Figure 1.
Diffractograms of (a) Sn1.5PMo12O40 catalyst and (b) its precursor H3PMo12O40.
Figure 1.
Diffractograms of (a) Sn1.5PMo12O40 catalyst and (b) its precursor H3PMo12O40.
Figure 2.
SEM images of (a) Precursor H3PMo12O40 and (b) Sn1.5PMo12O40 catalyst.
Figure 2.
SEM images of (a) Precursor H3PMo12O40 and (b) Sn1.5PMo12O40 catalyst.
Figure 3.
Texture properties of Sn1.5PMo12O40 catalyst: N2 adsorption and desorption curves and pores diameter distribution curve.
Figure 3.
Texture properties of Sn1.5PMo12O40 catalyst: N2 adsorption and desorption curves and pores diameter distribution curve.
Figure 4.
FTIR spectra of (a) Sn1.5PMo12O40 and (b) its precursor H3PMo12O40.
Figure 4.
FTIR spectra of (a) Sn1.5PMo12O40 and (b) its precursor H3PMo12O40.
Figure 5.
Experimental molar concentrations (symbols) and kinetic simulations (lines) as a function of time at 363 K and a reaction time of 8 h.
Figure 5.
Experimental molar concentrations (symbols) and kinetic simulations (lines) as a function of time at 363 K and a reaction time of 8 h.
Figure 6.
Parity graph of the experimental data and theoretical data (model) of glycerol conversion and product selectivities.
Figure 6.
Parity graph of the experimental data and theoretical data (model) of glycerol conversion and product selectivities.
Figure 7.
Reactive distillation column for glycerol etherification with TBA (reflux ratio = 1.1, Reactive stages = 6, TBA:Gly = 5).
Figure 7.
Reactive distillation column for glycerol etherification with TBA (reflux ratio = 1.1, Reactive stages = 6, TBA:Gly = 5).
Figure 8.
For reactive stages = 6, TBA: Gly = 5, (a) unreacted glycerol flow rate as a function of the reflux ratio, and (b) glycerol ether compositions in the bottom stream as a function of the reflux ratio.
Figure 8.
For reactive stages = 6, TBA: Gly = 5, (a) unreacted glycerol flow rate as a function of the reflux ratio, and (b) glycerol ether compositions in the bottom stream as a function of the reflux ratio.
Figure 9.
For Reflux ratio = 1.1, TBA:Gly = 5, (a) Unreacted glycerol flow rate at the bottom stream as a function of reactive stages. (b) Glycerol, DTBG, and TTBG mole fractions in the bottom stream as a function of reactive stages.
Figure 9.
For Reflux ratio = 1.1, TBA:Gly = 5, (a) Unreacted glycerol flow rate at the bottom stream as a function of reactive stages. (b) Glycerol, DTBG, and TTBG mole fractions in the bottom stream as a function of reactive stages.
Figure 10.
For Reflux ratio = 1.1, reactive stages = 6, (a) Unreacted glycerol in the bottom stream as a function of TBA feed flow rate, (b) Unreacted glycerol, DTBG, and TTBG mole fractions in the bottom stream as a function of TBA feed flow rate.
Figure 10.
For Reflux ratio = 1.1, reactive stages = 6, (a) Unreacted glycerol in the bottom stream as a function of TBA feed flow rate, (b) Unreacted glycerol, DTBG, and TTBG mole fractions in the bottom stream as a function of TBA feed flow rate.
Figure 11.
Process flow diagram (PFD) of the glycerol etherification with TBA using reactive distillation.
Figure 11.
Process flow diagram (PFD) of the glycerol etherification with TBA using reactive distillation.
Figure 12.
Residue curves map of the TBA-water-glycerol mixture at a pressure of 1 bar.
Figure 12.
Residue curves map of the TBA-water-glycerol mixture at a pressure of 1 bar.
Figure 13.
Heat-integrated process flow diagram (PFD) of glycerol etherification with TBA by reactive distillation.
Figure 13.
Heat-integrated process flow diagram (PFD) of glycerol etherification with TBA by reactive distillation.
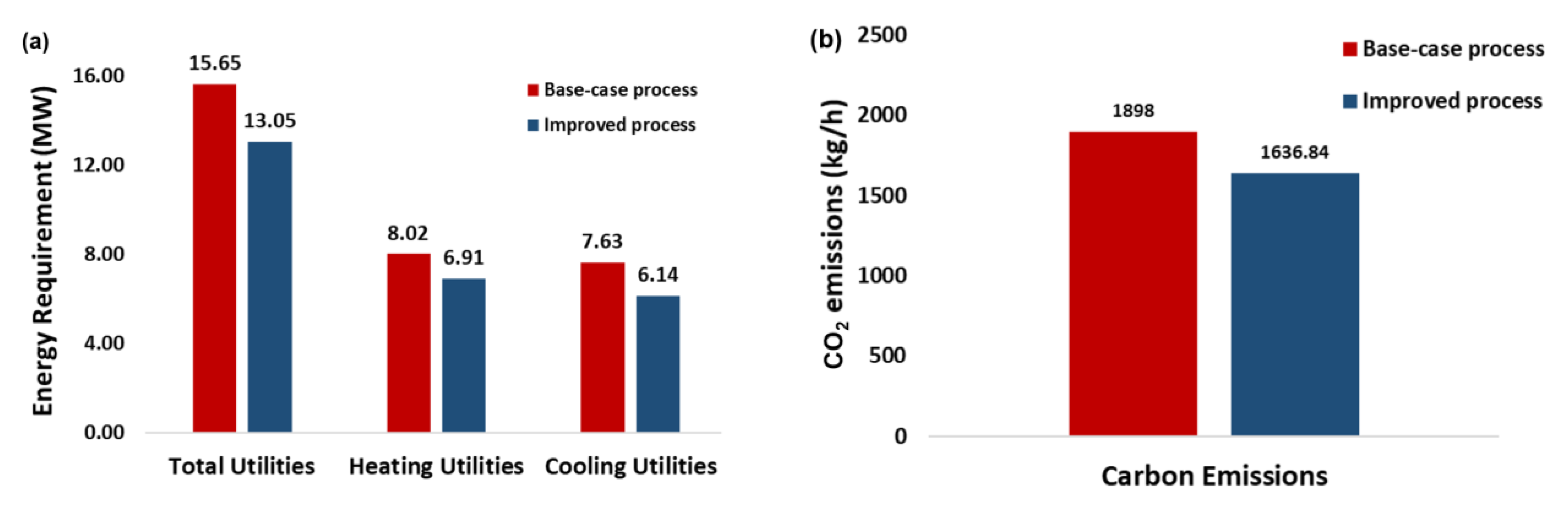
Figure 14.
Comparison between the base-case and integrated processes of, (a) energy requirements, and (b) carbon emissions.
Figure 14.
Comparison between the base-case and integrated processes of, (a) energy requirements, and (b) carbon emissions.
Figure 15.
Purchased costs distribution of the main equipment for the glycerol etherification process based on reactive distillation.
Figure 15.
Purchased costs distribution of the main equipment for the glycerol etherification process based on reactive distillation.
Figure 16.
Costs distribution of utilities for glycerol etherification process.
Figure 16.
Costs distribution of utilities for glycerol etherification process.
Figure 17.
Annual cost distribution of TPC of the glycerol etherification process.
Figure 17.
Annual cost distribution of TPC of the glycerol etherification process.
Figure 18.
Glycerol etherification reactions with TBA.
Figure 18.
Glycerol etherification reactions with TBA.
Figure 19.
Experimental and equilibrium values of, (a) Glycerol conversion as a function of temperature, (b) Component selectivity as a function of temperature.
Figure 19.
Experimental and equilibrium values of, (a) Glycerol conversion as a function of temperature, (b) Component selectivity as a function of temperature.
Table 1.
Energy dispersive spectroscopy analysis of Sn1.5PMo12O40 catalyst and its precursor H3PMo12O40 *.
Table 1.
Energy dispersive spectroscopy analysis of Sn1.5PMo12O40 catalyst and its precursor H3PMo12O40 *.
| Mass (%) | H3PMo12O40 | Sn1.5PMo12O40 |
|---|
| Theoretical | EDS | Theoretical | EDS |
|---|
| H | 0.2 | - | 0 | - |
| Sn | 0 | 0 | 8.5 | 9.5 |
| P | 1.7 | 2.3 | 1.5 | 2.4 |
| Mo | 63.1 | 62.1 | 58.0 | 57.7 |
| O | 35.0 | 35.6 | 32.0 | 30.4 |
Table 2.
Texture properties of H3PMo12O40 and Sn1.5PMo12O40 salt.
Table 2.
Texture properties of H3PMo12O40 and Sn1.5PMo12O40 salt.
| Catalyst | Surface Area
m2 g−1 | Pore Volume
(×10−2 cm3/g) | Pore Diameter
(nm) |
|---|
| H3PMo12O40 | 11.8 | 1.7 | 3.8 |
| Sn1.5PMo12O40 | 11.2 | 1.4 | 2.9 |
Table 3.
Mass balances of the reactants and products.
Table 3.
Mass balances of the reactants and products.
| Component | Mass Balance Equation | Component | Mass Balance Equation |
|---|
| Glycerol | | DTBG | |
| TBA | | TTBG | |
| MTBG | | | |
Table 4.
Frequency factors (k0) and activation energies (Ea) of the kinetic model.
Table 4.
Frequency factors (k0) and activation energies (Ea) of the kinetic model.
| Pre-Exponential Factor (m3/mol·s) | Activation Energy (kJ/mol) |
|---|
| | | 91 |
| | | 52.5 |
| | | 61 |
| | | 30 |
| | | 66 |
| | | 80 |
Table 5.
Streams information of glycerol with TBA etherification process.
Table 5.
Streams information of glycerol with TBA etherification process.
| Stream | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| Temperature (K) | 298 | 363 | 298 | 363 | 391 | 308 | 298 |
| Pressure (bar) | 1 | 4 | 1 | 4 | 4 | 2 | 1 |
| Enthalpy flow (kW) | −3708 | −3635 | −4119 | −9530 | −8670 | −4201 | −30 |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Molar flow (kmol/h) | 20 | 20 | 41.3 | 100.3 | 100.2 | 20.0 | 0 |
| Mass flow (kg/h) | 1842 | 1842 | 3061 | 7413 | 5137 | 4118 | 15 |
| Component Flowrates in (kmol/h) |
| Glycerol | 20.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 |
| TBA | 0.0 | 0.0 | 41.3 | 99.9 | 59.2 | 0.0 | 0.0 |
| MTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
| DTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 18.9 | 0.0 |
| TTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 |
| Water | 0.0 | 0.0 | 0.0 | 0.4 | 41.0 | 0.0 | 0.0 |
| Stream | 8 | 9 | 10 | 11 | 12 | 13 | |
| Temperature (K) | 308 | 371 | 360 | 464 | 364 | 308 | |
| Pressure (bar) | 1 | 2 | 1 | 2 | 1 | 1 | |
| Enthalpy flow (kW) | −27,695 | −8670 | −5619 | −29,369 | −3265 | −27,665 | |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | 0 | |
| Molar flow (kmol/h) | 149.7 | 100.3 | 59.0 | 191.4 | 41.4 | 149.8 | |
| Mass flow (kg/h) | 13,791 | 5137 | 4352 | 14,576 | 800 | 13,777 | |
| Component Flowrates in (kmol/h) |
| Glycerol | 149.7 | 0.0 | 0.0 | 149.7 | 0.1 | 149.5 | |
| TBA | 0.0 | 59.2 | 58.6 | 0.6 | 0.6 | 0.0 | |
| MTBG | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | |
| DTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| TTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Water | 0.3 | 41.0 | 0.4 | 41.0 | 40.6 | 0.3 | |
Table 6.
Stream information of the integrated glycerol etherification process.
Table 6.
Stream information of the integrated glycerol etherification process.
| Stream | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Temperature (K) | 298 | 363 | 298 | 363 | 391 | 560 |
| Pressure (bar) | 1 | 5 | 1 | 5 | 4 | 4 |
| Enthalpy flow (kW) | −3708 | −3635 | −4119 | −9530 | −8670 | −3499 |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | 0 |
| Molar flow (kmol/h) | 20 | 20 | 41.3 | 100.3 | 100.3 | 20.0 |
| Mass flow (kg/h) | 1842 | 1842 | 3061 | 7413 | 5137 | 4118 |
| Component Flowrates in (kmol/h) |
| Glycerol | 20.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| TBA | 0.0 | 0.0 | 41.3 | 99.9 | 59.2 | 0.0 |
| MTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| DTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 18.9 |
| TTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| Water | 0.0 | 0.0 | 0.0 | 0.4 | 41.0 | 0.0 |
| Stream | 7 | 8 | 9 | 10 | 11 | 12 |
| Temperature (K) | 476 | 374 | 308 | 298 | 308 | 371 |
| Pressure (bar) | 2 | 2 | 2 | 1 | 1 | 2 |
| Enthalpy flow (kW) | −3769 | −4048 | −4201 | −30 | −27,695 | −8670 |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | 0 |
| Molar flow (kmol/h) | 20.0 | 20.0 | 20.0 | 0.2 | 150.0 | 100.3 |
| Mass flow (kg/h) | 4118 | 4118 | 4118 | 15 | 13,791 | 5137 |
| Component Flowrates in (kmol/h) |
| Glycerol | 0.1 | 0.1 | 0.1 | 0.2 | 149.7 | 0.0 |
| TBA | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 59.2 |
| MTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| DTBG | 18.9 | 18.9 | 18.9 | 0.0 | 0.0 | 0.0 |
| TTBG | 1.0 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 |
| Water | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 41.0 |
| Stream | 13 | 14 | 15 | 16 | 17 | |
| Temperature (K) | 360 | 464 | 364 | 572 | 308 | |
| Pressure (bar) | 1 | 2 | 1 | 1 | 1 | |
| Enthalpy flow (kW) | −5619 | −29,369 | −3265 | −25,051 | −27,665 | |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | |
| Molar flow (kmol/h) | 59.0 | 191.4 | 41.4 | 149.8 | 149.8 | |
| Mass flow (kg/h) | 4352 | 14576 | 800 | 13777 | 13777 | |
| Component Flowrates in (kmol/h) | |
| Glycerol | 0.0 | 149.7 | 0.1 | 149.5 | 149.5 | |
| TBA | 58.6 | 0.6 | 0.6 | 0.0 | 0.0 | |
| MTBG | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | |
| DTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| TTBG | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Water | 0.4 | 41.0 | 40.6 | 0.3 | 0.3 | |
Table 7.
Materials and utility costs for glycerol etherification process [
28,
29].
Table 7.
Materials and utility costs for glycerol etherification process [
28,
29].
| Item | Price |
|---|
| Glycerol ($/metric ton) | 445 |
| TBA ($/metric ton) | 1150 |
| Product ($/metric ton) | 1700 |
| Utilities | |
| Medium pressure steam (mps) ($/metric ton) | 6 |
| High pressure steam (hps) ($/metric ton) | 8 |
| Electricity ($/kWh) | 0.045 |
| Cooling water ($/m3) | 0.08 |
Table 8.
Details of total capital investment calculations [
29].
Table 8.
Details of total capital investment calculations [
29].
| | Fraction of Delivered Equipment for the Fluid Processing Plant | Calculated Values (million $) |
|---|
| Direct Costs |
| Purchased equipment, E′ | | 1.133 |
| Delivery, a fraction of E′ | 0.1 | 0.113 |
| Subtotal: delivered equipment | | 1.246 |
| Purchased equipment installation | 0.47 | 0.586 |
| Instrumentation &Controls (installed) | 0.36 | 0.449 |
| Piping (installed) | 0.68 | 0.847 |
| Electrical systems (installed) | 0.11 | 0.137 |
| Buildings (including services) | 0.18 | 0.224 |
| Yard improvements | 0.1 | 0.125 |
| Service facilities (installed) | 0.7 | 0.872 |
| Total direct costs | 2.6 | 4.487 |
| Indirect Costs |
| Engineering and supervision | 0.33 | 0.411 |
| Construction expenses | 0.41 | 0.511 |
| Legal expenses | 0.04 | 0.05 |
| Contractor’s fee | 0.22 | 0.274 |
| Contingency | 0.44 | 0.548 |
| Total indirect costs | 1.44 | 1.795 |
| Fixed capital investment (FCI) | 6.281 |
| Working capital (WC) | 0.89 | 1.109 |
| Total capital investment (TCI) | 7.391 |
Table 9.
Economic evaluation summary of the reactive distillation-based process for glycerol etherification.
Table 9.
Economic evaluation summary of the reactive distillation-based process for glycerol etherification.
| Item | Value |
|---|
| Fixed capital cost | $6,282,000 |
| Total capital cost | $7,391,000 |
| Return on investment (ROI) | 29.36% |
| Payback period | 2.2 |
| Net present value | $7,853,000 |
| The discounted cash flow rate of return | 15.18% |
Table 10.
Initial and final compositions at different temperatures.
Table 10.
Initial and final compositions at different temperatures.
| Temperature K | Moles of Glycerol | Moles of Products | Moles of TBA |
|---|
| 0h | 4h | MTBG | DTBG | TTBG | 0 h | 4 h |
|---|
| 353 | 0.0046 | 0.0013 | 0.0026 | 0.0005 | 0.0000 | 0.0368 | 0.0333 |
| 363 | 0.0046 | 0.0011 | 0.0027 | 0.0008 | 0.0000 | 0.0368 | 0.0325 |
| 373 | 0.0046 | 0.0012 | 0.0024 | 0.0010 | 0.0001 | 0.0368 | 0.0323 |
| 383 | 0.0046 | 0.0014 | 0.0023 | 0.0007 | 0.0000 | 0.0368 | 0.0331 |