The Synergistic Catalysis of Chloroaromatic Organics and NOx over Monolithic Vanadium-Based Catalysts at Low Temperature
Abstract
:1. Introduction
2. Results and Discussion
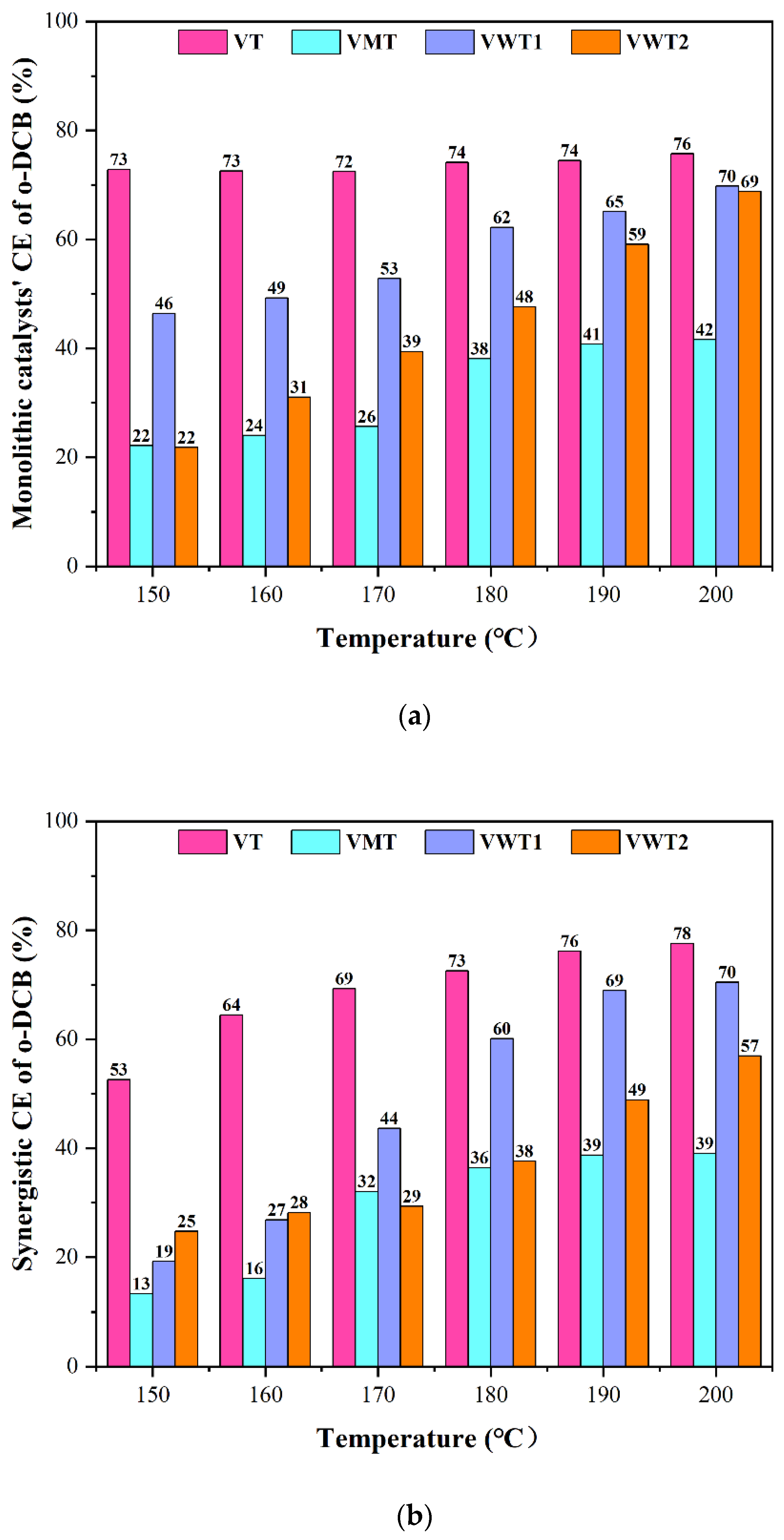
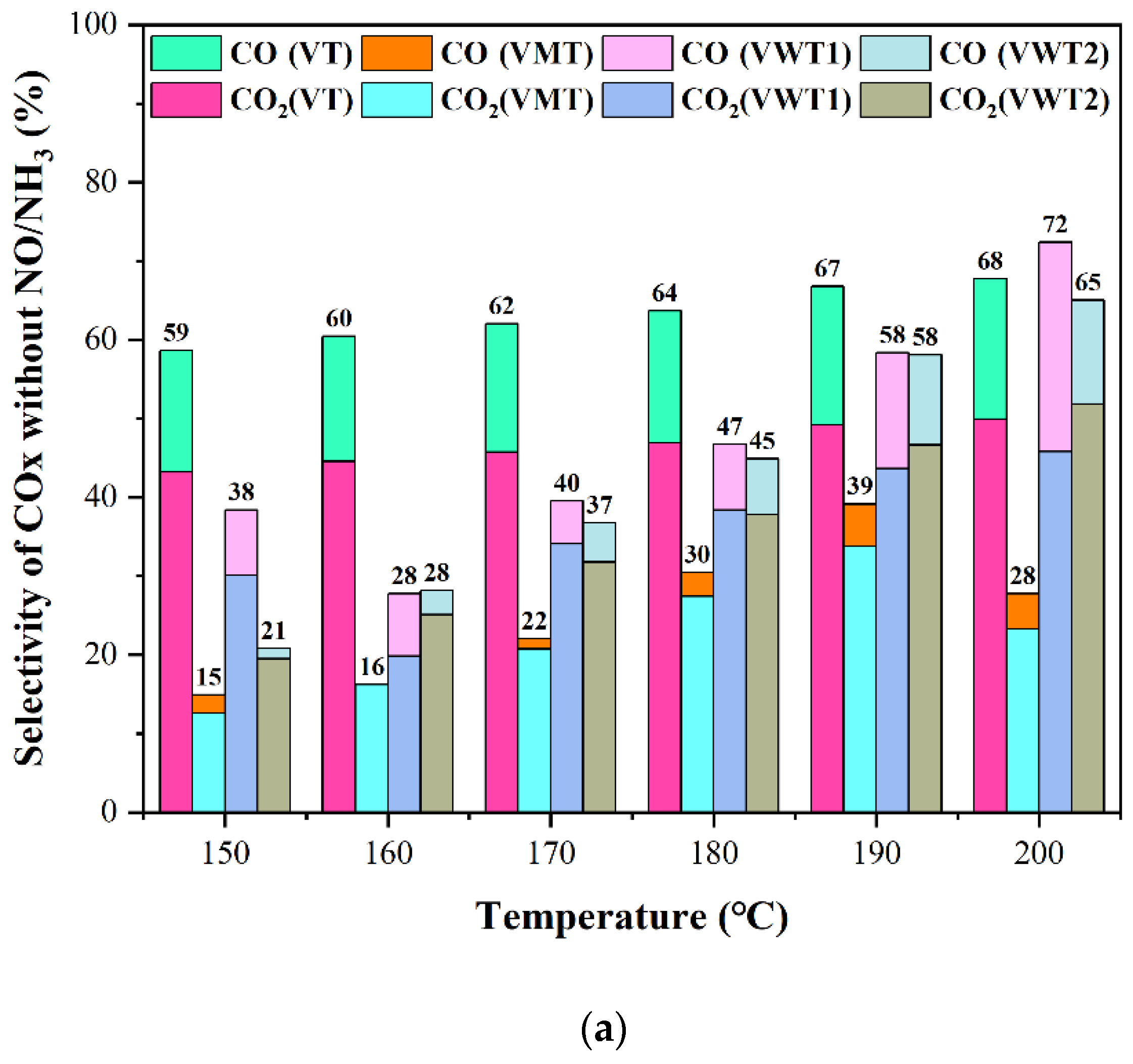
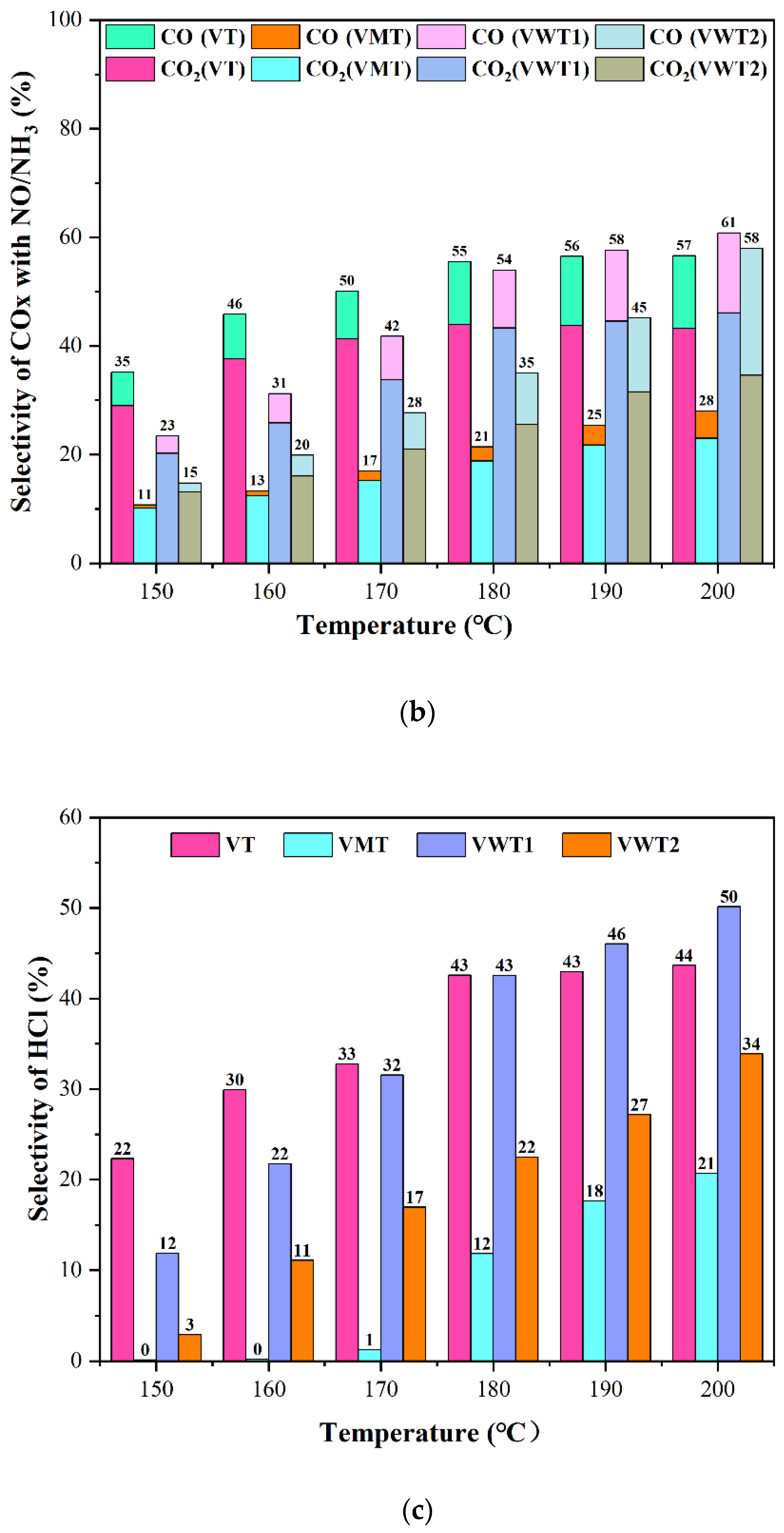
2.1. CE of o-DCB and Nox, and the Selectivity of Cox/HCl/N2O
2.2. BET and XRF Analysis
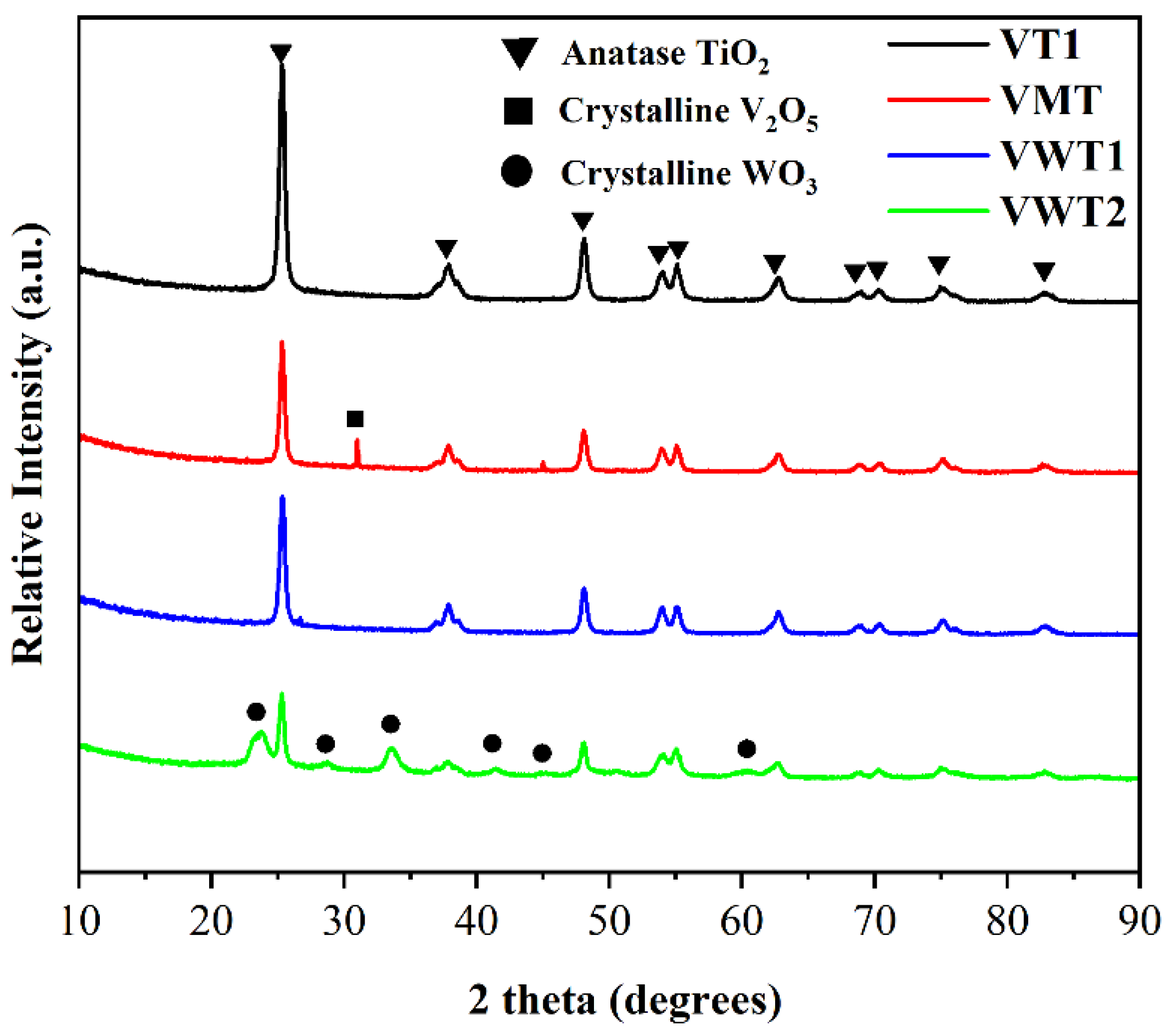
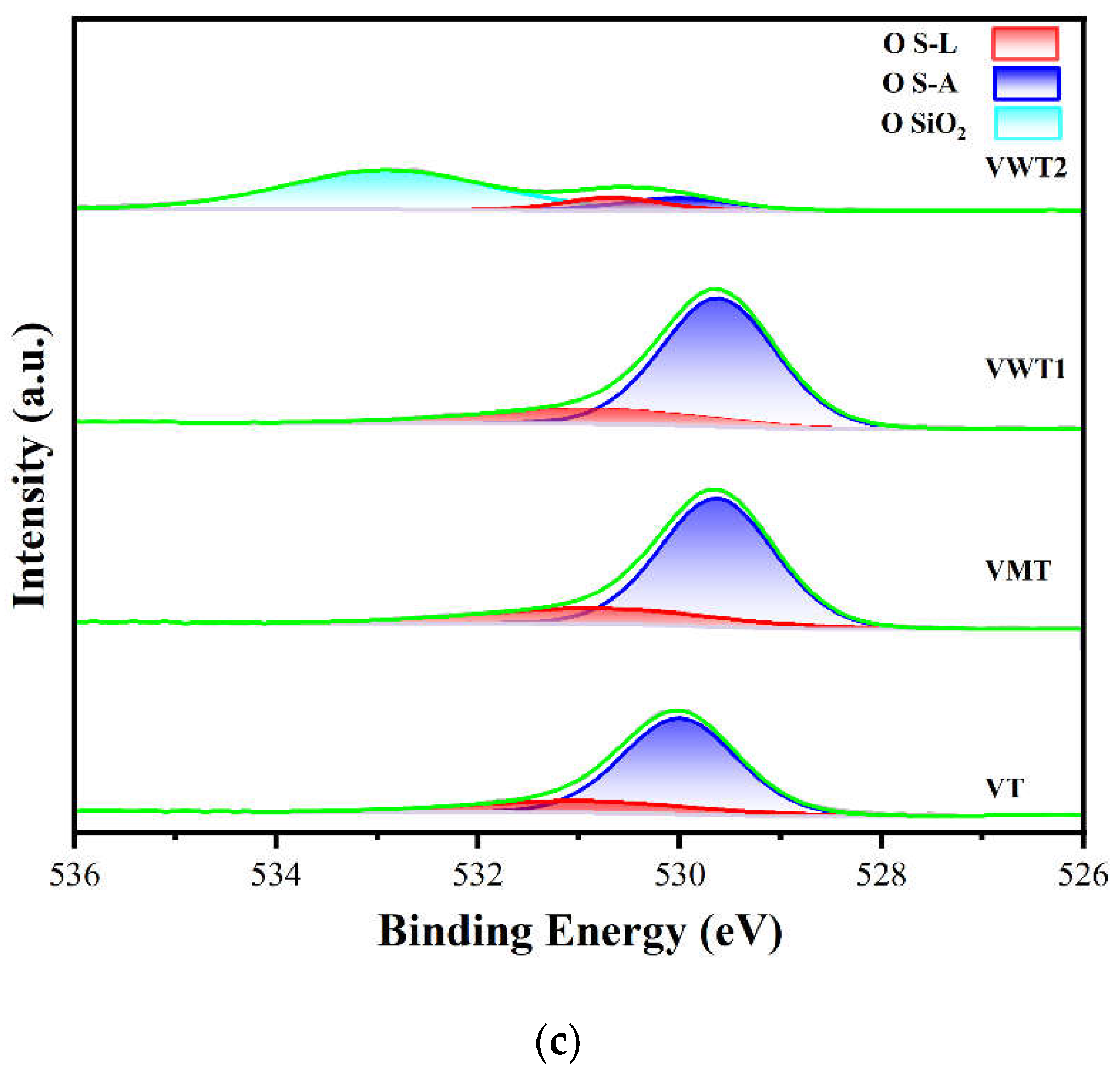
2.3. XRD and XPS Analysis
2.4. H2-TPR and NH3-TPD Analysis
3. Material and Methods
3.1. Catalysts Preparation and Characterization
3.2. Catalytic Tests and Reaction Set-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhavsar, S.P.; Reiner, E.J.; Hayton, A.; Fletcher, R.; Macpherson, K. Converting toxic equivalents (teq) of dioxins and dioxin-like compounds in fish from one toxic equivalency factor (tef) scheme to another. Environ. Int. 2008, 34, 915–921. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Kumar, A.; Ying, Q.; Vandenberghe, F.; Kleeman, M.J. Separately resolving nox and voc contributions to ozone formation. Atmos. Environ. 2022, 285, 119224. [Google Scholar] [CrossRef]
- Lu, S.; Xiang, Y.; Chen, Z.; Chen, T.; Lin, X.; Zhang, W.; Li, X.; Yan, J. Development of phosphorus-based inhibitors for pcdd/fs suppression. Waste Manag. 2021, 119, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Karademir, A.; Bakoglu, M.; Taspinar, F.; Ayberk, S. Removal of pcdd/fs from flue gas by a fixed-bed activated carbon filter in a hazardous waste incinerator. Environ. Sci. Technol. 2004, 38, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Fahlenkamp, H.; Mittelbach, G.; Hagenmaier, H.P.; Brunner, H.; Tichaczek, K.H. Catalytic oxidation. A technique for reducing pcdd/pcdf emissions from waste incinerators to less than 0.1 ng te/m3. VGB Kraftw. 1991, 71, 671–674. [Google Scholar]
- Mckay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (msw) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Hagenmaier, H. Katalystische oxidation halogenierter kohlenwasserstoffe unter besonderer berücksichtigung des dioxinproblems. VDI-Berichte 1989, 730, 239–254. [Google Scholar]
- Hagenmaier, H.; Horch, K.; Fahlenkamp, H.; Schetter, G. Destruction of PCDD and PCDF in refuse incineration plants by primary and secondary measures. Chemosphere 1991, 23, 1429–1437. [Google Scholar] [CrossRef]
- Okumura, M.; Akita, T.; Haruta, M.; Wang, X.; Kajikawa, O.; Okada, O. Multi-component noble metal catalysts prepared by sequential deposition precipitation for low temperature decomposition of dioxin. Appl. Catal. B Environ. 2003, 41, 43–52. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Uemichi, Y.; Ayame, A. Low-temperature hydrodechlorination mechanism of chlorobenzenes over platinum-supported and palladium-supported alumina catalysts. Appl. Catal. A Gen. 2005, 287, 89–97. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.; Liu, W.; Wang, X. Catalytic combustion of 1,2-dichlorobenzene at low temperature over mn-modified co3o4 catalysts. Appl. Catal. B Environ. 2015, 166–167, 393–405. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Stevens, W.R.; Lee, C.W.; Gullett, B.K.; Zhao, Y. Study on the decomposition of trace benzene over v2o5–WO3/TiO2-based catalysts in simulated flue gas. Appl. Catal. B Environ. 2014, 147, 322–329. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic oxidation of 1,2-dichlorobenzene over supported transition metal oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- Gan, L.; Li, K.; Niu, H.; Peng, Y.; Chen, J.; Huang, Y.; Li, J. Simultaneous removal of nox and chlorobenzene on v2o5/TiO2 granular catalyst: Kinetic study and performance prediction. Front. Environ. Sci. Eng. 2020, 15, 70. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Ren, Y.; Chen, T.; Lu, S.; Yan, J. Low temperature oxidation of pcdd/fs by TiO2-based v2o5/WO3 catalyst. Environ. Prog. Sustain. 2016, 35, 1265–1273. [Google Scholar] [CrossRef]
- Kuma, R.; Kitano, T.; Tsujiguchi, T.; Tanaka, T. Effect of molybdenum on the structure and performance of v2o5/TiO2–sio2–moo3 catalysts for the oxidative degradation of o-chlorotoluene. Appl. Catal. A Gen. 2020, 595, 117496. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Y.; Gao, Y.; Wang, H.; Zong, Y.; Chang, Z.; Zhang, S.; Han, P.; Yu, Y. Promotional effect of phosphorus addition on improving the so2 resistance of v2o5-moo3/TiO2 catalyst for nh3-scr of no. J. Phys. Chem. Solids 2022, 163, 110566. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based scr catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Chin, S.; Jurng, J.; Lee, J.; Moon, S. Catalytic conversion of 1,2-dichlorobenzene using v2o5/TiO2 catalysts by a thermal decomposition process. Chemosphere 2009, 75, 1206–1209. [Google Scholar] [CrossRef]
- Huang, X.; Peng, Y.; Liu, X.; Li, K.; Deng, Y.; Li, J. The promotional effect of moo3 doped v2o5/TiO2 for chlorobenzene oxidation. Catal. Commun. 2015, 69, 161–164. [Google Scholar] [CrossRef]
- Wu, M.; Ung, K.C.; Dai, Q.; Wang, X. Catalytic combustion of chlorinated vocs over vox/TiO2 catalysts. Catal. Commun. 2012, 18, 72–75. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; González-Marcos, M.P.; Markaide-Aiastui, B.A.; González-Marcos, J.A.; González-Velasco, J.R. Effect of vanadia loading on acidic and redox properties of vox/TiO2 for the simultaneous abatement of pcdd/fs and nox. J. Ind. Eng. Chem. 2020, 81, 440–450. [Google Scholar] [CrossRef]
- Albonetti, S.; Blasioli, S.; Bonelli, R.; Mengou, J.E.; Scirè, S.; Trifirò, F. The role of acidity in the decomposition of 1,2-dichlorobenzene over TiO2-based v2o5/WO3 catalysts. Appl. Catal. A Gen. 2008, 341, 18–25. [Google Scholar] [CrossRef]
- Cai, M.; Bian, X.; Xie, F.; Wu, W.; Cen, P. Formation and performance of monolithic catalysts for selective catalytic reduction of nitrogen oxides: A critical review. Chemistryselect 2021, 6, 12331–12341. [Google Scholar] [CrossRef]
- Zhan, M.X.; Fu, J.Y.; Ji, L.J.; Deviatkin, I.; Lu, S.Y. Comparative analyses of catalytic degradation of pcdd/fs in the laboratory vs. Industrial conditions. Chemosphere 2018, 191, 895–902. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Lai, J.; Lin, X.; Zhang, H.; Du, H.; Long, J.; Yan, J.; Li, X. Implication of operation time on low-temperature catalytic oxidation of chloroaromatic organics over vox/TiO2 catalysts: Deactivation mechanism analysis. J. Clean. Prod. 2022, 372, 133477. [Google Scholar] [CrossRef]
- Weber, R.; Sakurai, T.; Hagenmaier, H. Low temperature decomposition of pcdd/pcdf, chlorobenzenes and pahs by TiO2-based v2o5–WO3 catalysts. Appl. Catal. B Environ. 1999, 20, 249–256. [Google Scholar] [CrossRef]
- Ma, Y.; Lai, J.; Li, X.; Lin, X.; Li, L.; Jing, H.; Liu, T.; Yan, J. Field study on pcdd/f decomposition over vox/TiO2 catalyst under low-temperature: Mechanism and kinetics analysis. Chem. Eng. J. 2022, 429, 132222. [Google Scholar] [CrossRef]
- Xiao, G.; Guo, Z.; Lin, B.; Fu, M.; Ye, D.; Hu, Y. Cu-vwt catalysts for synergistic elimination of nox and volatile organic compounds from coal-fired flue gas. Environ. Sci. Technol. 2022, 56, 10095–10104. [Google Scholar] [CrossRef] [PubMed]
- Bertinchamps, F.; Treinen, M.; Blangenois, N.; Mariage, E.; Gaigneaux, E.M. Positive effect of nox on the performances of vox/TiO2-based catalysts in the total oxidation abatement of chlorobenzene. J. Catal. 2005, 230, 493–498. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A review on selective catalytic reduction of nox by nh3 over mn–based catalysts at low temperatures: Catalysts, mechanisms, kinetics and dft calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Topsøe, N. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line fourier transform infrared spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Ramis, G.; Yi, L.; Busca, G.; Turco, M.; Kotur, E.; Willey, R.J. Adsorption, activation, and oxidation of ammonia over scr catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Su, W.; Chang, H.; Peng, Y.; Zhang, C.; Li, J. Reaction pathway investigation on the selective catalytic reduction of no with nh3 over cu/ssz-13 at low temperatures. Environ. Sci. Technol. 2015, 49, 467–473. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, J.; Yu, X.; Feng, Y.; Wang, Q.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Insight into the mars-van krevelen mechanism for production 2,5-diformylfuran over fenx@c catalyst. Biomass Bioenergy 2022, 156, 106320. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.; Wachs, I.E. Formation of n2o greenhouse gas during scr of no with nh3 by supported vanadium oxide catalysts. Appl. Catal. B Environ. 2018, 224, 836–840. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Mao, L.; Fu, M.; Zhao, H.; Sun, L.; Xiao, Y.; Dong, G. A relationship between the v4+/v5+ ratio and the surface dispersion, surface acidity, and redox performance of v2o5–WO3/TiO2 scr catalysts. Rsc. Adv. 2018, 8, 31081–31093. [Google Scholar] [CrossRef] [Green Version]
- Went, G.T.; Leu, L.; Rosin, R.R.; Bell, A.T. The effects of structure on the catalytic activity and selectivity of v2o5/TiO2 for the reduction of no by nh3. J. Catal. 1992, 134, 492–505. [Google Scholar] [CrossRef]
- Lietti, L. Reactivity of v2o5&z.sbnd;WO3TiO2 de-nox catalysts by transient methods. Appl. Catal. B Environ. 1996, 10, 281–297. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, S.; Lee, M.; Ye, B.; Kim, T.; Kim, H.; Lee, J.W.; Lee, D.H. Effect of catalyst crystallinity on v-based selective catalytic reduction with ammonia. Nanomaterials 2021, 11, 1452. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Brückner, A.; Hipler, F.; Auer, G.; Löffler, E.; Grünert, W. A new view on the relations between tungsten and vanadium in v2o5WO3/TiO2 catalysts for the selective reduction of no with nh3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Lian, Z.; Wei, J.; Shan, W.; Yu, Y.; Radjenovic, P.M.; Zhang, H.; He, G.; Liu, F.; Li, J.; Tian, Z.; et al. Adsorption-induced active vanadium species facilitate excellent performance in low-temperature catalytic nox abatement. J. Am. Chem. Soc. 2021, 143, 10454–10461. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in xps analysis of first row transition metals, oxides and hydroxides: Sc, ti, v, cu and zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of nox with nh3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, M.J.; Boyano, A.; Herrera, C.; Larrubia, M.A.; Alemany, L.J.; Moliner, R. Vanadium loaded carbon-based monoliths for the on-board no reduction: Influence of vanadia and tungsten loadings. Chem. Eng. J. 2009, 155, 68–75. [Google Scholar] [CrossRef]
- Wang, X.; Du, X.; Yang, G.; Xue, J.; Chen, Y.; Zhang, L. Chemisorption of no2 on v-based scr catalysts: A fundamental study toward the mechanism of “fast scr” reaction. J. Phys. Chem. C 2019, 123, 20451–20458. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of nox and the selective catalytic oxidation of nh3. An ft-ir study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bertinchamps, F.; Blangenois, N.; Eloy, P.; Gaigneaux, E.M. On the impact of the choice of model voc in the evaluation of v-based catalysts for the total oxidation of dioxins: Furan vs. Chlorobenzene. Appl. Catal. B Environ. 2007, 74, 223–232. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Wang, B.; Sun, Q.; Xu, W.; Zhu, T. Effects of mox (m=mn, cu, sb, la) on v–mo–ce/ti selective catalytic reduction catalysts. J. Rare Earths 2020, 38, 157–166. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Sun, J.; Liu, Q.; Zhao, D.; Gao, W.; Liu, L. Effects of mercury oxidation on v2o5–WO3/TiO2 catalyst properties in nh3-scr process. Catal. Commun. 2015, 59, 78–82. [Google Scholar] [CrossRef]
- Wang, P.; Gao, S.; Wang, H.; Chen, S.; Chen, X.; Wu, Z. Enhanced dual resistance to alkali metal and phosphate poisoning: Mo modifying vanadium-titanate nanotubes scr catalyst. Appl. Catal. A Gen. 2018, 561, 68–77. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q. Surface characterization studies on the interaction of v2o5–WO3/TiO2 catalyst for low temperature scr of no with nh3. J. Solid State Chem. 2015, 221, 49–56. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhong, Q. Promotional effect of f-doped v2o5–WO3/TiO2 catalyst for nh3-scr of no at low-temperature. Appl. Catal. A Gen. 2012, 435–436, 156–162. [Google Scholar] [CrossRef]
- Besselmann, S.; Freitag, C.; Hinrichsen, O.; Muhler, M. Temperature-programmed reduction and oxidation experiments with v 2 o 5/tio 2 catalysts. Phys. Chem. Chem. Phys. 2001, 3, 4633–4638. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on scr performance of v2o5WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Dang, J.; Zhang, G.H.; Chou, K.C.; Reddy, R.G.; He, Y.; Sun, Y. Kinetics and mechanism of hydrogen reduction of moo3 to moo2. Int. J. Refract. Met. H 2013, 41, 216–223. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. In situ drifts investigation of the low-temperature reaction mechanism over mn-doped co3o4 for the selective catalytic reduction of nox with nh3. J. Phys. Chem. C 2015, 119, 22924–22933. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, B.; Du, J.; Tang, Q.; Liu, Z.; Liu, R.; Tao, C. The monolithic cordierite supported v2o5–moo3/TiO2 catalyst for nh3-scr. Chem. Eng. J. 2016, 294, 264–272. [Google Scholar] [CrossRef]





| Ea1 | Ea2 | R12 | R22 | |
|---|---|---|---|---|
| VT | 4.2 | 24.3 | 0.92 | 0.97 |
| VMT | 26.9 | 44.4 | 0.95 | 0.91 |
| VWT1 | 21.6 | 62.9 | 0.99 | 0.95 |
| VWT2 | 35.6 | 51.9 | 0.99 | 0.97 |
| BET Surface Area SBET (m2/g) | VOx (wt%) | WOx (MoOx) (wt%) | TiO2 (wt%) | Other Species (wt%) | VOx Surface Density (VOx/nm2) | Forms | |
|---|---|---|---|---|---|---|---|
| VT | 91.98 | 6.58% | / | 92.12% | 1.30% | 8.45 | Granular |
| VMT | 59.65 | 3.63% | 6.76% (MoOx) | 78.90% | 10.71% | 7.19 | Honeycomb |
| VWT1 | 74.53 | 2.99% | 2.62% (WOx) | 84.46% | 9.93% | 4.74 | Honeycomb |
| VWT2 | 78.90 | 2.87% | 12.14% (WOx) | 54.63% | 30.36% | 4.30 | Corrugated |
| V4+ | V5+ | V4+/V5+ | OS-A/OS-L | |||||
|---|---|---|---|---|---|---|---|---|
| Fresh | Used | Fresh | Used | Fresh | Used | Fresh | Used | |
| VT | 12.51% | 68.24% | 77.99% | 17.02% | 0.16 | 4.01 | 0.24 | 0.64 |
| VMT | 51.96% | 56.07% | 26.97% | 32.04% | 1.73 | 2.39 | 0.25 | 0.62 |
| VWT1 | 27.85% | 58.12% | 62.77% | 20.62% | 0.44 | 2.82 | 0.34 | 0.87 |
| VWT2 | 11.56% | 50.22% | 65.86% | 33.32% | 0.18 | 1.51 | 0.91 | 1.10 |
| Brønsted Acidity µmol NH3/g | Lewis Acidity µmol NH3/g | Total Acidity µmol NH3/g | |
|---|---|---|---|
| VT | 0.29 | 0.23 | 0.52 |
| VMT | 0.27 | 0.53 | 0.80 |
| VWT1 | 0.11 | 0.74 | 0.85 |
| VWT2 | 0.12 | 0.07 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.; Ma, Y.; Wu, J.; Yu, H.; Li, X.; Lin, X. The Synergistic Catalysis of Chloroaromatic Organics and NOx over Monolithic Vanadium-Based Catalysts at Low Temperature. Catalysts 2022, 12, 1342. https://doi.org/10.3390/catal12111342
Lai J, Ma Y, Wu J, Yu H, Li X, Lin X. The Synergistic Catalysis of Chloroaromatic Organics and NOx over Monolithic Vanadium-Based Catalysts at Low Temperature. Catalysts. 2022; 12(11):1342. https://doi.org/10.3390/catal12111342
Chicago/Turabian StyleLai, Jianwen, Yunfeng Ma, Jiayao Wu, Hong Yu, Xiaodong Li, and Xiaoqing Lin. 2022. "The Synergistic Catalysis of Chloroaromatic Organics and NOx over Monolithic Vanadium-Based Catalysts at Low Temperature" Catalysts 12, no. 11: 1342. https://doi.org/10.3390/catal12111342





