Insight into the Effects of Inorganic Element Catalysis and Basic Fuel Properties on the Self-Sustained Smoldering Process of Sewage Sludge
Abstract
1. Introduction
2. Results and Discussion
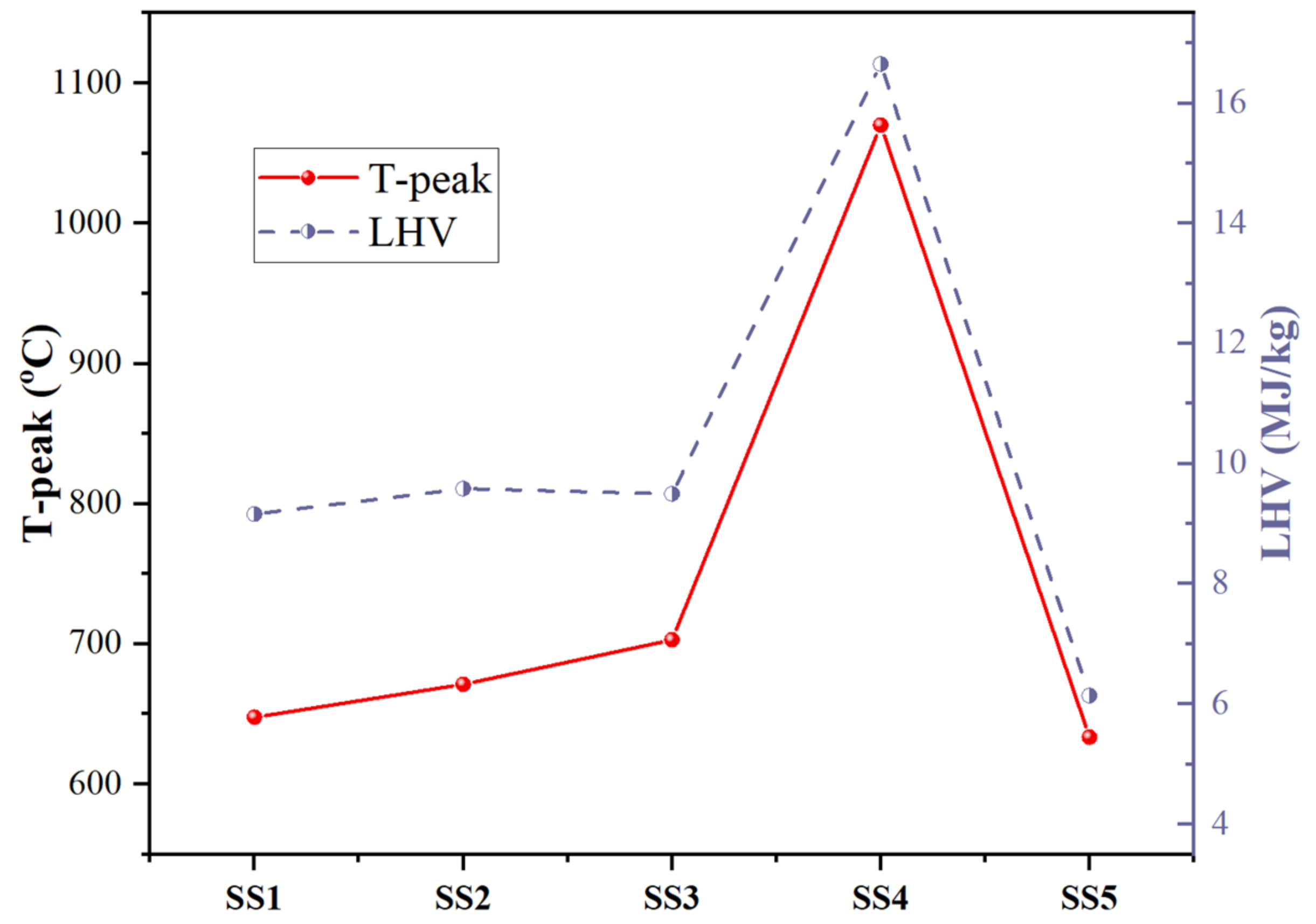
2.1. Basic Fuel Properties of SS
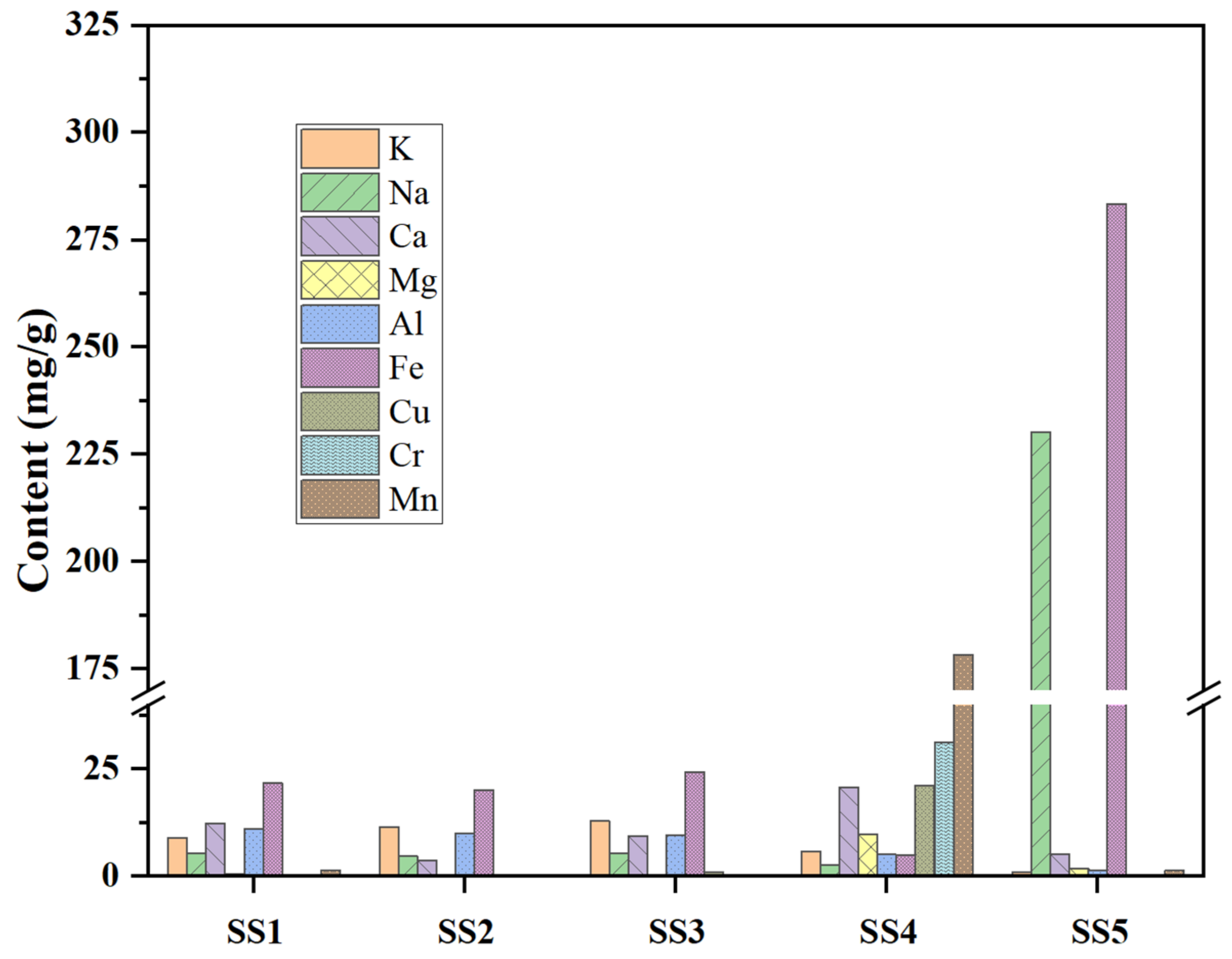
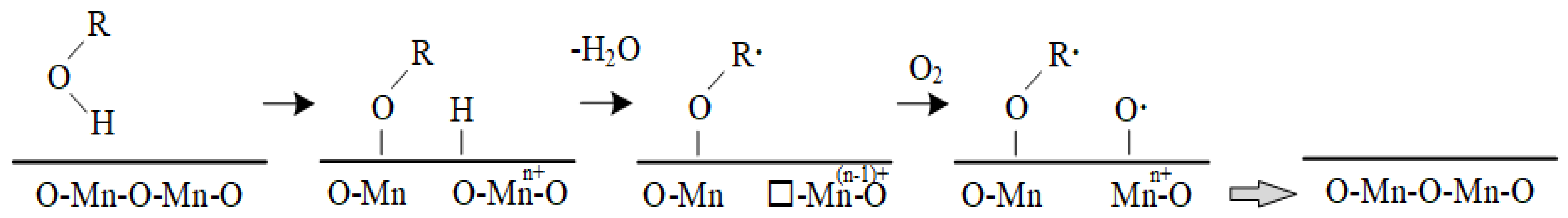
2.2. Characteristics of Inorganic Elements
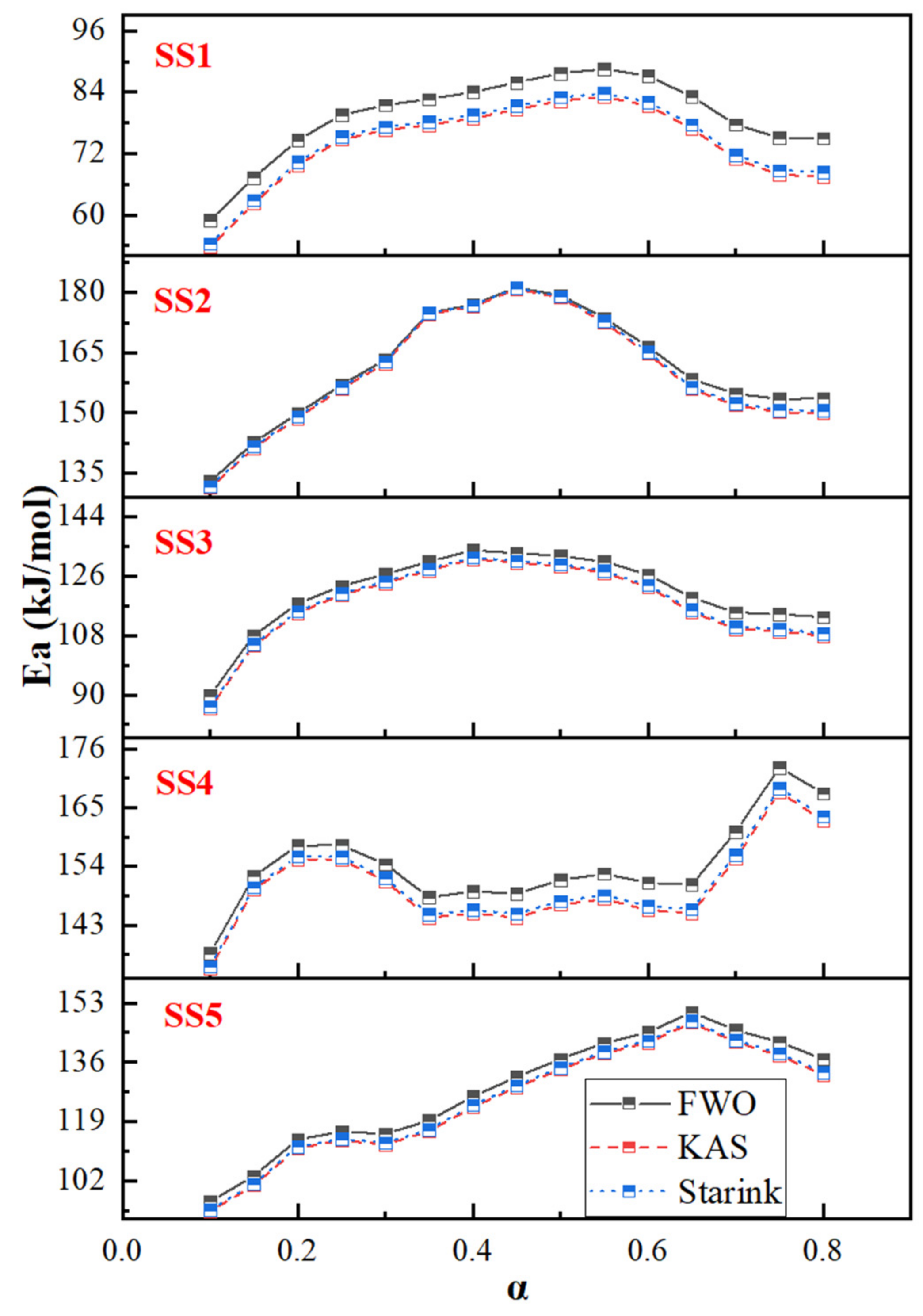
2.3. Thermogravimetric and Kinetic Analysis
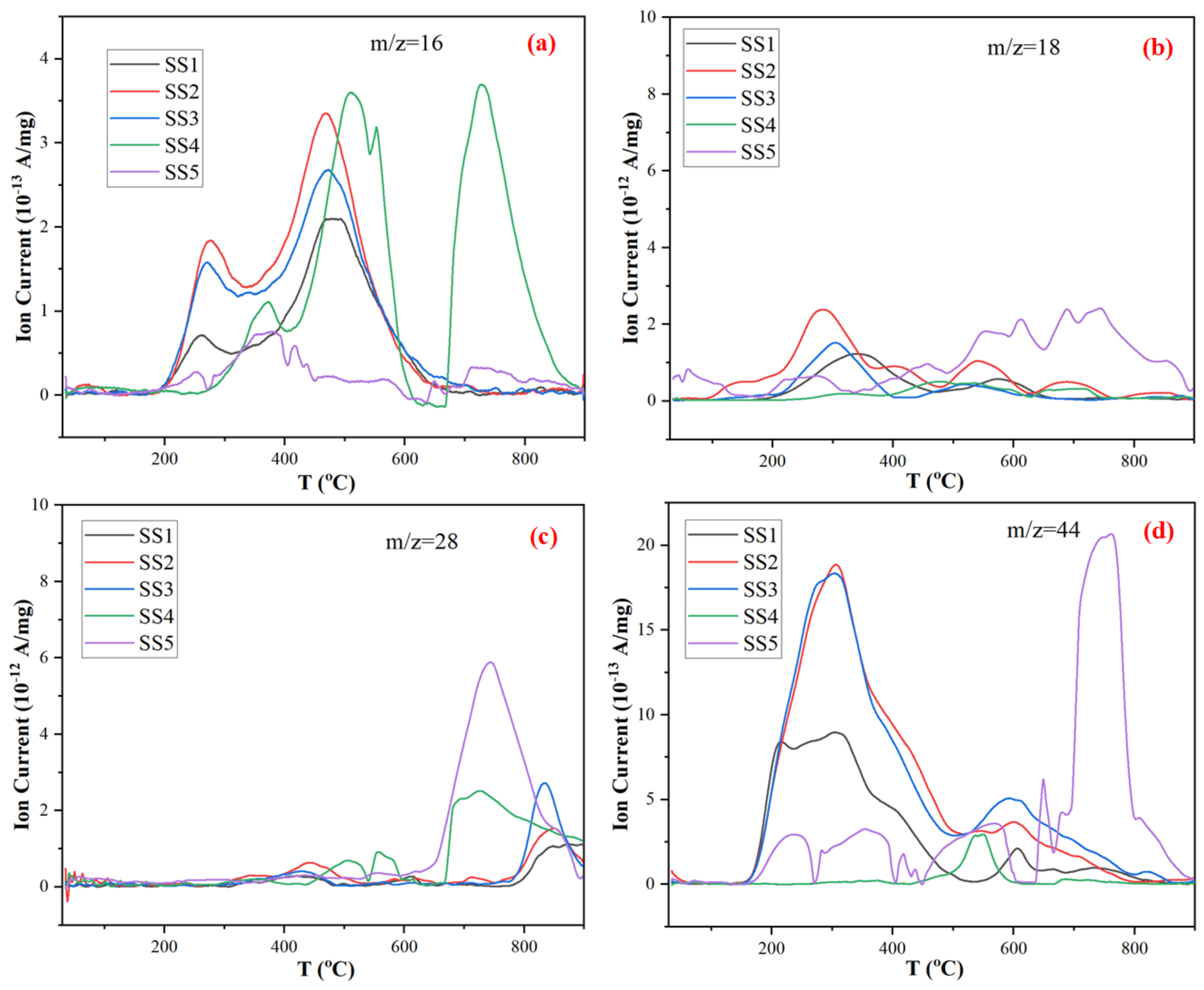
2.4. TG–FTIR–MS for the Gaseous Products
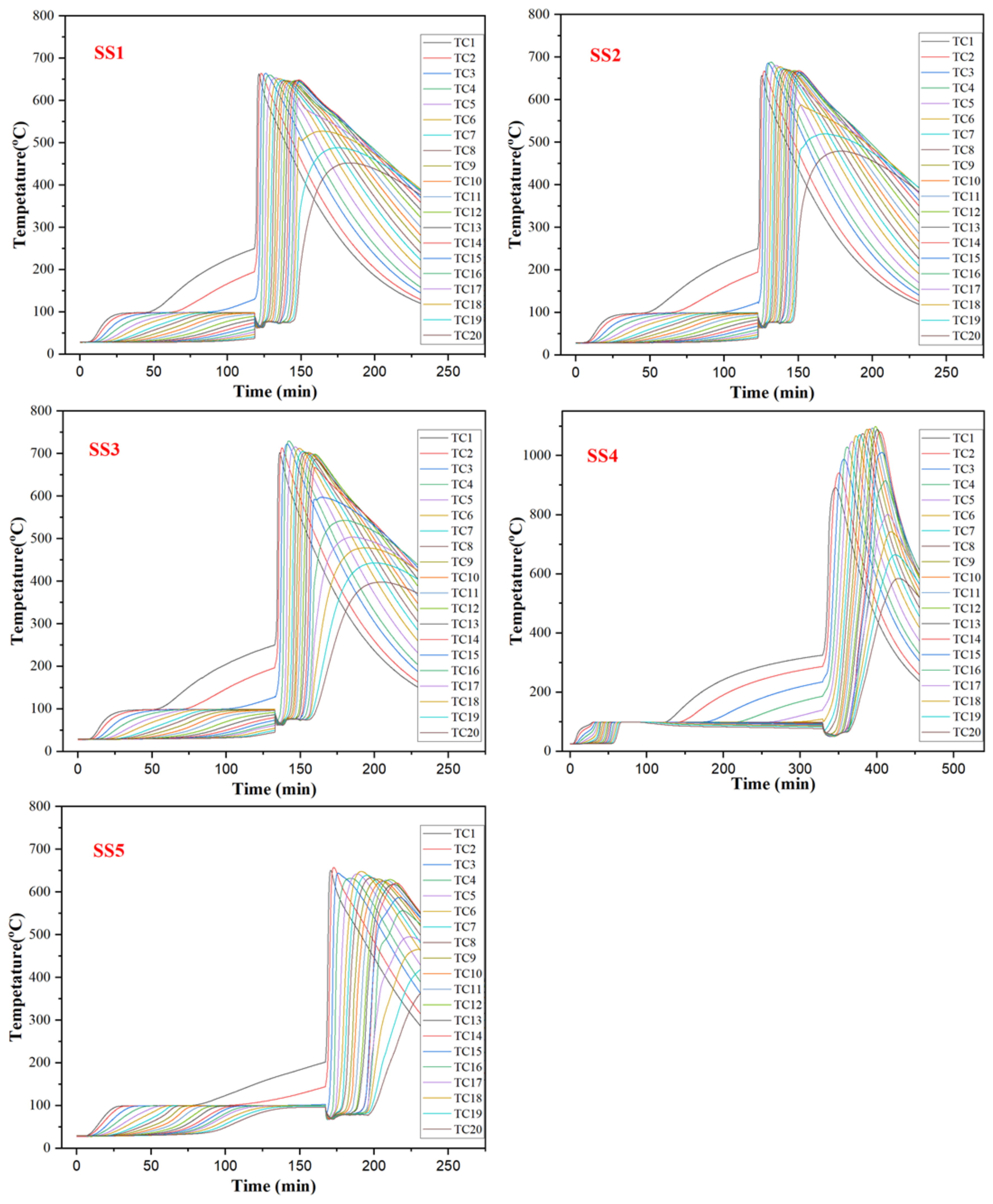
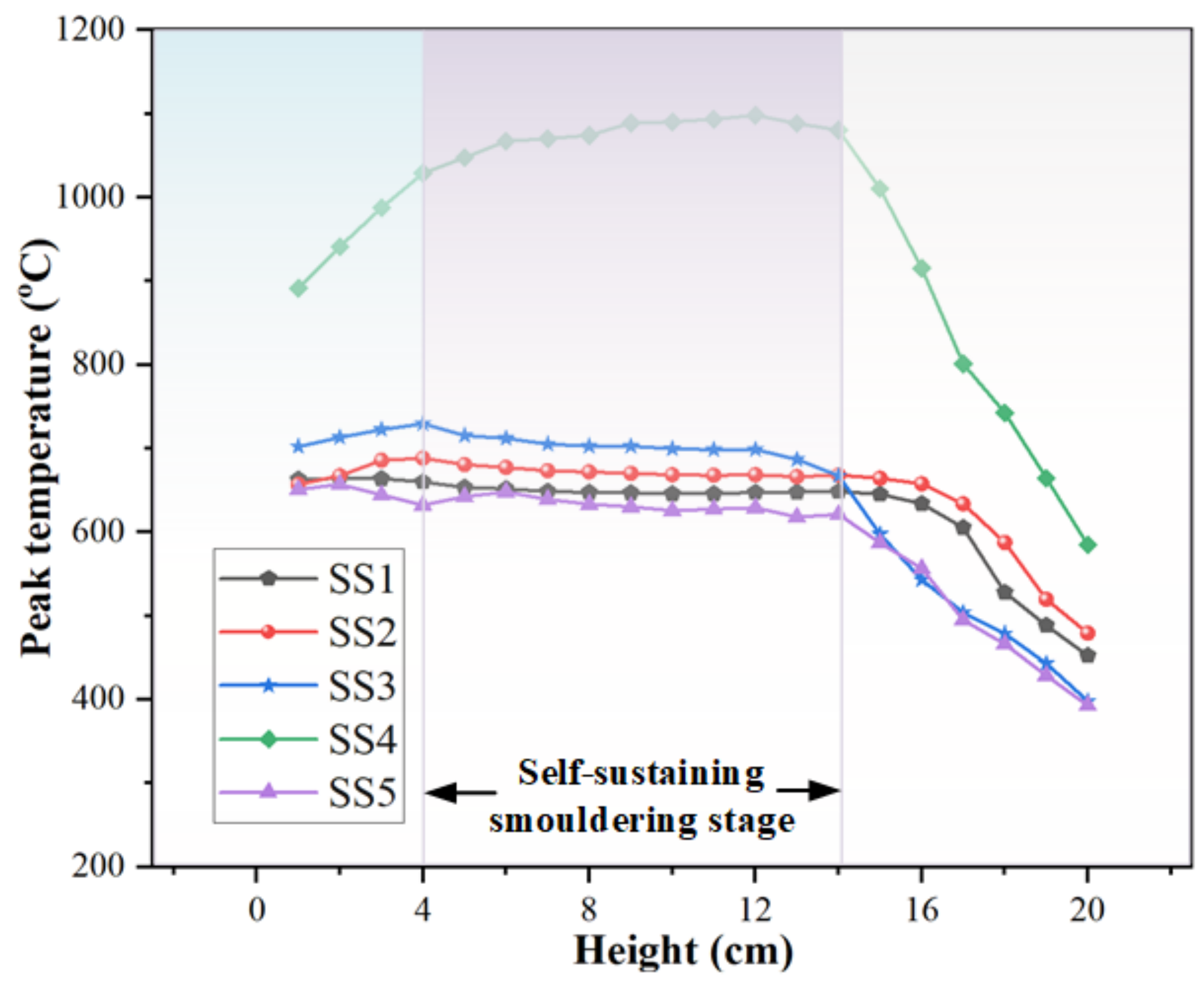
2.5. Smoldering Combustion
3. Materials and Methods
3.1. Materials
3.2. Kinetics Theory
3.3. Apparatus and Methods
3.3.1. Proximate, Ultimate and Heating Value Analyses
3.3.2. Inorganic Element Analysis
3.3.3. Thermogravimetric Analysis
3.3.4. TG–FTIR–MS Analysis
3.3.5. Smoldering Combustion Setup
4. Conclusions and Outlook
- (1)
- The basic fuel properties and inorganic element composition of various sludges are quite different, especially between industrial sludges. The pyrolysis process of the SS can be divided into two stages, which are basically determined by the organic and inorganic compositions of the fuel.
- (2)
- The iso-conversional methods (FWO, KAS and Starink) are appropriate for evaluating the apparent activation energy of the complex mixtures of sludge, as indicated by the dependence of the apparent activation energy on the conversions. The apparent activation energy of municipal sludges shows a tendency of increasing and then decreasing with the conversion rate, ranging from 53 to 182 kJ/mol overall, whereas the industrial sludges do not exhibit a uniform trend. It can be considered that the organic components include proteins, lipids, polysaccharides and humic acid, while the inorganic components mainly play a catalytic role in the process of decomposition.
- (3)
- Employing a multi-device coupled online system (TG–FTIR–MS) for real-time qualitative and semi-quantitative analysis of pyrolysis products can be useful and necessary to provide more accurate and detailed information for revealing the genuine pyrolysis process. It reveals that the main gaseous products released during the heating process are CO2, H2O, CH4 and CO, which are produced by the pyrolysis of organic macromolecules and inorganic substances.
- (4)
- In the smoldering experiment, it is found that the smoldering front and the drying front propagated at stable but different speeds. Furthermore, the propagation velocity of the char oxidation front was significantly affected by the carbon density and inorganic element catalysis of sewage sludge, in which the carbon density mainly determines the heat release in the oxidation process and the inorganic elements play a significant catalytic role at different temperatures.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.; Zhou, Z.; Luo, J.; Yuan, H.; Zhu, N.; Lou, Z. Quantifying the thermochemical pathways of soluble organics in sewage sludge flocs during pyrolysis for precursor optimization and by-product control. Chem. Eng. J. 2022, 444, 136627. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Wang, L.; Skjevrak, G.; Hustad, J.E.; Grønli, M.G. Effects of sewage sludge and marble sludge addition on slag characteristics during wood waste pellets combustion. Energy Fuels 2011, 25, 5775–5785. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 2009, 88, 1344–1350. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, J.; He, Y.; Evrendilek, F.; Buyukada, M.; Xie, W.; Sun, S. Co-pyrolytic mechanisms, kinetics, emissions and products of biomass and sewage sludge in N2, CO2 and mixed atmospheres. Chem. Eng. J. 2020, 397, 125372. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, Y.; Lu, Q.; Gao, M.; Ouyang, Z. Experimental investigation of gasification and incineration characteristics of dried sewage sludge in a circulating fluidized bed. Fuel 2015, 150, 441–447. [Google Scholar] [CrossRef]
- Qianshi, S.; Xiaohan, W.; Haowen, L.; Zixin, Y.; Yue, Y.; Jiepeng, H. Comprehensive study on the influence of preparation conditions on the physicochemical structure of char and the adaptability of the char reactivity model. Chem. Eng. J. 2022, 431, 133903. [Google Scholar] [CrossRef]
- Edifor, S.Y.; Nguyen, Q.D.; van Eyk, P.; Biller, P.; Lewis, D.M. Rheological studies of municipal sewage sludge slurries for hydrothermal liquefaction biorefinery applications. Chem. Eng. Res. Des. 2021, 166, 148–157. [Google Scholar] [CrossRef]
- Yermán, L.; Wall, H.; Torero, J.; Gerhard, J.I.; Cheng, Y.L. Smoldering combustion as a treatment technology for feces: Sensitivity to key parameters. Combust. Sci. Technol. 2016, 188, 968–981. [Google Scholar] [CrossRef]
- Torero, J.L.; Gerhard, J.I.; Martins, M.F.; Zanoni, M.A.; Rashwan, T.L.; Brown, J.K. Processes defining smouldering combustion: Integrated review and synthesis. Prog. Energy Combust. Sci. 2020, 81, 100869. [Google Scholar] [CrossRef]
- Pironi, P.; Switzer, C.; Rein, G.; Fuentes, A.; Gerhard, J.I.; Torero, J.L. Small-scale forward smouldering experiments for remediation of coal tar in inert media. Proc. Combust. Inst. 2009, 32, 1957–1964. [Google Scholar] [CrossRef]
- Gascó, G.; Blanco, C.G.; Guerrero, F.; Lázaro, A.M. The influence of organic matter on sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2005, 74, 413–420. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Liu, L.; Kumar, S.; Wang, Z.; He, Y.; Liu, J.; Cen, K. Catalytic effect of metal chlorides on coal pyrolysis and gasification part I. Combined TG-FTIR study for coal pyrolysis. Thermochim. Acta 2017, 655, 331–336. [Google Scholar] [CrossRef]
- Shao, J.; Yan, R.; Chen, H.; Yang, H.; Lee, D.H. Catalytic effect of metal oxides on pyrolysis of sewage sludge. Fuel Process. Technol. 2010, 91, 1113–1118. [Google Scholar] [CrossRef]
- Sert, M.; Ballice, L.; Yüksel, M.; Sağlam, M. Effect of demineralization on product yield and composition at isothermal pyrolysis of Eynez lignites. Ind. Eng. Chem. Res. 2011, 50, 10400–10406. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.; Su, S.; Xu, K.; He, L.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Kai, X.; Yang, T.; Huang, Y.; Sun, Y.; He, Y.; Li, R. The effect of biomass components on the co-combustion characteristics of biomass with coal. In Proceedings of the 2011 Second International Conference on Digital Manufacturing & Automation, Zhangjiajie, China, 5–7 August 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1274–1278. [Google Scholar]
- Shen, B. Study on MSW catalytic combustion by TGA. Energy Convers. Manag. 2006, 47, 1429–1437. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, X.; Liu, A. Kinetic studies on catalytic combustion of rice and wheat straw under air- and oxygen-enriched atmospheres, by using thermogravimetric analysis. Biomass Bioenergy 2008, 32, 1046–1055. [Google Scholar]
- Eom, I.Y.; Kim, J.Y.; Kim, T.S.; Lee, S.M.; Choi, D.; Choi, I.G.; Choi, J.W. Effect of essential inorganic metals on primary thermal degradation of lignocellulosic biomass. Bioresour. Technol. 2012, 104, 687–694. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Zuo, W.; Chen, L.; Cui, Y.; Tan, T. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge. Environ. Sci. Technol. 2013, 47, 3498–3505. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Zhang, Z. Effect of inherent minerals on sewage sludge pyrolysis: Product characteristics, kinetics and thermodynamics. Waste Manag. 2018, 80, 175–185. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Serret, M.D.; Araus, J.L. The nitrogen contribution of different plant parts to wheat grains: Exploring genotype, water, and nitrogen effects. Front. Plant Sci. 2017, 7, 1986. [Google Scholar] [CrossRef]
- Miller, S.F.; Miller B, G. The occurrence of inorganic elements in various biofuels and its effect on ash chemistry and behavior and use in combustion products. Fuel Process. Technol. 2007, 88, 1155–1164. [Google Scholar] [CrossRef]
- Song, Q.; Wang, X.; Gu, C.; Wang, N.; Li, H.; Su, H.; Huo, J.; Qiao, Y. A comprehensive model of biomass char-CO2 gasification reactivity with inorganic element catalysis in the kinetic control zone based on TGA analysis. Chem. Eng. J. 2020, 398, 125624. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass. Fuel Process. Technol. 2012, 104, 189–197. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Jiaqiang, E.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Taylor S, H. Designing oxidation catalysts. Catal. Today 1999, 49, 105–113. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Li, C.Z.; Sathe, C.; Kershaw, J.R.; Pang, Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel 2000, 79, 427–438. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; He, Y.; Yuan, Y.; Liu, L.; Zhu, Y.; Cen, K. Catalytic effect of metal chloride additives on the volatile gas release characteristics for high-temperature lignite pyrolysis. Energy Fuels 2019, 33, 9437–9445. [Google Scholar] [CrossRef]
- Han, Y.F.; Chen, F.; Zhong, Z.; Ramesh, K.; Chen, L.; Widjaja, E. Controlled synthesis, characterization, and catalytic properties of Mn2O3 and Mn3O4 nanoparticles supported on mesoporous silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef] [PubMed]
- Najjar, H.; Batis, H. Development of Mn-based perovskite materials: Chemical structure and applications. Catal. Rev. 2016, 58, 371–438. [Google Scholar] [CrossRef]
- Karayildirim, T.; Yanik, J.; Yuksel, M.; Bockhorn, H. Characterisation of products from pyrolysis of waste sludges. Fuel 2006, 85, 1498–1508. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.; Ma, P.; Zhang, X.; Wu, Y.; Huang, L. Effect of alkali and alkaline earth metals on co-pyrolysis characteristics of municipal solid waste and biomass briquettes. J. Therm. Anal. Calorim. 2020, 139, 489–498. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, K.; Kim, S.S.; Brown, R.C. Kinetic understanding of the effect of Na and Mg on pyrolytic behavior of lignin using a distributed activation energy model and density functional theory modeling. Green Chem. 2019, 21, 1099–1107. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Kuo, J.; Buyukada, M.; Evrendilek, F. Thermal characteristics, kinetics, gas emissions and thermodynamic simulations of (co-) combustions of textile dyeing sludge and waste tea. J. Clean. Prod. 2019, 239, 118113. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H. A comprehensive study on pyrolysis kinetics of microalgal biomass. Energy Convers. Manag. 2017, 131, 109–116. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y.; Peng, X.; Ma, X. Co-pyrolysis kinetics of sewage sludge and oil shale thermal decomposition using TGA–FTIR analysis. Energy Convers. Manag. 2016, 118, 345–352. [Google Scholar] [CrossRef]
- Wang, T.; Fu, T.; Chen, K.; Cheng, R.; Chen, S.; Liu, J.; Mei, M.; Li, J.; Xue, Y. Co-combustion behavior of dyeing sludge and rice husk by using TG-MS: Thermal conversion, gas evolution, and kinetic analyses. Bioresour. Technol. 2020, 311, 123527. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.M.; Siddiqui, M.Z.; Park, Y.K. Different pyrolysis kinetics and product distribution of municipal and livestock manure sewage sludge. Environ. Pollut. 2021, 285, 117197. [Google Scholar] [CrossRef]
- Bi, P.; Yuan, Y.; Fan, M.; Jiang, P.; Zhai, Q.; Li, Q. Production of aromatics through current-enhanced catalytic conversion of bio-oil tar. Bioresour. Technol. 2013, 136, 222–229. [Google Scholar] [CrossRef]
- Peng, X.; Ma, X.; Lin, Y.; Guo, Z.; Hu, S.; Ning, X.; Cao, Y.; Zhang, Y. Co-pyrolysis between microalgae and textile dyeing sludge by TG–FTIR: Kinetics and products. Energy Convers. Manag. 2015, 100, 391–402. [Google Scholar] [CrossRef]
- Schult, D.A.; Matkowsky, B.J.; Volpert, V.A.; Fernandez-Pello, A.C. Forced forward smolder combustion. Combust. Flame 1996, 104, 1–26. [Google Scholar] [CrossRef]
- Torero, J.L.; Fernandez-Pello, A.C. Forward smolder of polyurethane foam in a forced air flow. Combust. Flame 1996, 106, 89–109. [Google Scholar] [CrossRef]
- Vantelon, J.P.; Lodeho, B.; Pignoux, S.; Ellzey, J.L.; Torero, J.L. Experimental observations on the thermal degradation of a porous bed of tires. Proc. Combust. Inst. 2005, 30, 2239–2246. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Lu, Z. Structure and extinction of reverse smolder waves in the presence of heat losses: A premixed-flame perspective. Combust. Flame 2022, 242, 112201. [Google Scholar] [CrossRef]
- Wyn, H.K.; Konarova, M.; Beltramini, J.; Perkins, G.; Yermán, L. Self-sustaining smouldering combustion of waste: A review on applications, key parameters and potential resource recovery. Fuel Process. Technol. 2020, 205, 106425. [Google Scholar] [CrossRef]
- Mishra, G.; Bhaskar, T. Non-isothermal model free kinetics for pyrolysis of rice straw. Bioresour. Technol. 2014, 169, 614–621. [Google Scholar] [CrossRef]
- Starink, M.J. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of iso-conversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]




| Proximate Analysis | Ultimate Analysis | HHV e MJ/kg | LHV f MJ/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V a % | A b % | FC c % | C % | H % | N % | S % | O d % | |||
| SS1 | 37.0 | 57.7 | 5.4 | 20.4 | 2.9 | 3.6 | 0.6 | 72.5 | 9.4 | 9.2 |
| SS2 | 43.0 | 49.9 | 7.1 | 23.9 | 3.6 | 4.0 | 0.8 | 67.7 | 10.3 | 9.6 |
| SS3 | 42.2 | 50.1 | 7.7 | 23.9 | 3.6 | 4.1 | 1.0 | 67.4 | 10.2 | 9.5 |
| SS4 | 30.1 | 55.0 | 14.9 | 35.5 | 3.5 | 0.2 | 0.3 | 60.6 | 17.4 | 16.7 |
| SS5 | 38.7 | 60.6 | 0.6 | 15.9 | 3.0 | 2.4 | 4.9 | 73.8 | 6.8 | 6.1 |
| Sample | °C | °C | %/°C | % |
|---|---|---|---|---|
| SS1 | 207.88 | 272.04 | 1.11 | 65.01 |
| SS2 | 223.68 | 279.25 | 1.39 | 58.43 |
| SS3 | 224.02 | 275.92 | 1.44 | 53.75 |
| SS4 | 319.02 | 545.64 | 1.10 | 65.47 |
| SS5 | 160.01 | 355.83 | 0.78 | 48.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, X.; Song, Q.; Chen, Q.; Li, H.; Yang, Z.; Wang, X. Insight into the Effects of Inorganic Element Catalysis and Basic Fuel Properties on the Self-Sustained Smoldering Process of Sewage Sludge. Catalysts 2022, 12, 1173. https://doi.org/10.3390/catal12101173
Zhang W, Wang X, Song Q, Chen Q, Li H, Yang Z, Wang X. Insight into the Effects of Inorganic Element Catalysis and Basic Fuel Properties on the Self-Sustained Smoldering Process of Sewage Sludge. Catalysts. 2022; 12(10):1173. https://doi.org/10.3390/catal12101173
Chicago/Turabian StyleZhang, Wei, Xiaowei Wang, Qianshi Song, Qianyi Chen, Haowen Li, Zixin Yang, and Xiaohan Wang. 2022. "Insight into the Effects of Inorganic Element Catalysis and Basic Fuel Properties on the Self-Sustained Smoldering Process of Sewage Sludge" Catalysts 12, no. 10: 1173. https://doi.org/10.3390/catal12101173
APA StyleZhang, W., Wang, X., Song, Q., Chen, Q., Li, H., Yang, Z., & Wang, X. (2022). Insight into the Effects of Inorganic Element Catalysis and Basic Fuel Properties on the Self-Sustained Smoldering Process of Sewage Sludge. Catalysts, 12(10), 1173. https://doi.org/10.3390/catal12101173







