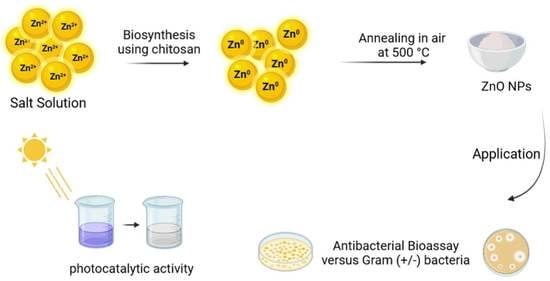
Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystallinity and Crystallite Size
2.2. Morphological Investigation
2.3. UV-Vis Spectroscopy Analysis
2.4. Antibacterial Activities
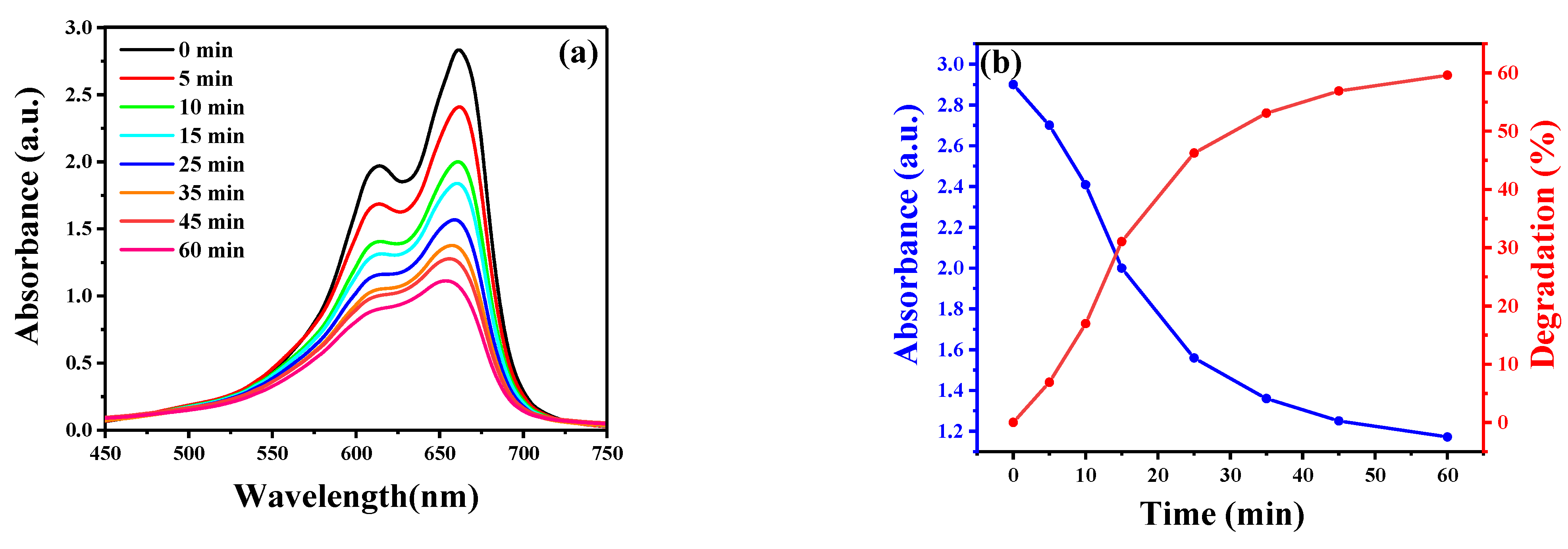
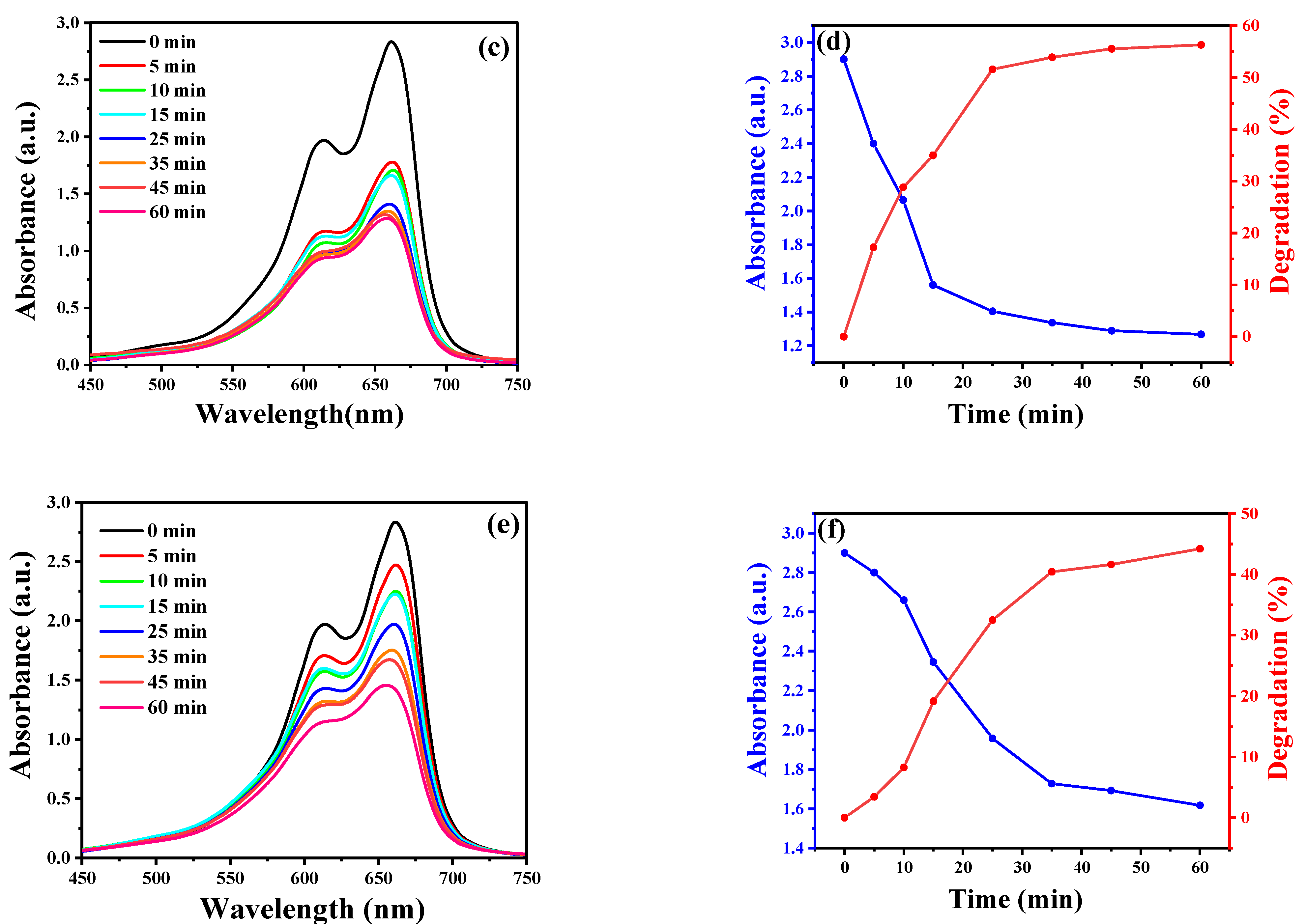
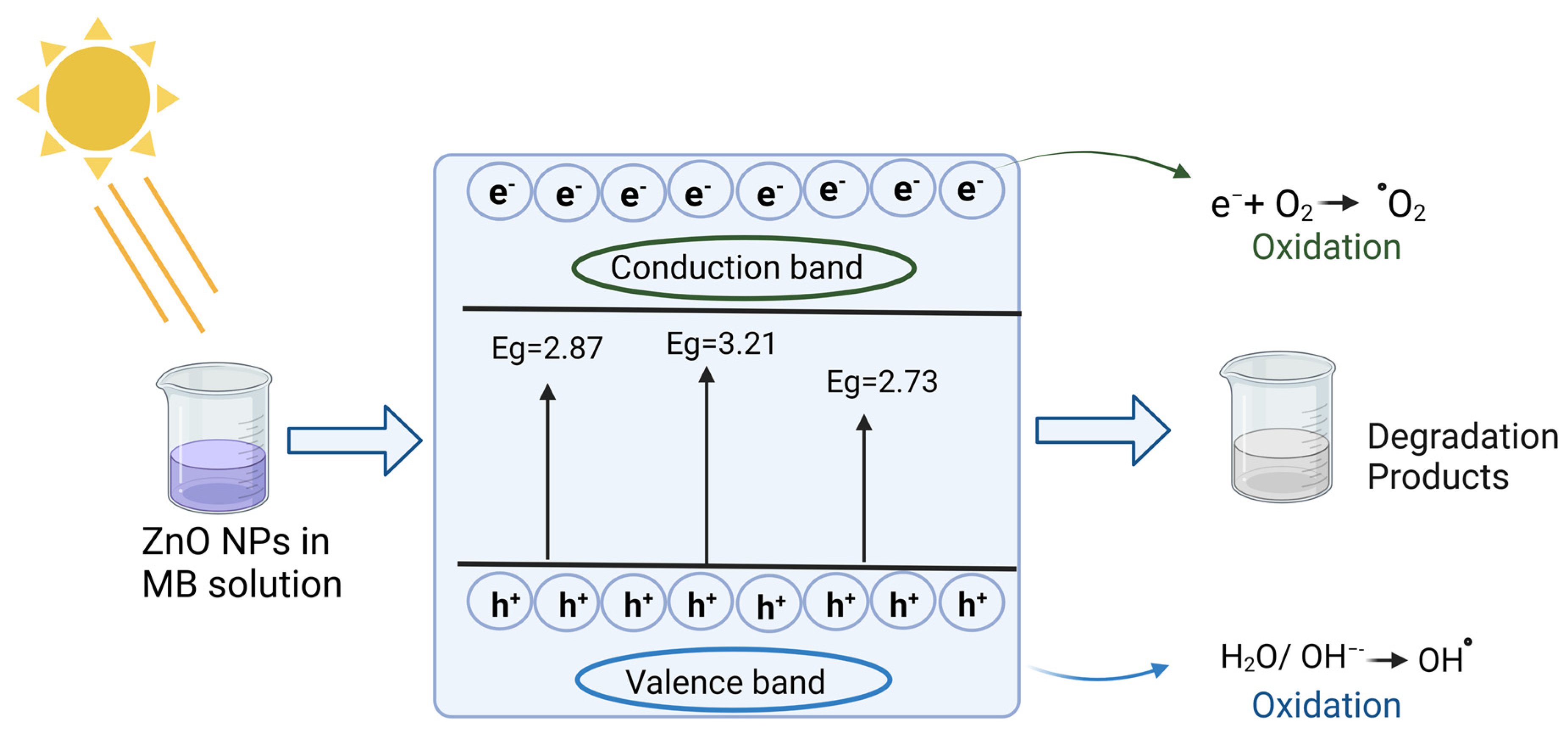
2.5. Photocatalytic Ability to MB Degradation
| Modifier | AZO Dye | Time (min) | Dye Removal (%) | Ref. |
|---|---|---|---|---|
| Extract of Becium grandiflorum | Methylene Blue | 60 | 30 | [41] |
| Ruellia tuberosa extract | Malachite green (MG) | 60 | 59 | [42] |
| Myrica esculenta fruits extract | Methylene Blue | 60 | 29 | [43] |
| leaf extract of the plant Ruta Chalepensis | Methyl Red | 60 | 74 | [44] |
| Ulva lactuca seaweed extract | Methylene Blue | 60 | 45 | [45] |
| Chitosan of shrimp shells | Methylene Blue | 60 | 60 | This work |
| Chitodan of crab shells | Methylene Blue | 60 | 56 | |
| Chitosan of Streptomyces griseus bacteria | Methylene Blue | 60 | 44 |
3. Experimental
3.1. Materials
3.2. Sol-Gel Synthesis of ZnO NPs Using Different Chitosan Sources
3.3. Physicochemical Characterization of ZnO NPs
3.4. Bioassay for Antibacterial
3.5. Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barhoum, A.; Melcher, J.; Van Assche, G.; Rahier, H.; Bechelany, M.; Fleisch, M.; Bahnemann, D. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: Porous microparticles versus nonporous nanoparticles. J. Mater. Sci. 2017, 52, 2746–2762. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Seghir, B.B.; Amor, I.B.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A Review on Biogenic Green Synthesis of ZnO Nanoparticles by Plant Biomass and their Applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Turky, A.O.; Barhoum, A.; MohamedRashad, M.; Bechlany, M. Enhanced the structure and optical properties for ZnO/PVP nanofibers fabricated via electrospinning technique. J. Mater. Sci. Mater. Electron. 2017, 28, 17526–17532. [Google Scholar] [CrossRef]
- Barhoum, A.; Van Assche, G.; Rahier, H.; Fleisch, M.; Bals, S.; Delplancked, M.-P.; Leroux, F.; Bahnemann, D. Sol-gel hot injection synthesis of ZnO nanoparticles into a porous silica matrix and reaction mechanism. Mater. Des. 2017, 119, 270–276. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Bashal, A.H.; Riyadh, S.M.; Alharbi, W.; Alharbi, K.H.; Farghaly, T.A.; Khalil, K.D. Bio-Based (Chitosan-ZnO) Nanocomposite: Synthesis, Characterization, and Its Use as Recyclable, Ecofriendly Biocatalyst for Synthesis of Thiazoles Tethered Azo Groups. Polymers 2022, 14, 386. [Google Scholar] [CrossRef]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnology 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.M. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Dodson, K.; LeJeune, J. Escherichia coli O157: H7, Campylobacter jejuni, and Salmonella prevalence in cull dairy cows marketed in northeastern Ohio. J. Food Prot. 2005, 68, 927–931. [Google Scholar] [CrossRef]
- Jin, T.; Sun, D.; Su, J.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J. Food Sci. 2009, 74, M46–M52. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayaman, V.; Chandarshekar, B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Varma, A.; Deshpande, S.; Kennedy, J. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Amor, I.B.; Hemmami, H.; Laouini, S.E.; Temam, H.B.; Zaoui, H.; Barhoum, A. Biosynthesis MgO and ZnO nanoparticles using chitosan extracted from Pimelia Payraudi Latreille for antibacterial applications. World J. Microbiol. Biotechnol. 2022, 39, 19. [Google Scholar] [CrossRef]
- Pillai, P.S.; Prajapati, D.I.; Ameta, R.; Ali, Y. Preparation of C-TiO2 nanophotocatalyst and its used for degradation of evans blue. Sci. Rev. Chem. Commun. 2016, 6, 12–18. [Google Scholar]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of pH Value on the Bandgap Energy and Particles Size for Biosynthesis of ZnO Nanoparticles: Efficiency for Photocatalytic Adsorption of Methyl Orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Jha, S.; Singh, A.K.; Mishra, S.K.; Pathak, A.K.; Ojha, R.P.; Yadav, R.S.; Dikshit, A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals 2022, 12, 1063. [Google Scholar] [CrossRef]
- Rezaei, M.; Khajenoori, M.; Nematollahi, B. Preparation of nanocrystalline MgO by surfactant assisted precipitation method. Mater. Res. Bull. 2011, 46, 1632–1637. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Saied, E.; Farag, M.M.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus Oryzae-mediated green synthesis of magnesium oxide nanoparticles (MgO-NPs): A promising tool for antimicrobial, mosquitocidal action, and tanning effluent treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Najoom, S.; Fozia, F.; Ahmad, I.; Wahab, A.; Ahmad, N.; Ullah, R.; Gul, A.; Bari, A.; Khan, M.Y.; Khan, A.A. Effective Antiplasmodial and Cytotoxic Activities of Synthesized Zinc Oxide Nanoparticles Using Rhazya stricta Leaf Extract. Evid.-Based Complement. Altern. Med. 2021, 2021, 5586740. [Google Scholar] [CrossRef] [PubMed]
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Oje, O.O.; Emma-Uba, C.O.; Mgbii, S.I.; Olowo, O.J.; Nwanji, O.L. Green synthesis of biogenic zinc oxide nanoflower as dual agent for photodegradation of an organic dye and tyrosinase inhibitor. J. Inorg. Organomet. Polym. Mater. 2021, 31, 886–897. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Amézaga-Madrid, P.; Pizá-Ruiz, P.; Antúnez-Flores, W.; Miki-Yoshida, M. Optical band gap estimation of ZnO nanorods. Mater. Res. 2016, 19, 33–38. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Vaseem, M.; Lee, K.-M.; Shin, J.-K.; Hahn, Y.-B. Synthesis of ZnO nanoparticles and their ink-jetting behavior. J. Nanosci. Nanotechnol. 2012, 12, 2380–2386. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 nanocomposites for antimicrobial coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 1–19. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.A.; Zerefa, E.; Adimasu, Y. PVA assisted ZnO based mesoporous ternary metal oxides nanomaterials: Synthesis, optimization, and evaluation of antibacterial activity. Mater. Res. Express 2020, 7, 045011. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Barhoum, A.; Nada, A.A.; Moustafa, Y.M.; Seliman, S.M.; Youssef, A.M.; Bechelany, M. Synthesis of mesoporous core-shell CdS@ TiO2 (0D and 1D) photocatalysts for solar-driven hydrogen fuel production. J. Photochem. Photobiol. A Chem. 2018, 351, 261–270. [Google Scholar] [CrossRef]
- Anisuzzaman, S.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Uttam, P.; Varunkumar, K.; Chong, K.F.; Ali, G.A. Photocatalytic performance of a novel semiconductor nanocatalyst: Copper doped nickel oxide for phenol degradation. Mater. Chem. Phys. 2020, 242, 122520. [Google Scholar] [CrossRef]
- Pang, Y.L.; Law, Z.X.; Lim, S.; Chan, Y.Y.; Shuit, S.H.; Chong, W.C.; Lai, C.W. Enhanced photocatalytic degradation of methyl orange by coconut shell–derived biochar composites under visible LED light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 27457–27473. [Google Scholar] [CrossRef]
- Li, H.; Yin, S.; Wang, Y.; Sato, T. Efficient persistent photocatalytic decomposition of nitrogen monoxide over a fluorescence-assisted CaAl2O4:(Eu, Nd)/(Ta, N)-codoped TiO2/Fe2O3. Appl. Catal. B Environ. 2013, 132, 487–492. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Su, Q.; Lv, C.; Jin, X.; Wen, X. UV-induced photodegradation of naproxen using a nano γ-FeOOH composite: Degradation kinetics and photocatalytic mechanism. Front. Chem. 2019, 7, 847. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grandiflorum for antimicrobial activity and adsorption of methylene blue. Appl. Water Sci. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Kalpana, V.; Rajalakshmi, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Enhanced photocatalytic degradation of water pollutants using bio-green synthesis of zinc oxide nanoparticles (ZnO NPs). J. Environ. Chem. Eng. 2021, 9, 105772. [Google Scholar] [CrossRef]
- Lal, S.; Verma, R.; Chauhan, A.; Dhatwalia, J.; Guleria, I.; Ghotekar, S.; Thakur, S.; Mansi, K.; Kumar, R.; Kumari, A. Antioxidant, antimicrobial, and photocatalytic activity of green synthesized ZnO-NPs from Myrica esculenta fruits extract. Inorg. Chem. Commun. 2022, 141, 109518. [Google Scholar] [CrossRef]
- Kumar, M.A.; Ravikumar, C.; Nagaswarupa, H.; Purshotam, B.; Gonfa, B.; Murthy, H.A.; Sabir, F.K.; Tadesse, S. Evaluation of bi-functional applications of ZnO nanoparticles prepared by green and chemical methods. J. Environ. Chem. Eng. 2019, 7, 103468. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef]
- Senthilraja, A.; Subash, B.; Krishnakumar, B.; Rajamanickam, D.; Swaminathan, M.; Shanthi, M. Synthesis, characterization and catalytic activity of co-doped Ag–Au–ZnO for MB dye degradation under UV-A light. Mater. Sci. Semicond. Process. 2014, 22, 83–91. [Google Scholar] [CrossRef]

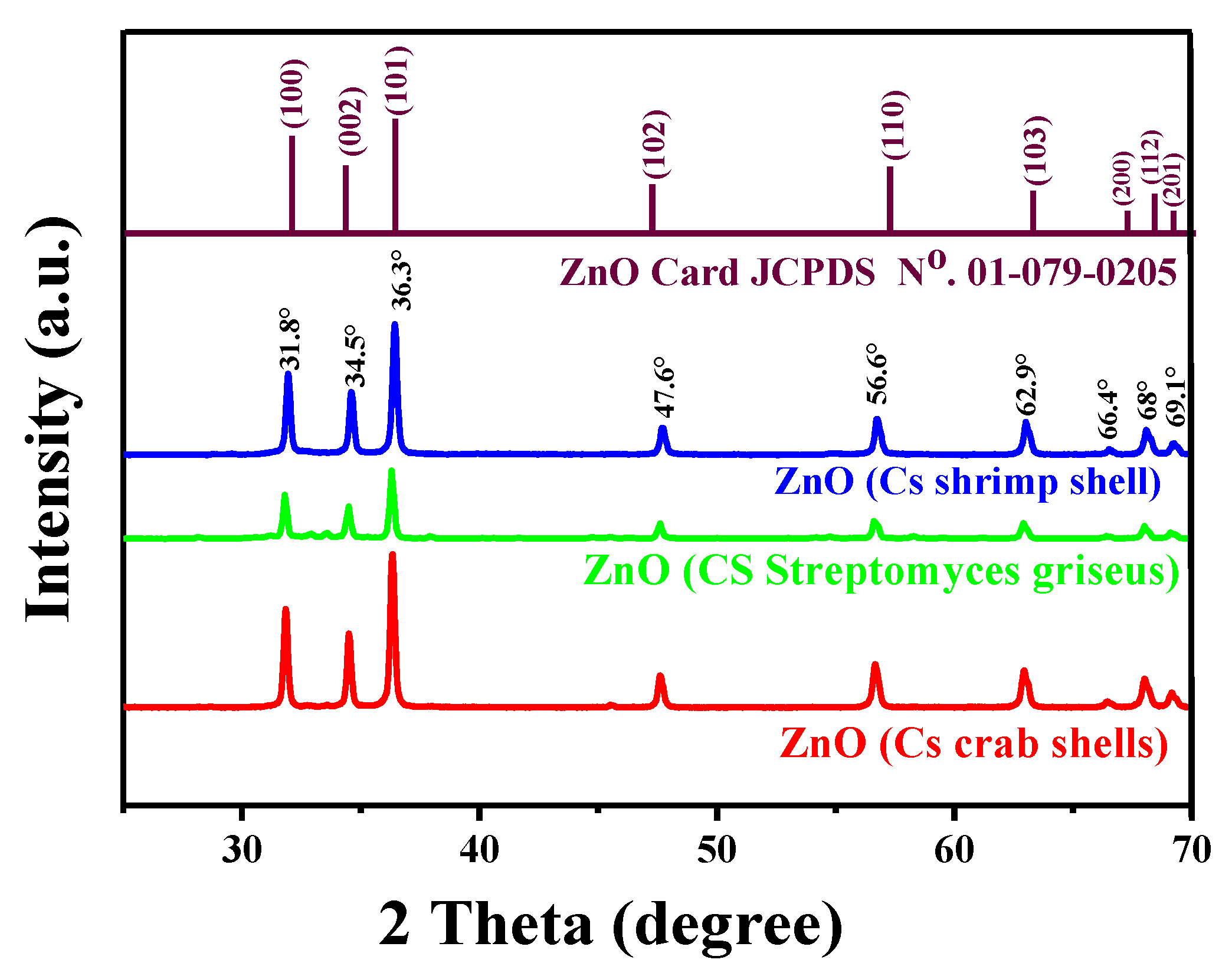

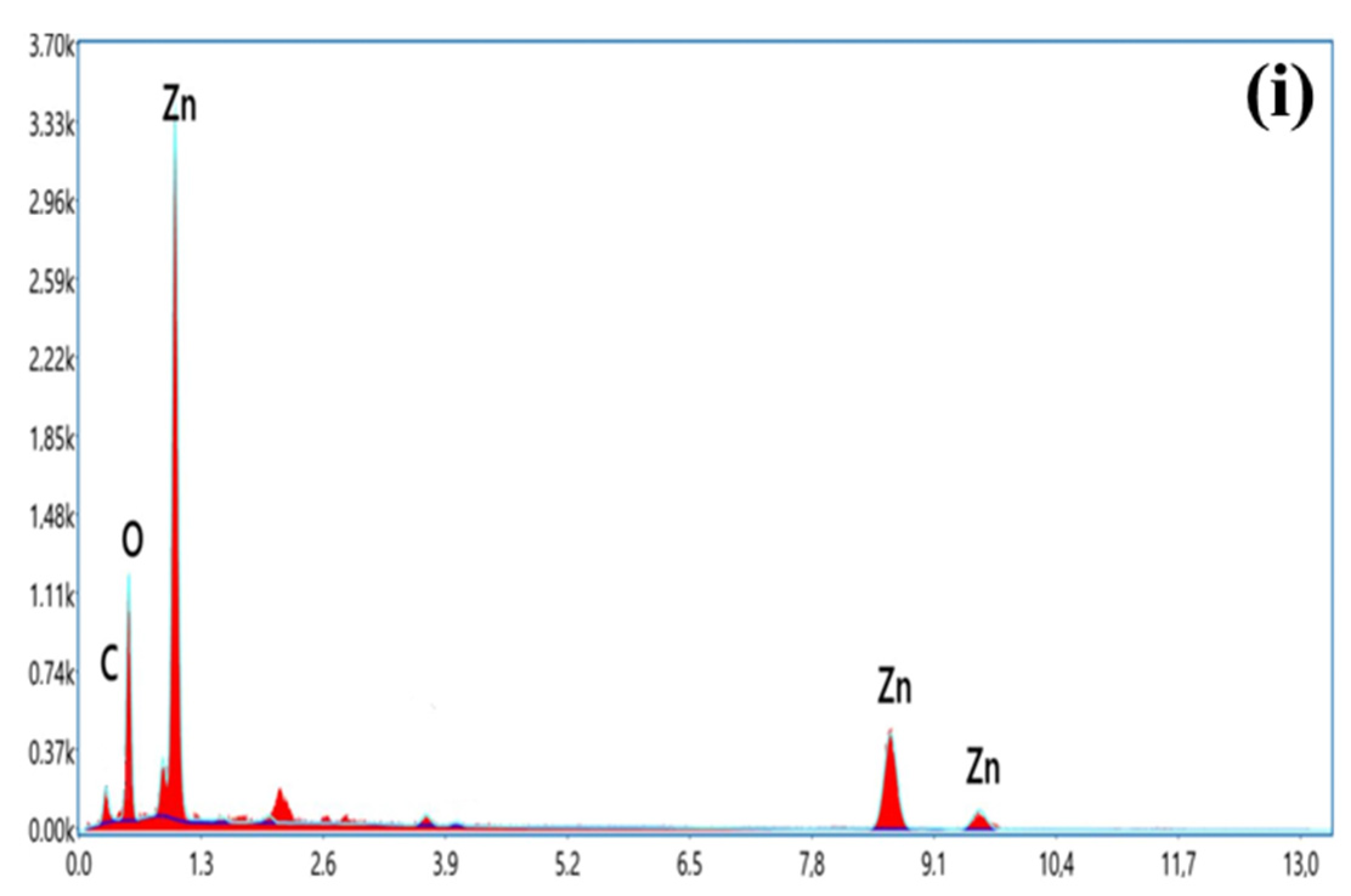


| Chitosan Source | 2θ (°) | FHWM | Crystallite Size (nm) | Lattice Parameter (Å) | |
|---|---|---|---|---|---|
| A | C | ||||
| ZnO NPs by CS of shrimp shells | 36.3 | 0.2657 | 30.9 | 3.25 | 5.19 |
| ZnO NPs by CS of crab shells | 36.5 | 0.253 | 33.6 | 3.23 | 5.17 |
| ZnO NPs by CS of Streptomyces griseus bacteria | 36.3 | 0.2423 | 35.8 | 3.24 | 5.19 |
| Compound | Composition of ZnO | |
|---|---|---|
| ZnO NPs by shrimp shells | Element | Atomic percentage % |
| O K | 52.01 | |
| Zn K | 47.99 | |
| Totals | 100 | |
| ZnO NPs by crab shells | C K | 12.43 |
| O K | 52.59 | |
| Ne K | 0.82 | |
| Zn K | 34.16 | |
| Totals | 100 | |
| ZnO NPs by Streptomyces griseus bacteria | C K | 22.07 |
| O K | 51.13 | |
| Zn K | 26.8 | |
| Totals | 100 | |
| Sample | Conc. | Zone of Inhibition a (mm) | ||||
|---|---|---|---|---|---|---|
| Gram-Negative | Gram-Positive | |||||
| Pseudomonas aeruginosa | Salmonella typhimuruim | Staphulococcus aureus | Listeria innocua | Bacillus subtiliis | ||
| ZnO NPs by CS of shrimp shells | 2 mg⁄mL | 13 ± 0.25 | 7 ± 0.15 | 12 ± 0.10 | 12 ± 0.12 | 20 ± 0.30 |
| 4 mg⁄mL | 13 ± 0.20 | 11 ± 0.15 | 19 ± 0.30 | 15 ± 0.15 | 16 ± 0.35 | |
| 6 mg⁄mL | 11 ± 0.15 | 14 ± 0.2 | 22 ± 0.35 | 16 ± 0.19 | 19 ± 025 | |
| ZnO NPs by CS of crab shells | 2 mg⁄mL | 11 ± 0.10 | 1 ± 0.05 | 0.5 ± 0.05 | 16 ± 0.2 | 12 ± 0.35 |
| 4 mg⁄mL | 12 ± 0.15 | 9 ± 0.15 | 0.8 ± 0.05 | 17 ± 0.25 | 20 ± 0.1 | |
| 6 mg⁄mL | 15 ± 0.25 | 12 ± 0.25 | 1 ± 0.1 | 16 ± 0.3 | 20 ± 0.2 | |
| ZnO NPs by CS of Streptomyces griseus bacteria | 2 mg⁄mL | 0.5 ± 0.15 | 1 ± 0.1 | 0.4 ± 0.12 | 4 ± 0.15 | 10 ± 0.20 |
| 4 mg⁄mL | 0.75 ± 0.05 | 3 ± 0.1 | 0.7 ± 0.04 | 13 ± 0.2 | 14 ± 0.25 | |
| 6 mg⁄mL | 1.25 ± 0.05 | 7 ± 0.15 | 1.1 ± 0.05 | 11 ± 0.20 | 15 ± 0.30 | |
| ciprofloxacin (CIP-5) | 50 µg | 22 ± 0.4 | 17 ± 0.15 | 14 ± 0.2 | 24 ± 0.3 | 24 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Amor, I.; Hemmami, H.; Laouini, S.E.; Mahboub, M.S.; Barhoum, A. Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye. Catalysts 2022, 12, 1611. https://doi.org/10.3390/catal12121611
Ben Amor I, Hemmami H, Laouini SE, Mahboub MS, Barhoum A. Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye. Catalysts. 2022; 12(12):1611. https://doi.org/10.3390/catal12121611
Chicago/Turabian StyleBen Amor, Ilham, Hadia Hemmami, Salah Eddine Laouini, Mohammed Sadok Mahboub, and Ahmed Barhoum. 2022. "Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye" Catalysts 12, no. 12: 1611. https://doi.org/10.3390/catal12121611
APA StyleBen Amor, I., Hemmami, H., Laouini, S. E., Mahboub, M. S., & Barhoum, A. (2022). Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye. Catalysts, 12(12), 1611. https://doi.org/10.3390/catal12121611