Electro-Chemical Degradation of Norfloxacin Using a PbO2-NF Anode Prepared by the Electrodeposition of PbO2 onto the Substrate of Nickel Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication and Characterization of PbO2-NF Electrode
2.3. Electrolysis
2.4. Analytical Methods
3. Results and Discussion
3.1. Properties of PbO2-FN Electrode
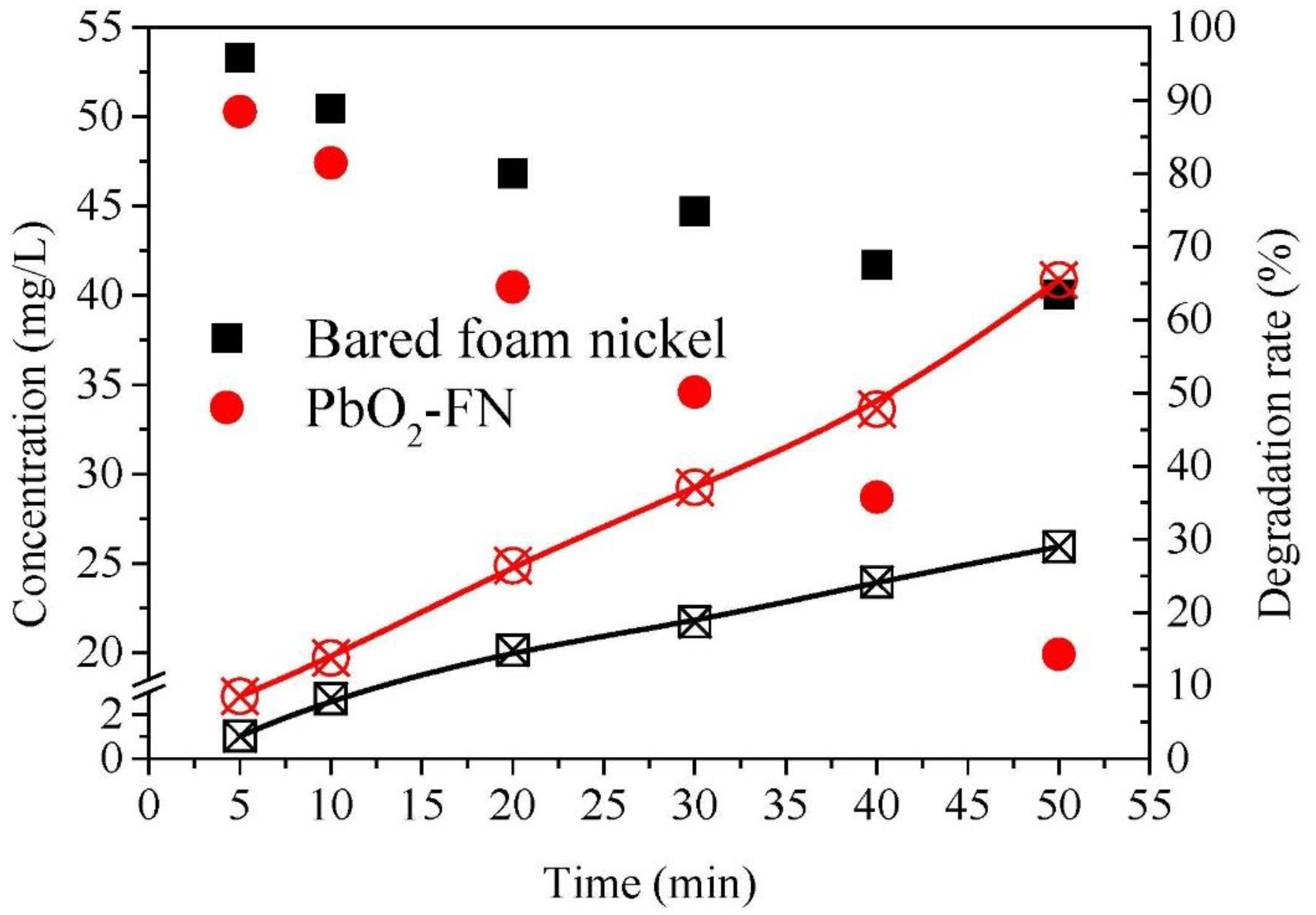
3.1.1. Comparison of Electrical Decomposition Performance
3.1.2. Physicochemical Properties
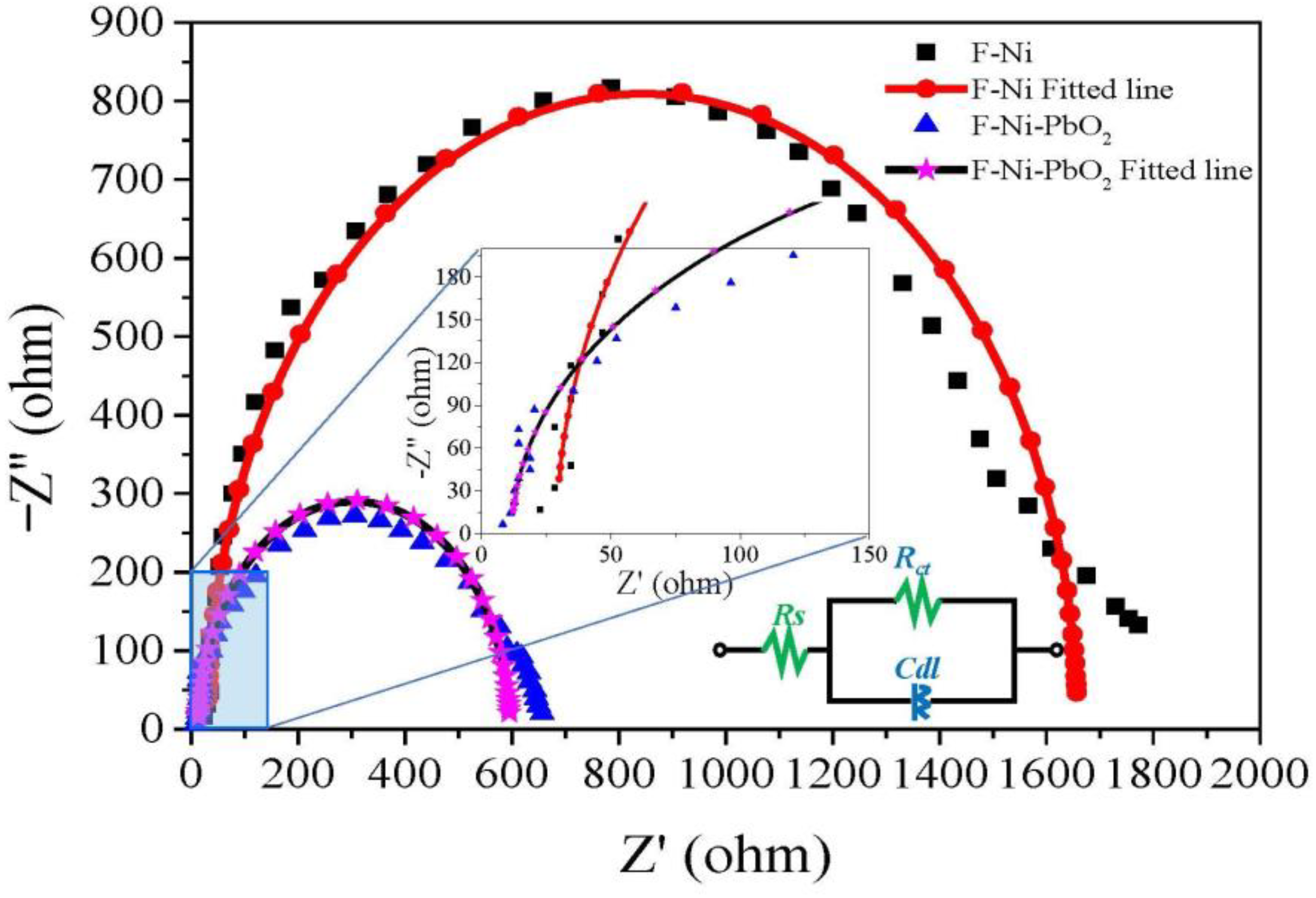
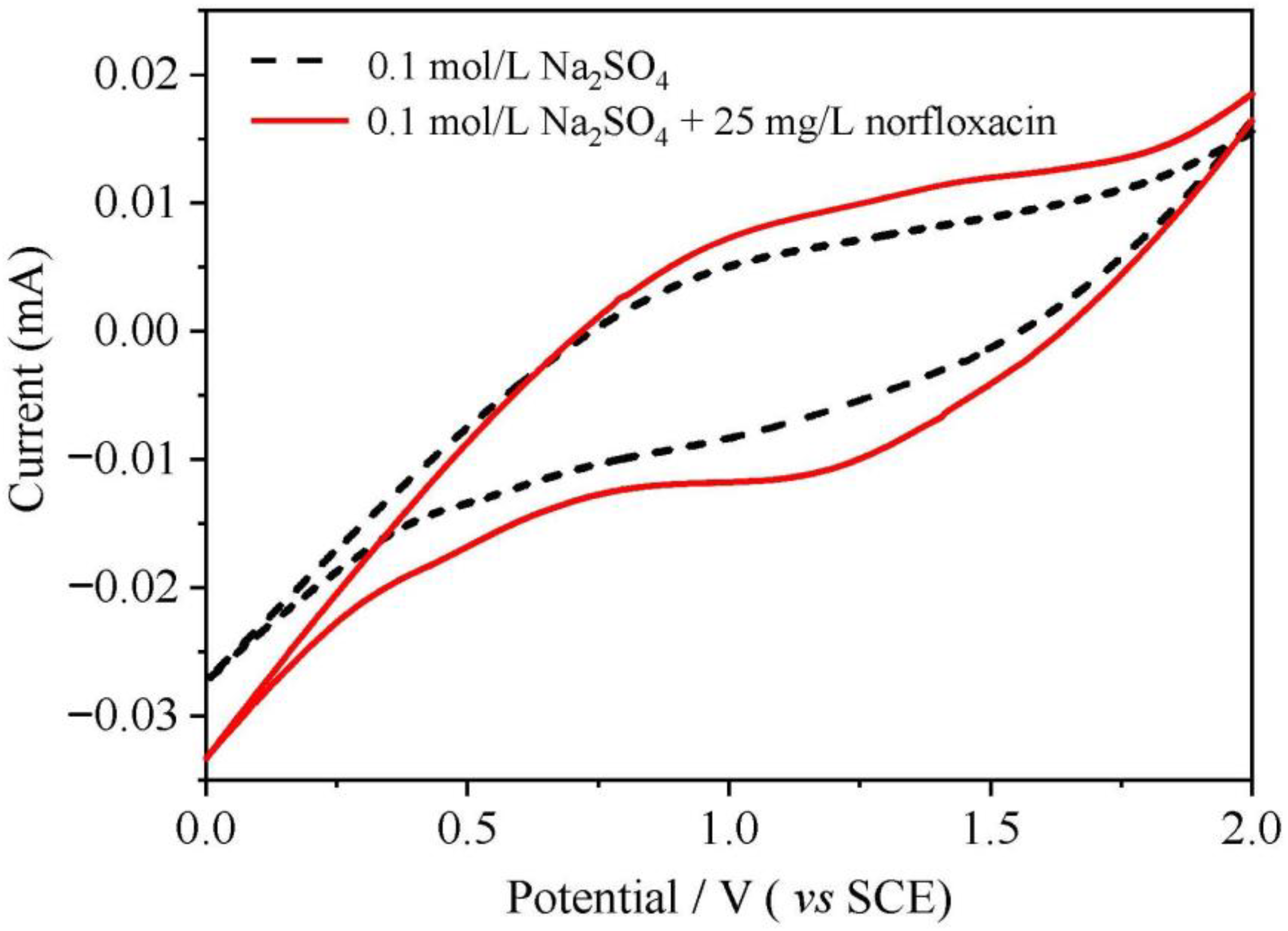
3.1.3. Electrochemical Properties
3.2. Electrochemical Oxidation
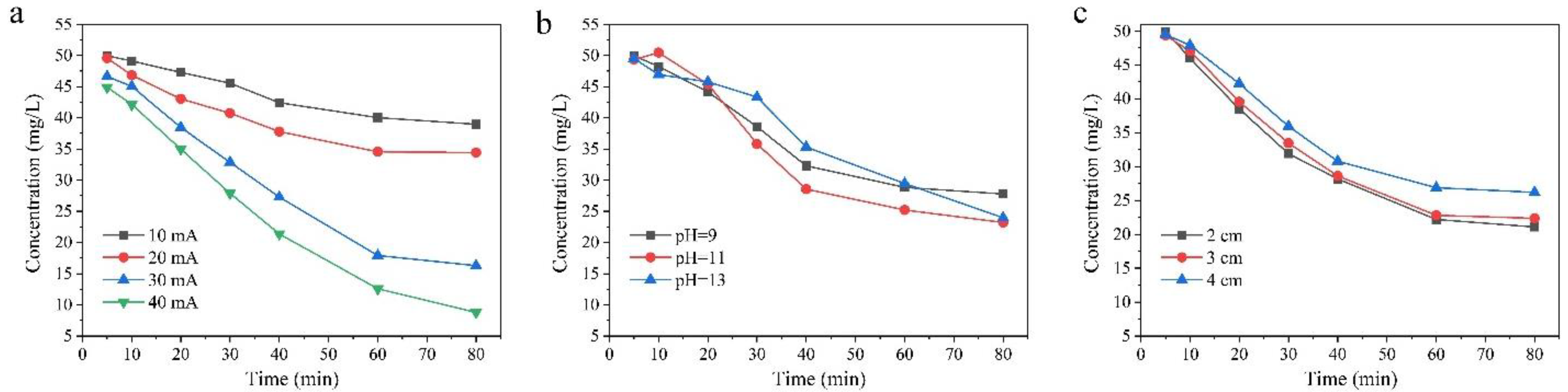
3.2.1. Effects of Applied Current Density
3.2.2. Effect of pH
3.2.3. Effect of Inter-Electrode Distance
3.3. Degradation Kinetics
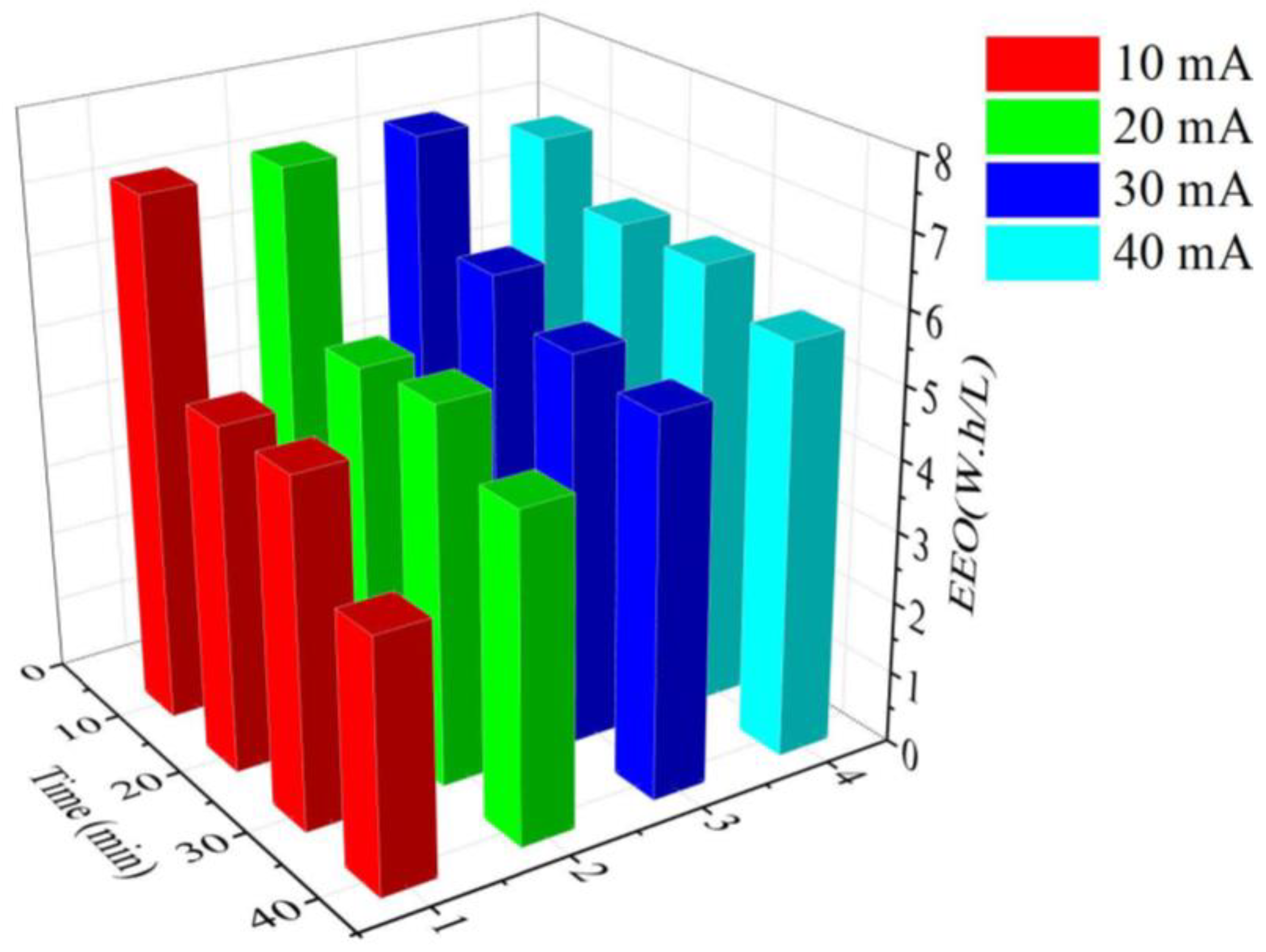
3.4. Energy Consumption
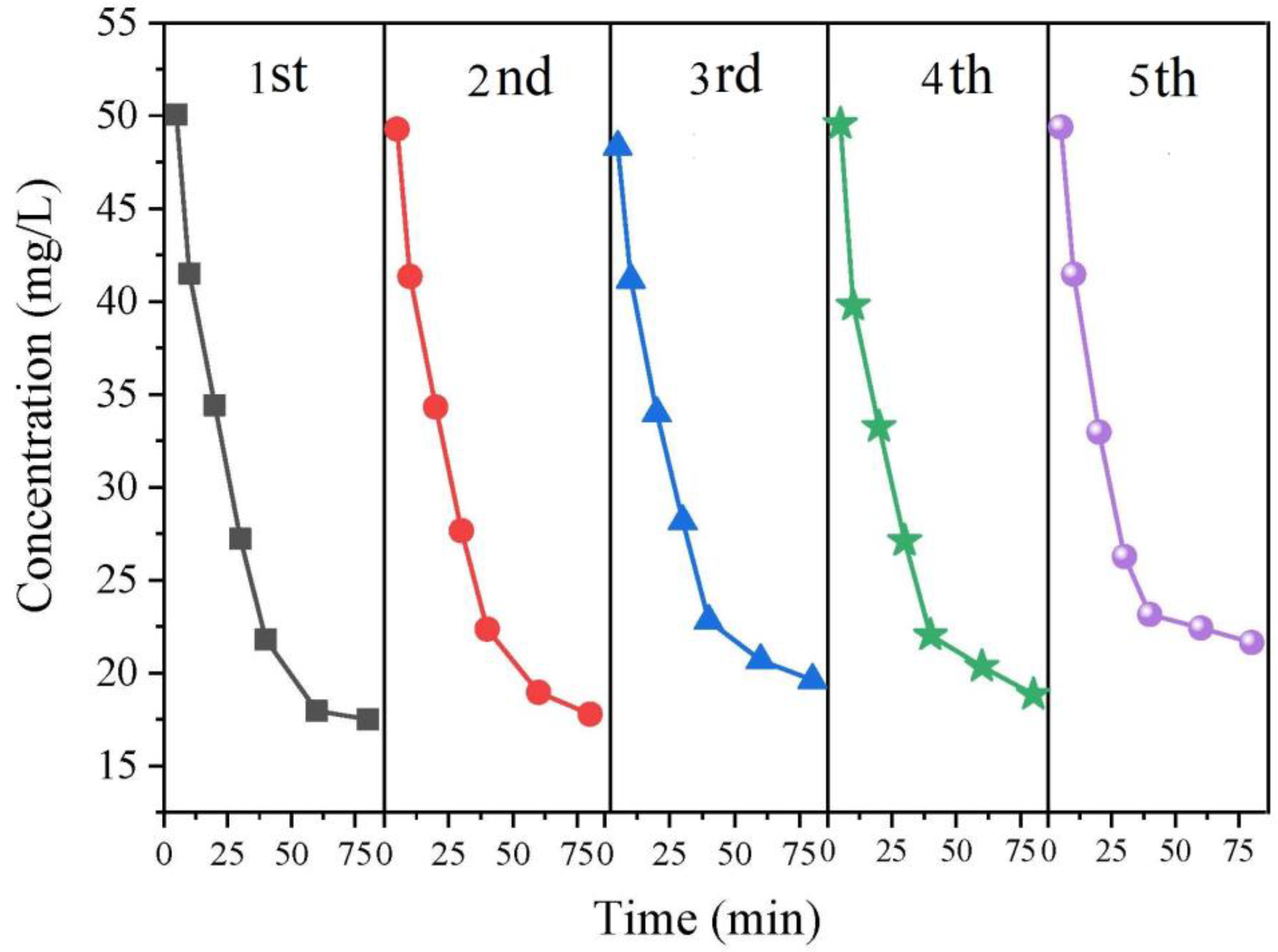
3.5. Reusability and Sustainability of PbO2-NF Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priya, A.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment—A review. Environ. Res. 2021, 204, 112298. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarestrup, F.M. Veterinary Drug Usage and Antimicrobial Resistance in Bacteria of Animal Origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.M.F.; Dias, M.; Teixeira, A.C.S.C.; Palma, M.S.A. Environmental contamination by fluoroquinolones. Braz. J. Pharm. Sci. 2014, 50, 41–54. [Google Scholar] [CrossRef]
- Xu, Z.; Li, T.; Bi, J.; Wang, C. Spatiotemporal heterogeneity of antibiotic pollution and ecological risk assessment in Taihu Lake Basin, China. Sci. Total Environ. 2018, 643, 12–20. [Google Scholar] [CrossRef]
- Wang, Q.; Tu, S.; Wang, W.; Liu, L.; Duan, X.; Chang, L. Fabrication of In2O3 doped PbO2 anode and its application for electrochemical degradation of norfloxacin in aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106462. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2018, 646, 1385–1397. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, H.; Qi, C.; Zhao, Y.; Wen, Y.; Xu, C.; Zhong, Q.; Sun, D.; Zhou, S.; Yang, B.; et al. L-cysteine boosted Fe(III)-activated peracetic acid system for sulfamethoxazole degradation: Role of L-cysteine and mechanism. Chem. Eng. J. 2023, 451, 138588. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: Influencing factors and mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe(III)/peroxymonosulfate process with L-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2020, 409, 128176. [Google Scholar] [CrossRef]
- Mora-Gomez, J.; Ortega, E.; Mestre, S.; Pérez-Herranz, V.; García-Gabaldón, M. Electrochemical degradation of norfloxacin using BDD and new Sb-doped SnO2 ceramic anodes in an electrochemical reactor in the presence and absence of a cation-exchange membrane. Sep. Purif. Technol. 2019, 208, 68–75. [Google Scholar] [CrossRef]
- Altıncı, O.C.; Körbahti, B.K. Graphene oxide-polyaniline conducting composite film deposited on platinum-iridium electrode by electrochemical polymerization of aniline: Synthesis and environmental electrochemistry application. Appl. Surf. Sci. Adv. 2022, 7, 100212. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Zhao, M.; Zhang, L.; Dong, H.; Yu, H. Norfloxacin degradation by a green carbon black-Ti/SnO2-Sb electrochemical system in saline water. Catal. Today 2018, 327, 308–314. [Google Scholar] [CrossRef]
- Carneiro, J.F.; Aquino, J.M.; Silva, B.F.; Silva, A.J.; Rocha-Filho, R.C. Comparing the electrochemical degradation of the fluoroquinolone antibiotics norfloxacin and ciprofloxacin using distinct electrolytes and a BDD anode: Evolution of main oxidation byproducts and toxicity. J. Environ. Chem. Eng. 2020, 8, 104433. [Google Scholar] [CrossRef]
- Montañés, M.; García-Gabaldón, M.; Roca-Pérez, L.; Giner-Sanz, J.; Mora-Gómez, J.; Pérez-Herranz, V. Analysis of norfloxacin ecotoxicity and the relation with its degradation by means of electrochemical oxidation using different anodes. Ecotoxicol. Environ. Saf. 2019, 188, 109923. [Google Scholar] [CrossRef]
- Meng, J.; Li, D.; Zhang, L.; Gao, W.; Huang, K.; Geng, C.; Guan, Y.; Ming, H.; Jiang, W.; Liang, J. Degradation of Norfloxacin by Electrochemical Oxidation Using Ti/Sno2-Sb Electrode Doped with Ni or Mo. Electrocatalysis 2021, 12, 436–446. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Zhou, L.; Dong, F.; Yang, D.; Lei, H.; Du, L.; Jia, L.; Zhou, S. Optimized terbium doped Ti/PbO2 dimensional stable anode as a strong tool for electrocatalytic degradation of imidacloprid waste water. Ecotoxicol. Environ. Saf. 2019, 188, 109921. [Google Scholar] [CrossRef] [PubMed]
- Montes, I.S.; Neto, J.R.F.; Silva, B.F.; da Silva, A.J.; Aquino, J.M.; Rocha-Filho, R.C. Evolution of the antibacterial activity and oxidation intermediates during the electrochemical degradation of norfloxacin in a flow cell with a PTFE-doped β-PbO2 anode: Critical comparison to a BDD anode. Electrochimica Acta 2018, 284, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Duan, X.; Xu, F.; Chang, L. Fabrication of three-dimensional networked PbO2 anode for electrochemical oxidation of organic pollutants in aqueous solution. J. Taiwan Inst. Chem. Eng. 2019, 100, 74–84. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wei, F.; Teng, G.; Yao, Y. Preparation and characterization of hydrophobic stearic acid-Yb-PbO2 anode and its application on the electrochemical degradation of naproxen sodium. J. Electroanal. Chem. 2020, 868, 114191. [Google Scholar] [CrossRef]
- Samet, Y.; Elaoud, S.C.; Ammar, S.; Abdelhedi, R. Electrochemical degradation of 4-chloroguaiacol for wastewater treatment using PbO2 anodes. J. Hazard. Mater. 2006, 138, 614–619. [Google Scholar] [CrossRef]
- Polcaro, A.M.; Palmas, S.; Renoldi, F.; Mascia, M. On the performance of Ti/SnO2 and Ti/PbO2 anodesin electrochemical degradation of 2-chlorophenolfor wastewater treatment. J. Appl. Electrochem. 1999, 29, 147–151. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Dai, Q. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochimica Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- He, M.; Cao, L.; Li, W.; Chang, X.; Zheng, X.; Ren, Z. NiO nanoflakes decorated needle-like MnCo2O4 hierarchical structure on nickle foam as an additive-free and high performance supercapacitor electrode. J. Mater. Sci. 2021, 56, 8613–8626. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Guo, X.; Wang, R.; Zhang, H. Facile Growth of Caterpillar-like NiCo2S4 Nanocrystal Arrays on Nickle Foam for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 18774–18781. [Google Scholar] [CrossRef]
- Yang, G.; Mu, X.; Jin, Y.; Fan, T.; Wang, S.; Yuan, F.; Ma, J. In-situ chemical corrosive nickel foam as high-efficient electrocatalyst for 5-hydroxymethylfurfural oxidation. Appl. Surf. Sci. 2022, 594, 153432. [Google Scholar] [CrossRef]
- Guo, G. Direct fabrication of mixed metal–organic frameworks (Ni/Cu-MOF) and C@NiCu2O4 onto Ni foam as binder-free high performance electrode for supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 16287–16301. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, T.; Su, Q.; Tang, Y.; Xu, X.; Akram, M.; Jiang, B. Highly efficient and mild electrochemical degradation of bentazon by nano-diamond doped PbO2 anode with reduced Ti nanotube as the interlayer. J. Colloid Interface Sci. 2020, 575, 254–264. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Yang, Y.; Cui, L.; Guo, L. Electrochemical degradation of insecticide hexazinone with Bi-doped PbO2 electrode: Influencing factors, intermediates and degradation mechanism. Chemosphere 2018, 216, 812–822. [Google Scholar] [CrossRef]
- Ao, X.; Wang, W.; Sun, W.; Lu, Z.; Li, C. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: An investigation of the role of pH. Water Res. 2021, 203, 117458. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Guo, J.; Yang, J.; Lian, J.; Luo, X.; Wang, X.; Wang, K.; Wang, L. Studies on the electrochemical degradation of Acid Orange II wastewater with cathodes modified by quinones. J. Ind. Eng. Chem. 2014, 20, 752–758. [Google Scholar] [CrossRef]
- Ramírez, C.; Saldaña, A.; Hernández, B.; Acero, R.; Guerra, R.; Garcia-Segura, S.; Brillas, E.; Peralta-Hernández, J.M. Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J. Ind. Eng. Chem. 2013, 19, 571–579. [Google Scholar] [CrossRef]
- Yang, L.; Huang, C.; Yin, Z.; Meng, J.; Guo, M.; Feng, L.; Liu, Y.; Zhang, L.; Du, Z. Rapid electrochemical reduction of a typical chlorinated organophosphorus flame retardant on copper foam: Degradation kinetics and mechanisms. Chemosphere 2021, 264, 128515. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Xu, Z.; Niu, J.; Hou, L.-A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]


| Treatment Conditions | Electrical Parameters | First-Order Dynamic Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Current Density (mA) | pH | Distance (cm) | Average Voltage (V) | EEO (Wh/L) | Initial Concentration | Degradation Rate Constant (k) | Half Life (t1/2) | Adj. R-Square |
| 20 | 7 | 4 | 13.561 | 9.63 | 49.201 | 0.00525 | 132 | 0.90804 |
| 30 | 7 | 2 | 16.613 | 5.584 | 54.276 | 0.01261 | 54.956 | 0.98811 |
| 40 | 11 | 2 | 16.264 | 5.431 | 55.284 | 0.02375 | 29.179 | 0.99614 |
| 40 | 9 | 2 | 13.069 | 10.937 | 52.114 | 0.00925 | 74.919 | 0.94135 |
| 30 | 11 | 3 | 13.16 | 6.637 | 54.377 | 0.01694 | 40.909 | 0.92431 |
| 30 | 13 | 3 | 15.582 | 10.169 | 53.475 | 0.00959 | 72.263 | 0.96139 |
| 40 | 7 | 2 | 16.01 | 9.078 | 51.823 | 0.01373 | 50.473 | 0.95407 |
| 40 | 7 | 3 | 12.785 | 7.501 | 51.795 | 0.01281 | 54.098 | 0.95522 |
| 30 | 7 | 4 | 13.561 | 7.549 | 51.053 | 0.0102 | 67.941 | 0.94067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Cheng, Z.; Li, H.; Xiang, L. Electro-Chemical Degradation of Norfloxacin Using a PbO2-NF Anode Prepared by the Electrodeposition of PbO2 onto the Substrate of Nickel Foam. Catalysts 2022, 12, 1297. https://doi.org/10.3390/catal12111297
Tang J, Cheng Z, Li H, Xiang L. Electro-Chemical Degradation of Norfloxacin Using a PbO2-NF Anode Prepared by the Electrodeposition of PbO2 onto the Substrate of Nickel Foam. Catalysts. 2022; 12(11):1297. https://doi.org/10.3390/catal12111297
Chicago/Turabian StyleTang, Jianshe, Zhubin Cheng, Hao Li, and Li Xiang. 2022. "Electro-Chemical Degradation of Norfloxacin Using a PbO2-NF Anode Prepared by the Electrodeposition of PbO2 onto the Substrate of Nickel Foam" Catalysts 12, no. 11: 1297. https://doi.org/10.3390/catal12111297





