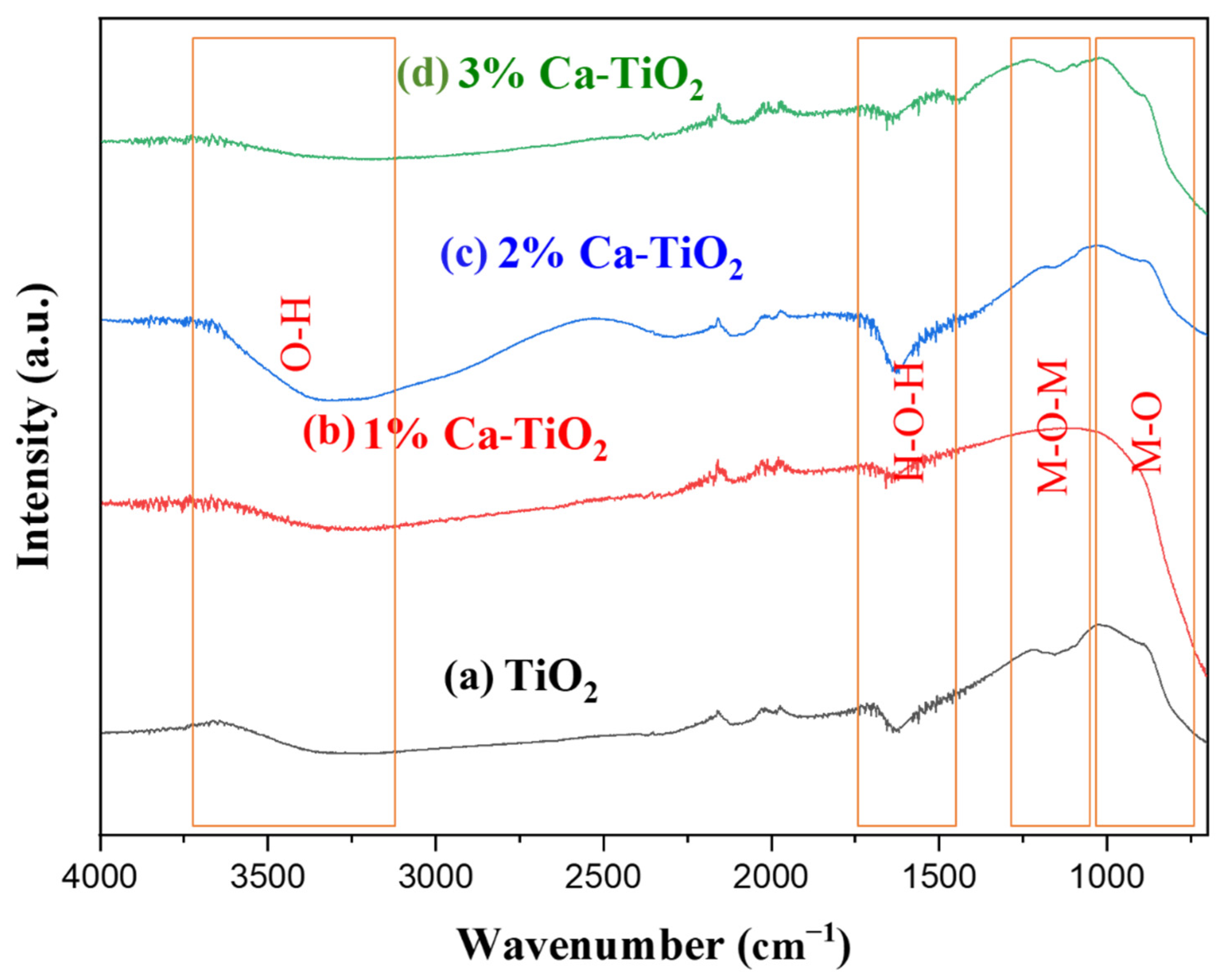
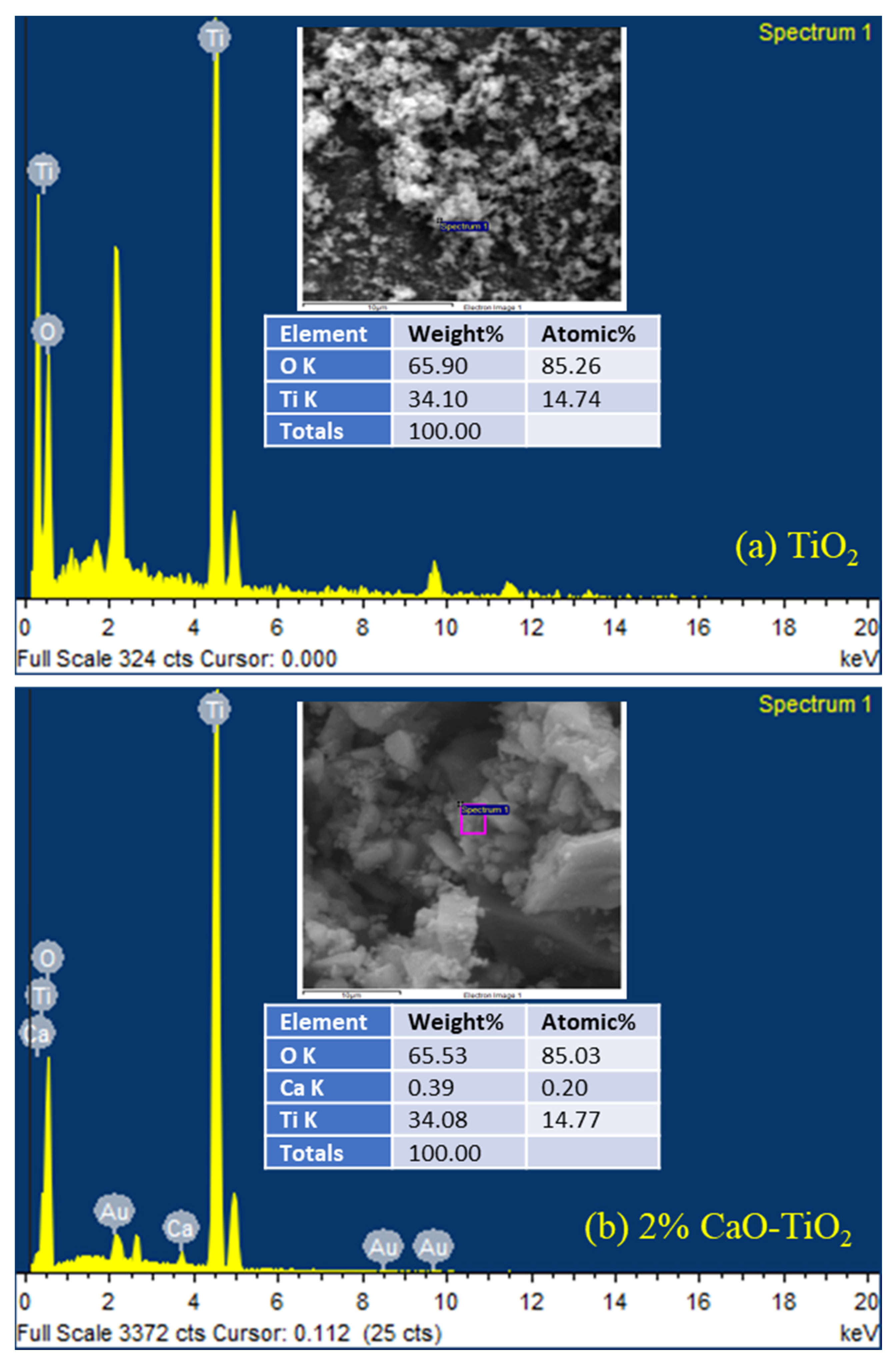
2.1. Physicochemical Properties of the Catalyst
2.2. Catalyst Performance Analysis
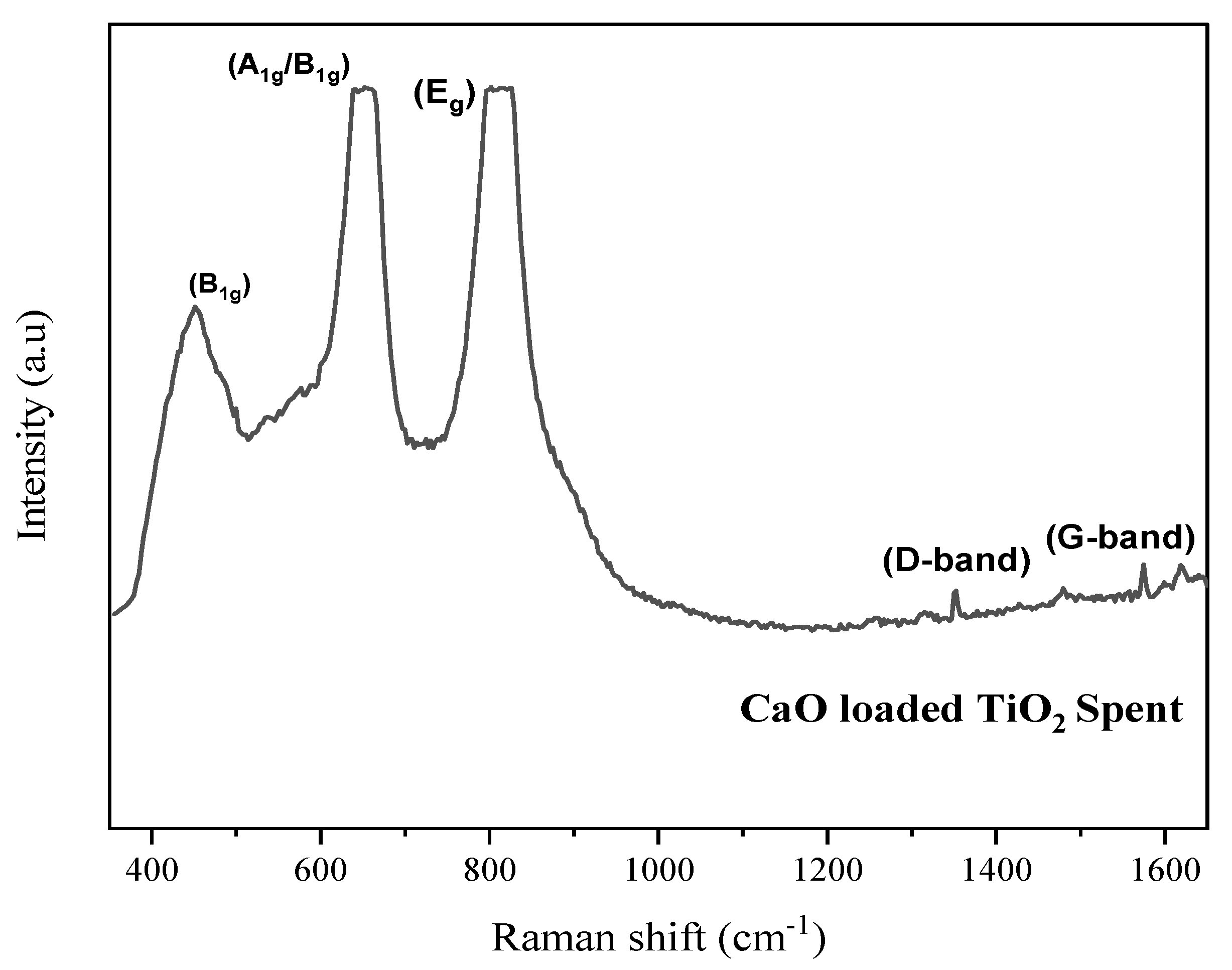
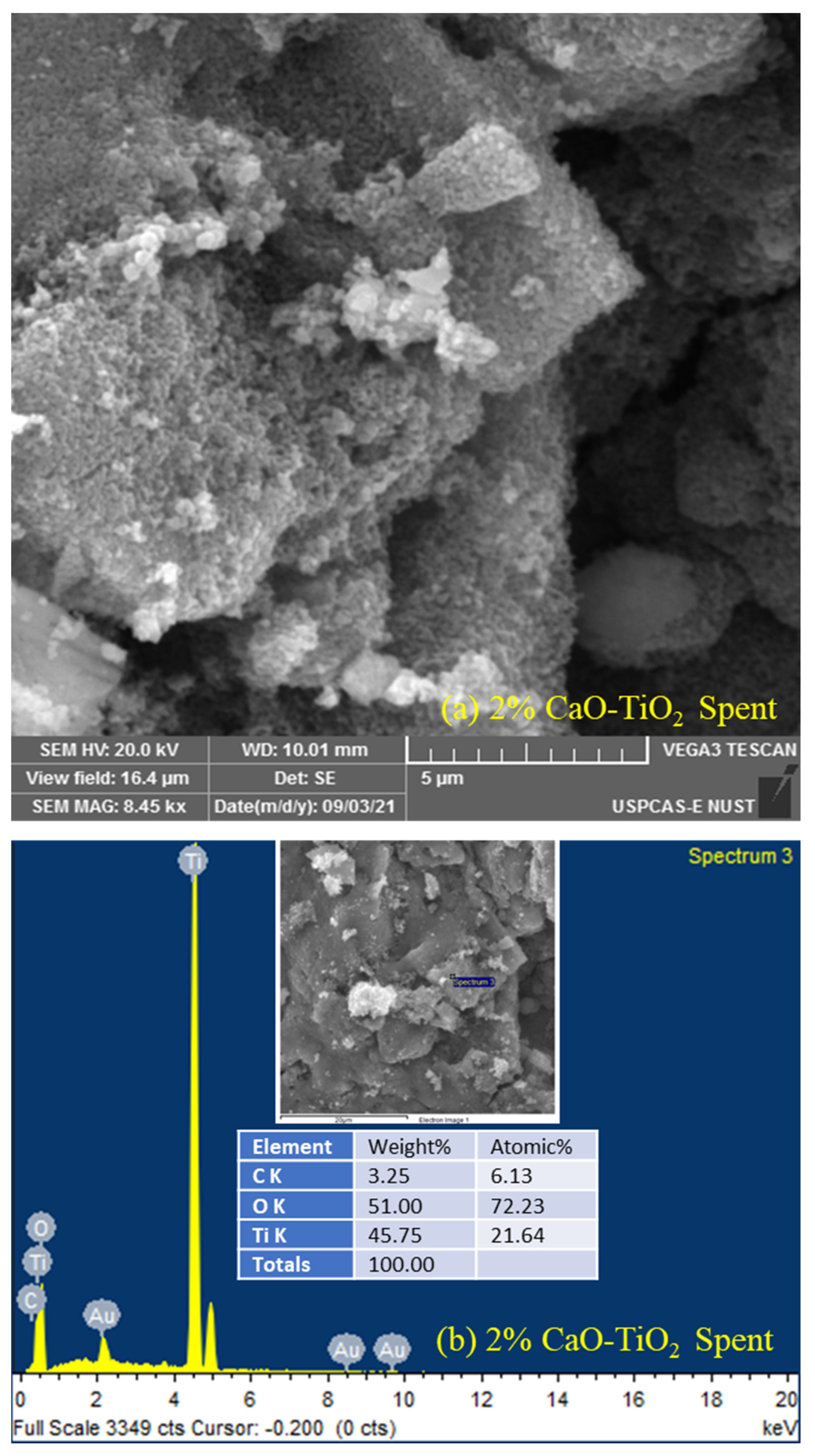
2.3. Characterization of Spent Catalyst
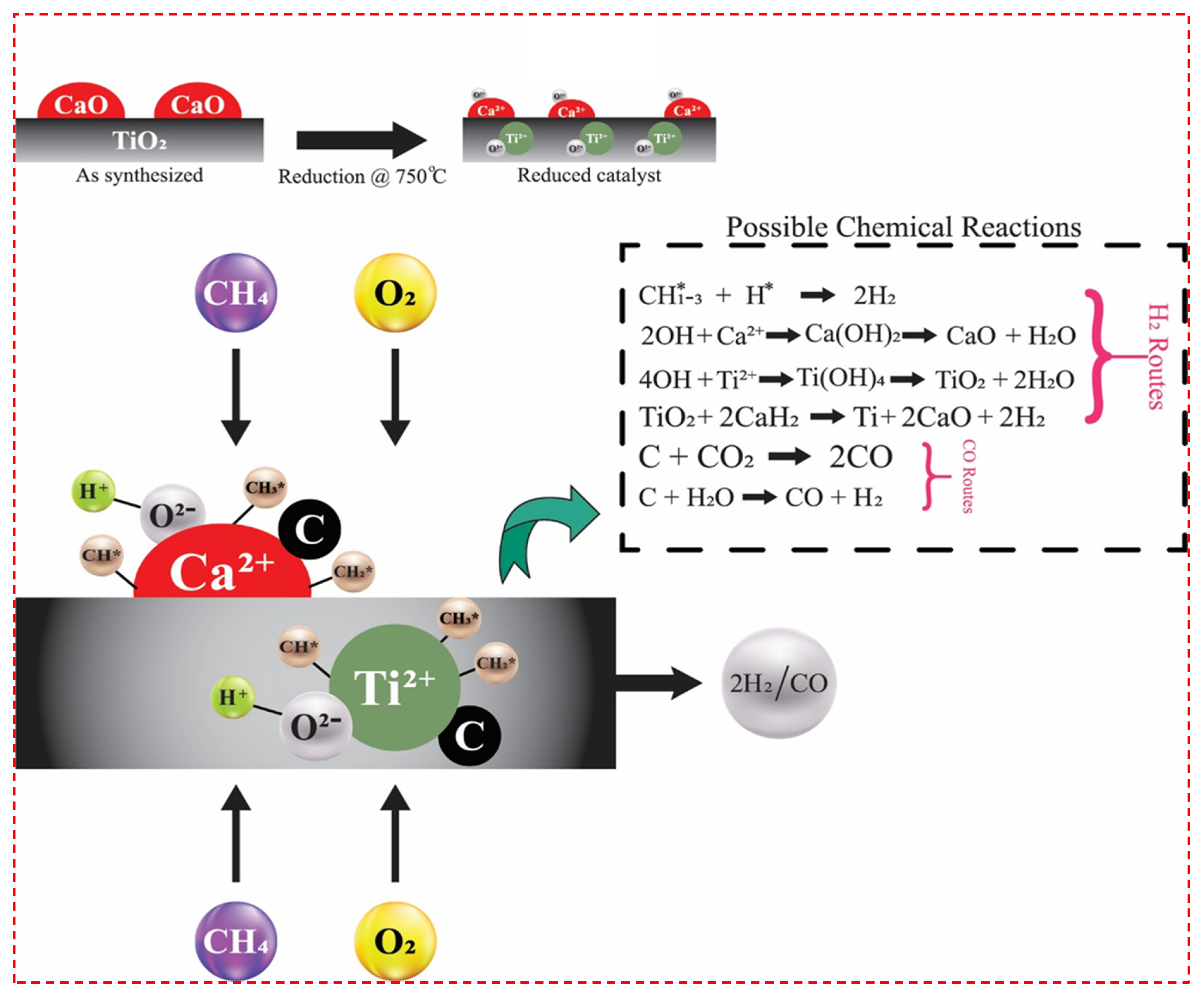
2.4. Reaction Mechanism
3. Materials and Methods
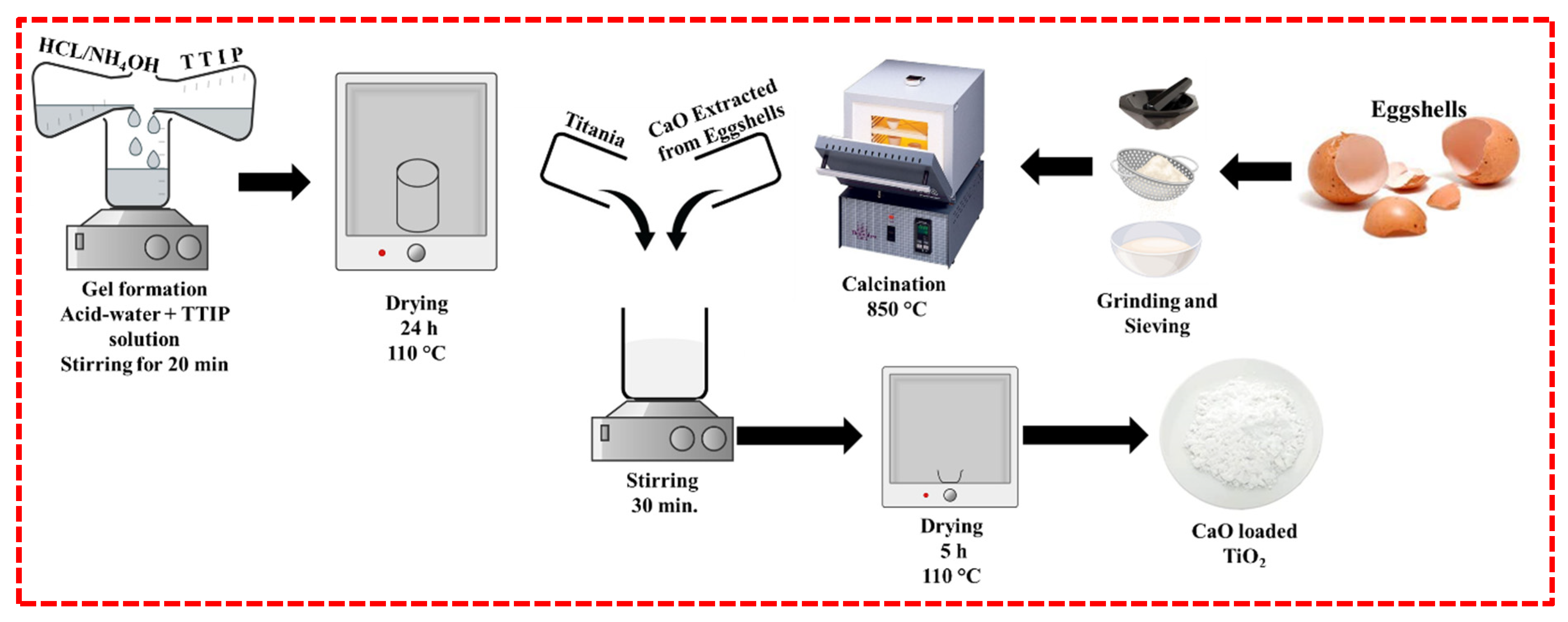
3.1. Synthesis of CaO Doped TiO2
3.2. Material Characterisation
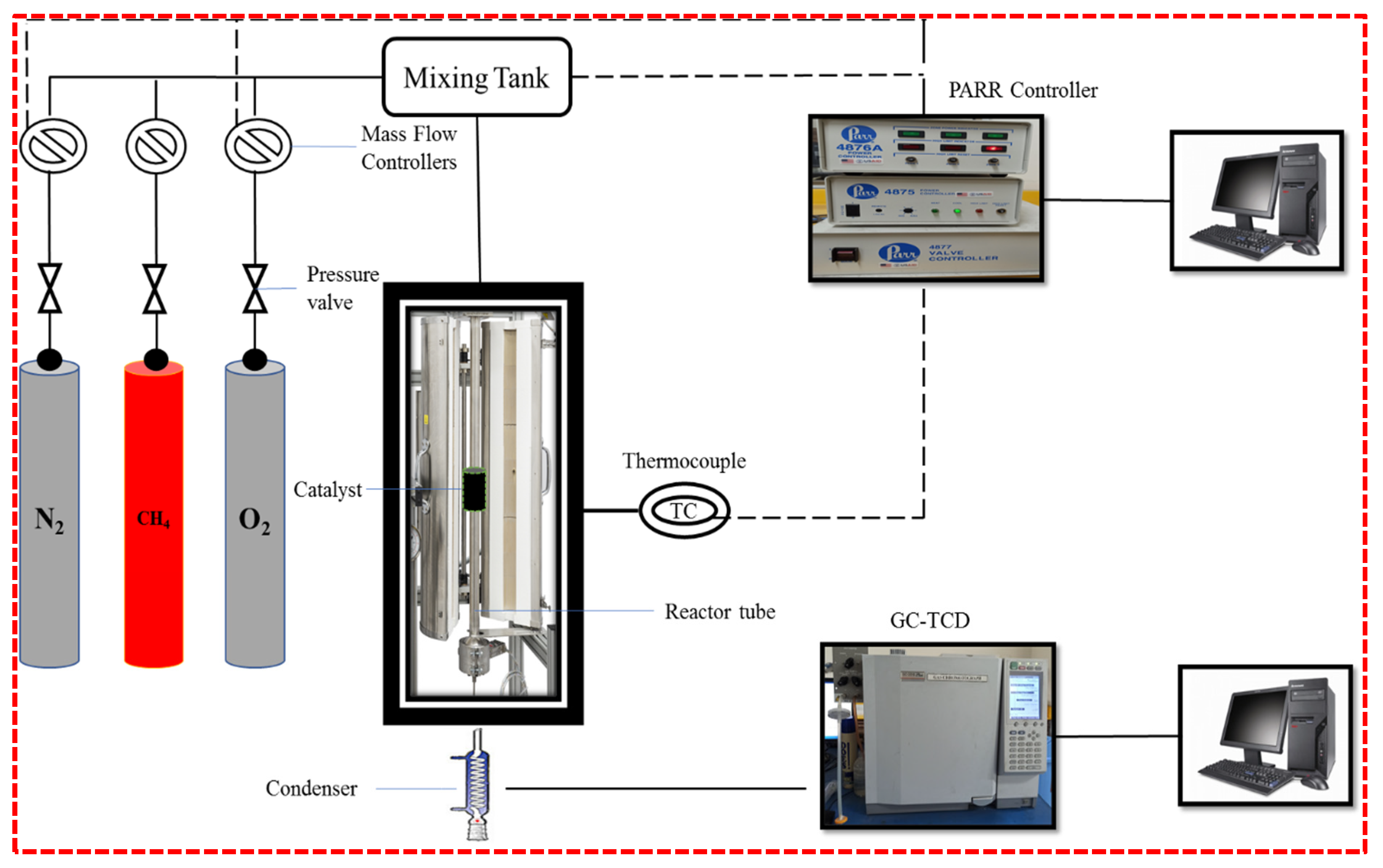
3.3. Partial Oxidation of Methane Setup
3.4. Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.; Mirzaei, M.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, G.; Gao, X.; Yamane, N.; Zhang, P.; Liu, G.; Tsubaki, N. Ni/Silicalite-1 coating being coated on SiC foam: A tailor-made monolith catalyst for syngas production using a combined methane reforming process. Chem. Eng. J. 2017, 327, 465–473. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Mathew, T.; May, R.B.; Prakash, G.S. Single step bi-reforming and oxidative bi-reforming of methane (natural gas) with steam and carbon dioxide to metgas (CO-2H2) for methanol synthesis: Self-sufficient effective and exclusive oxygenation of methane to methanol with oxygen. J. Am. Chem. Soc. 2015, 137, 8720–8729. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Gao, X.; Liu, G.; Yang, R.; Zhang, H.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Facile one-step synthesis of mesoporous Ni-Mg-Al catalyst for syngas production using coupled methane reforming process. Fuel 2018, 211, 1–10. [Google Scholar] [CrossRef]
- Xing, C.; Yang, G.; Wu, M.; Yang, R.; Tan, L.; Zhu, P.; Wei, Q.; Li, J.; Mao, J.; Yoneyama, Y. Hierarchical zeolite Y supported cobalt bifunctional catalyst for facilely tuning the product distribution of Fischer–Tropsch synthesis. Fuel 2015, 148, 48–57. [Google Scholar] [CrossRef]
- Ding, H.; Xu, Y.; Luo, C.; Wang, Q.; Shen, C.; Xu, J.; Zhang, L. A novel composite perovskite-based material for chemical-looping steam methane reforming to hydrogen and syngas. Energy Convers. Manag. 2018, 171, 12–19. [Google Scholar] [CrossRef]
- Horn, R.; Schlögl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C. CO2 reforming of methane combined with steam reforming or partial oxidation of methane to syngas over NdCoO3 perovskite-type mixed metal-oxide catalyst. Appl. Energy 2006, 83, 1024–1032. [Google Scholar] [CrossRef]
- Cao, D.; Ding, H.; Luo, C.; Wu, F.; Li, X.; Zhang, L. Development of a cordierite monolith reactor coated with CeO2-supported BaSrCo-based perovskite for chemical looping steam methane reforming. Fuel Processing Technol. 2021, 220, 106889. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Khajenoori, M. Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrogen Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yamaki, T.; Taniguchi, S.; Endo, A.; Kataoka, S. Integrating life cycle assessment for design and optimization of methanol production from combining methane dry reforming and partial oxidation. J. Clean. Prod. 2021, 292, 125970. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva Junior, R.B.; Frety, R.; Neto, É.T.; Brandão, S.T. Study of nickel, lanthanum and niobium-based catalysts applied in the partial oxidation of methane. Catal. Today 2020, 344, 15–23. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic partial oxidation of methane to syngas: Review of perovskite catalysts and membrane reactors. Catal. Rev. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Zhao, Y.-J.; Yi, Q.; Wei, G.-Q.; Shi, L.-J.; Zhou, H. NiO/κ-CeZrO4 functional oxygen carriers with Niδ+ and oxygen vacancy synergy for chemical looping partial oxidation reforming of methane. Fuel Process. Technol. 2021, 219, 106875. [Google Scholar] [CrossRef]
- Kim, H.W.; Kang, K.M.; Kwak, H.-Y. Preparation of supported Ni catalysts with a core/shell structure and their catalytic tests of partial oxidation of methane. Int. J. Hydrogen Energy 2009, 34, 3351–3359. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, L.; Fang, Y.; Li, H.; Zhang, J.; Zhao, T.-S.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Combined methane dry reforming and methane partial oxidization for syngas production over high dispersion Ni based mesoporous catalyst. Fuel Process. Technol. 2019, 188, 98–104. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.; Hansen, J.B. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Jin, R.; Chen, Y.; Li, W.; Cui, W.; Ji, Y.; Yu, C.; Jiang, Y. Mechanism for catalytic partial oxidation of methane to syngas over a Ni/Al2O3 catalyst. Appl. Catal. A Gen. 2000, 201, 71–80. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni catalysts on different supports to obtain synthesis gas. Int. J. Hydrogen Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Ding, C.; Duan, Q.; Ma, Q.; Zhang, K.; Liu, P. Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane. Int. J. Hydrogen Energy 2018, 43, 6603–6613. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Fundamentals and their applications in organic reactions. Appl. Catal. A Gen. 2015, 504, 103–109. [Google Scholar] [CrossRef]
- Kwong, T.-L.; Yung, K.-F. Heterogeneous alkaline earth metal–transition metal bimetallic catalysts for synthesis of biodiesel from low grade unrefined feedstock. RSC Adv. 2015, 5, 83748–83756. [Google Scholar] [CrossRef]
- Fleys, M.; Simon, Y.; Swierczynski, D.; Kiennemann, A.; Marquaire, P.-M. Investigation of the Reaction of Partial Oxidation of Methane over Ni/La2O3 Catalyst. Energy Fuels 2006, 20, 2321–2329. [Google Scholar] [CrossRef]
- Choudhary, V.; Rajput, A.; Rane, V. Low temperature oxidative conversion of methane to synthesis gas over Co/rare earth oxide catalysts. Catal. Lett. 1992, 16, 269–272. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Dedov, A.; Loktev, A.; Danilov, V.; Krasnobaeva, O.; Nosova, T.; Mukhin, I.; Baranchikov, A.; Yorov, K.E.; Bykov, M.; Moiseev, I. Catalytic Materials Based on Hydrotalcite-Like Aluminum, Magnesium, Nickel, and Cobalt Hydroxides: Effect of the Nickel/Cobalt Ratio on the Results of Partial Oxidation and Dry Reforming of Methane to Synthesis Gas. Pet. Chem. 2020, 60, 194–203. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Ma, J.-J.; Zhu, H.-L.; Zheng, Y.-Q. Self-supported beaded Au@ Co3O4 nanowire arrays perform electrocatalytic CO2 reduction in water to syngas and water oxidation to O2. New J. Chem. 2020, 44, 11808–11816. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Preparation of Ni/MeAl2O4-MgAl2O4 (Me = Fe, Co, Ni, Cu, Zn, Mg) nanocatalysts for the syngas production via combined dry reforming and partial oxidation of methane. Renew. Energy 2020, 149, 1053–1067. [Google Scholar] [CrossRef]
- Martínez-Navarro, B.; Sanchis, R.; Asedegbega-Nieto, E.; Solsona, B.; Ivars-Barceló, F. (Ag) Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature. Nanomaterials 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Barona, M.; Gaggioli, C.A.; Gagliardi, L.; Snurr, R.Q. DFT Study on the Catalytic Activity of ALD-Grown Diiron Oxide Nanoclusters for Partial Oxidation of Methane to Methanol. J. Phys. Chem. A 2020, 124, 1580–1592. [Google Scholar] [CrossRef]
- Khaleel, A.; Jobe, S.; Ahmed, M.a.; Al-Zuhair, S.; Tariq, S. Enhanced selectivity of syngas in partial oxidation of methane: A new route for promising Ni-alumina catalysts derived from Ni/γ-AlOOH with modified Ni dispersion. Int. J. Energy Res. 2020, 44, 12081–12099. [Google Scholar] [CrossRef]
- Świrk, K.; Grams, J.; Motak, M.; Da Costa, P.; Grzybek, T. Understanding of tri-reforming of methane over Ni/Mg/Al hydrotalcite-derived catalyst for CO2 utilization from flue gases from natural gas-fired power plants. J. CO2 Util. 2020, 42, 101317. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Hassan, M.; Liaquat, R.; Naqvi, S.R.; Mehran, M.T.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S. Optimization of Alkaline Earth Metal Oxide and Hydroxide Catalysts for Base-Catalyzed Reactions. In Advances in Catalysis; Gates, B.C., Knözinger, H., Eds.; Academic Press: Cambridge, MA, USA, 2006; Volume 49, pp. 239–302. [Google Scholar]
- Javed, A.H.; Shahzad, N.; Butt, F.A.; Khan, M.A.; Naeem, N.; Liaquat, R.; Khoja, A.H. Synthesis of bimetallic Co-Ni/ZnO nanoprisms (ZnO-NPr) for hydrogen-rich syngas production via partial oxidation of methane. J. Environ. Chem. Eng. 2021, 9, 106887. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, J.; Zhang, J. Effects of CeO2 and CaO composite promoters on the properties of eggshell Ni/MgO-Al2O3 catalysts for partial oxidation of methane to syngas. React. Kinet. Catal. Lett. 2008, 94, 351–357. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; González-Carrazán, S.R.; Soria, M.A.; Ruíz, P. Effect of the nature of TiO2 support over the performances of Rh/TiO2 catalysts in the partial oxidation of methane. Catal. Today 2013, 203, 158–162. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M.; Mohamed, M.; Hussain Khoja, A. Effect of support size for stimulating hydrogen production in phenol steam reforming using Ni-embedded TiO2 nanocatalyst. J. Environ. Chem. Eng. 2020, 8, 103604. [Google Scholar] [CrossRef]
- Yan, Q.; Weng, W.Z.; Wan, H.L.; Toghiani, H.; Toghiani, R.K.; Pittman, C. Activation of methane to syngas over a Ni/TiO2 catalyst. Appl. Catal. A Gen. 2003, 239, 43–58. [Google Scholar] [CrossRef]
- Perkas, N.; Zhong, Z.; Chen, L.; Besson, M.; Gedanken, A. Sonochemically prepared high dispersed Ru/TiO2 mesoporous catalyst for partial oxidation of methane to syngas. Catal. Lett. 2005, 103, 9–14. [Google Scholar] [CrossRef]
- Elmasides, C.; Kondarides, D.I.; Neophytides, S.G.; Verykios, X.E. Partial Oxidation of Methane to Synthesis Gas over Ru/TiO2 Catalysts: Effects of Modification of the Support on Oxidation State and Catalytic Performance. J. Catal. 2001, 198, 195–207. [Google Scholar] [CrossRef]
- Efstathiou, A.M.; Kladi, A.; Tsipouriari, V.A.; Verykios, X.E. Reforming of Methane with Carbon Dioxide to Synthesis Gas over Supported Rhodium Catalysts: II. A Steady-State Tracing Analysis: Mechanistic Aspects of the Carbon and Oxygen Reaction Pathways to Form CO. J. Catal. 1996, 158, 64–75. [Google Scholar] [CrossRef]
- Wu, T.; Yan, Q.; Wan, H. Partial oxidation of methane to hydrogen and carbon monoxide over a Ni/TiO2 catalyst. J. Mol. Catal. A Chem. 2005, 226, 41–48. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M. Metal-support interactions during the CO2 reforming of CH4 over model TiOx/Pt catalysts. Catal. Lett. 1997, 48, 31–38. [Google Scholar] [CrossRef]
- Znaidi, L.; Seraphimova, R.; Bocquet, J.; Colbeau-Justin, C.; Pommier, C. A semi-continuous process for the synthesis of nanosize TiO2 powders and their use as photocatalysts. Mater. Res. Bull. 2001, 36, 811–825. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, N.; Xiao, L.; Liu, Y.; Wang, H. MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J. Colloid Interface Sci. 2010, 352, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bazli, L.; Siavashi, M.; Shiravi, A. A review of carbon nanotube/TiO2 composite prepared via sol-gel method. J. Compos. Compd. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- You, Y.F.; Xu, C.H.; Xu, S.S.; Cao, S.; Wang, J.P.; Huang, Y.B.; Shi, S.Q. Structural characterization and optical property of TiO2 powders prepared by the sol–gel method. Ceram. Int. 2014, 40, 8659–8666. [Google Scholar] [CrossRef]
- Lai, C.W. Modification of one-dimensional TiO2 nanotubes with CaO dopants for high CO2 adsorption. Int. J. Photoenergy 2014, 2014, 471713. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Cheng, H.; Du, Y.; Han, X. Synthesis of nano-sized anatase TiO2 with reactive {001} facets using lamellar protonated titanate as precursor. Chem. Commun. 2012, 48, 6978–6980. [Google Scholar] [CrossRef]
- Da Cunha, T.; Maulu, A.; Guillot, J.; Fleming, Y.; Duez, B.; Lenoble, D.; Arl, D. Design of Silica Nanoparticles-Supported Metal Catalyst by Wet Impregnation with Catalytic Performance for Tuning Carbon Nanotubes Growth. Catalysts 2021, 11, 986. [Google Scholar] [CrossRef]
- Shakir, S.; Khan, Z.S.; Ali, A.; Akbar, N.; Musthaq, W. Development of copper doped titania based photoanode and its performance for dye sensitized solar cell applications. J. Alloys Compd. 2015, 652, 331–340. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Orlov, E.A.; Malikov, N.A.; Tarasov, Y.V.; Sheveyko, A.N.; Permyakova, E.S.; Kuptsov, K.A.; Dyatlov, I.A.; Ignatov, S.G.; Ilnitskaya, A.S.; et al. Ag(Pt) nanoparticles-decorated bioactive yet antibacterial Ca- and P-doped TiO2 coatings produced by plasma electrolytic oxidation and ion implantation. Appl. Surf. Sci. 2020, 516, 146068. [Google Scholar] [CrossRef]
- Rattana, T. The Structural, morphological and optical properties of Ca doped TiO2 thin films prepared by sol-gel method. SNRU J. Sci. Technol. 2018, 10, 1–5. [Google Scholar]
- Castro, Y.; Durán, A. Ca doping of mesoporous TiO2 films for enhanced photocatalytic efficiency under solar irradiation. J. Sol-Gel Sci. Technol. 2016, 78, 482–491. [Google Scholar] [CrossRef]
- Liu, Z.; Jian, Z.; Fang, J.; Xu, X.; Zhu, X.; Wu, S. Low-Temperature Reverse Microemulsion Synthesis, Characterization, and Photocatalytic Performance of Nanocrystalline Titanium Dioxide. Int. J. Photoenergy 2012, 2012, 702503. [Google Scholar] [CrossRef]
- Ramasamy, V.; Mohana, V.; Rajendran, V. Characterization of Ca doped CeO2 quantum dots and their applications in photocatalytic degradation. OpenNano 2018, 3, 38–47. [Google Scholar] [CrossRef]
- Kalaivani, T.; Anilkumar, P. Role of temperature on the phase modification of TiO2 nanoparticles synthesized by the precipitation method. Silicon 2018, 10, 1679–1686. [Google Scholar] [CrossRef]
- Rosa, S.; Nossol, A.; Nossol, E.; Zarbin, A.; Peralta-Zamora, P. Non-Synergistic UV-A Photocatalytic Degradation of Estrogens by Nano-TiO 2 Supported on Activated Carbon. J. Braz. Chem. Soc. 2017, 28, 582. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Abd Hamid, S.B. Biotemplated Synthesis of Anatase Titanium Dioxide Nanoparticles via Lignocellulosic Waste Material. BioMed Res. Int. 2014, 2014, 205636. [Google Scholar] [CrossRef] [PubMed]
- Siang, T.J.; Jalil, A.A.; Liew, S.Y.; Owgi, A.H.K.; Rahman, A.F.A. A review on state-of-the-art catalysts for methane partial oxidation to syngas production. Catal. Rev. 2022, 1–57. [Google Scholar] [CrossRef]
- Pulišová, P.; Boháček, J.; Šubrt, J.; Szatmáry, L.; Bezdička, P.; Večerníková, E.; Balek, V. Thermal behaviour of titanium dioxide nanoparticles prepared by precipitation from aqueous solutions. J. Therm. Anal. Calorim. 2010, 101, 607–613. [Google Scholar] [CrossRef]
- Hernández, J.V. Structural and Morphological Modification of TiO2 Doped Metal Ions and Investigation of Photo-Induced Charge Transfer Processes. 2017. Available online: https://tel.archives-ouvertes.fr/tel-01954392/document (accessed on 5 February 2022).
- Sridevi, D.V.; Vadivel, R.; Thirupathi, S.; Geetha, K.; Prabu, R.; Karthikeyan, J.; Rajarajan, K.; Ramachadran, K. Synthesis, Structural and Optical Properties of Co Doped TiO2 Nanocrystals by Sol-Gel Method. Mech. Mater. Sci. Eng. J. 2017, 9. Available online: https://mmse.xyz/en/synthesis-structural-and-optical-properties-of-co-doped-tio2-nanocrystals-by-sol-gel-method/ (accessed on 26 July 2022).
- Al-Taweel, S.S.; Saud, H.R. New route for synthesis of pure anatase TiO2 nanoparticles via utrasound-assisted sol-gel method. J. Chem. Pharm. Res 2016, 8, 620–626. [Google Scholar]
- Biru, M.; Qaderi, J.; Mamat, C.R.; Jalil, A.A. Preparation and Characterization of Copper, Iron, and Nickel Doped Titanium Dioxide Photocatalysts for Decolorization of Methylene Blue. Sains Malays. 2021, 50, 135–149. [Google Scholar]
- Lugo, V.R.; Mondragón-Galicia, G.; Gutiérrez-Martínez, A.; Gutiérrez-Wing, C.; González, O.R.; López, P.; Salinas-Hernández, P.; Tzompantzi, F.; Valderrama, M.I.R.; Pérez-Hernández, R. Pt–Ni/ZnO-rod catalysts for hydrogen production by steam reforming of methanol with oxygen. RSC Adv. 2020, 10, 41315–41323. [Google Scholar] [CrossRef]
- Matras, D.; Jacques, S.D.; Poulston, S.; Grosjean, N.; Estruch Bosch, C.; Rollins, B.; Wright, J.; Di Michiel, M.; Vamvakeros, A.; Cernik, R.J. Operando and postreaction diffraction imaging of the La–Sr/CaO catalyst in the oxidative coupling of methane reaction. J. Phys. Chem. C 2019, 123, 1751–1760. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Erjavec, B.; Pintar, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Rui, Z.; Feng, D.; Chen, H.; Ji, H. Anodic TiO2 nanotube array supported nickel–noble metal bimetallic catalysts for activation of CH4 and CO2 to syngas. Int. J. Hydrogen Energy 2014, 39, 16252–16261. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, S.; Sharma, S.K.; Sharma, M.; Singh, V.P.; Vaish, R. Antibacterial and photocatalytic active transparent TiO2 crystallized CaO–BaO–B2O3–Al2O3–TiO2–ZnO glass nanocomposites. J. Am. Ceram. Soc. 2019, 102, 3378–3390. [Google Scholar] [CrossRef]
- Kikuchi, E.; Chen, Y. Syngas formation by partial oxidation of methane in palladium membrane reactor. Stud. Surf. Sci. Catal. 1998, 119, 441–446. [Google Scholar] [CrossRef]
- De Santana Santos, M.; Neto, R.C.R.; Noronha, F.B.; Bargiela, P.; da Rocha, M.d.G.C.; Resini, C.; Carbó-Argibay, E.; Fréty, R.; Brandão, S.T. Perovskite as catalyst precursors in the partial oxidation of methane: The effect of cobalt, nickel and pretreatment. Catal. Today 2018, 299, 229–241. [Google Scholar] [CrossRef]
- Guo, D.; Wang, G.-C. Partial oxidation of methane on anatase and rutile defective TiO2 supported Rh4 cluster: A density functional theory study. J. Phys. Chem. C 2017, 121, 26308–26320. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Javid, M.; Wang, M.; Khushk, S. Enhanced H2 Production from Municipal Solid Waste Gasification Using Ni–CaO–TiO2 Bifunctional Catalyst Prepared by dc Arc Plasma Melting. Ind. Eng. Chem. Res. 2019, 58, 13408–13419. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Mastro, S.L.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Dychalska, A.; Popielarski, P.; Franków, W.; Fabisiak, K.; Paprocki, K.; Szybowicz, M. Study of CVD diamond layers with amorphous carbon admixture by Raman scattering spectroscopy. Mater. Sci. 2015, 33, 799–805. [Google Scholar] [CrossRef]
- Nath, B.D.; Takaishi, K.; Ema, T. Macrocyclic multinuclear metal complexes acting as catalysts for organic synthesis. Catal. Sci. Technol. 2020, 10, 12–34. [Google Scholar] [CrossRef]
- Ma, R.; Xu, B.; Zhang, X. Catalytic partial oxidation (CPOX) of natural gas and renewable hydrocarbons/oxygenated hydrocarbons—A review. Catal. Today 2019, 338, 18–30. [Google Scholar] [CrossRef]
- Osman, A.I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Liu, T.; Snyder, C.; Veser, G. Catalytic Partial Oxidation of Methane: Is a Distinction between Direct and Indirect Pathways Meaningful? Ind. Eng. Chem. Res. 2007, 46, 9045–9052. [Google Scholar] [CrossRef]
- Heitnes, K.; Lindberg, S.; Rokstad, O.A.; Holmen, A. Catalytic partial oxidation of methane to synthesis gas. Catal. Today 1995, 24, 211–216. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, J.; Zhang, J. Effect of CeO2 and CaO promoters on ignition performance for partial oxidation of methane over Ni/MgO-Al2O3 catalyst. J. Nat. Gas Chem. 2007, 16, 148–154. [Google Scholar] [CrossRef]
- Iizuka, T.; Hattori, H.; Ohno, Y.; Sohma, J.; Tanabe, K. Basic sites and reducing sites of calcium oxide and their catalytic activities. J. Catal. 1971, 22, 130–139. [Google Scholar] [CrossRef]
- Maestri, M.; Vlachos, D.G.; Beretta, A.; Forzatti, P.; Groppi, G.; Tronconi, E. Dominant reaction pathways in the catalytic partial oxidation of CH 4 on Rh. Top. Catal. 2009, 52, 1983. [Google Scholar] [CrossRef]
- Yazid, S.A.; Rosli, Z.M.; Juoi, J.M. Effect of titanium (IV) isopropoxide molarity on the crystallinity and photocatalytic activity of titanium dioxide thin film deposited via green sol–gel route. J. Mater. Res. Technol. 2019, 8, 1434–1439. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J.; Mohammadi, A. Sol–gel nanostructured titanium dioxide: Controlling the crystal structure, crystallite size, phase transformation, packing and ordering. Microporous Mesoporous Mater. 2008, 112, 392–402. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Cordero-Cabrera, M.C.; Fray, D.J.; Ghorbani, M. Preparation of high surface area titania (TiO2) films and powders using particulate sol–gel route aided by polymeric fugitive agents. Sens. Actuators B Chem. 2006, 120, 86–95. [Google Scholar] [CrossRef]
- Lim, C.S.; Ryu, J.H.; Kim, D.-H.; Cho, S.-Y.; Oh, W.-C. Reaction morphology and the effect of pH on the preparation of TiO2 nanoparticles by a sol-gel method. J. Ceram. Process. Res. 2010, 11, 736–741. [Google Scholar]
- Javed, A.H.; Shahzad, N.; Khan, M.A.; Ayub, M.; Iqbal, N.; Hassan, M.; Hussain, N.; Rameel, M.I.; Shahzad, M.I. Effect of ZnO nanostructures on the performance of dye sensitized solar cells. Sol. Energy 2021, 230, 492–500. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoja, A.H.; Javed, A.H.; Naqvi, S.R.; Shakir, S.; Ud Din, I.; Arshad, Z.; Rashid, U.; Qazi, U.Y.; Naeem, N. Partial Oxidation of Methane over CaO Decorated TiO2 Nanocatalyst for Syngas Production in a Fixed Bed Reactor. Catalysts 2022, 12, 1089. https://doi.org/10.3390/catal12101089
Khoja AH, Javed AH, Naqvi SR, Shakir S, Ud Din I, Arshad Z, Rashid U, Qazi UY, Naeem N. Partial Oxidation of Methane over CaO Decorated TiO2 Nanocatalyst for Syngas Production in a Fixed Bed Reactor. Catalysts. 2022; 12(10):1089. https://doi.org/10.3390/catal12101089
Chicago/Turabian StyleKhoja, Asif Hussain, Ahad Hussain Javed, Salman Raza Naqvi, Sehar Shakir, Israf Ud Din, Zafar Arshad, Umer Rashid, Umair Yaqub Qazi, and Nida Naeem. 2022. "Partial Oxidation of Methane over CaO Decorated TiO2 Nanocatalyst for Syngas Production in a Fixed Bed Reactor" Catalysts 12, no. 10: 1089. https://doi.org/10.3390/catal12101089








