Effects of Sn Promoter on the Ordered Mesoporous Co3O4-Al2O3 Mixed Metal Oxide for Fischer–Tropsch Synthesis Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural and Surface Properties of Sn/m-CoAlOx Catalysts
2.2. Reduction Behaviors and Surface Properties of Sn/m-CoAlOx
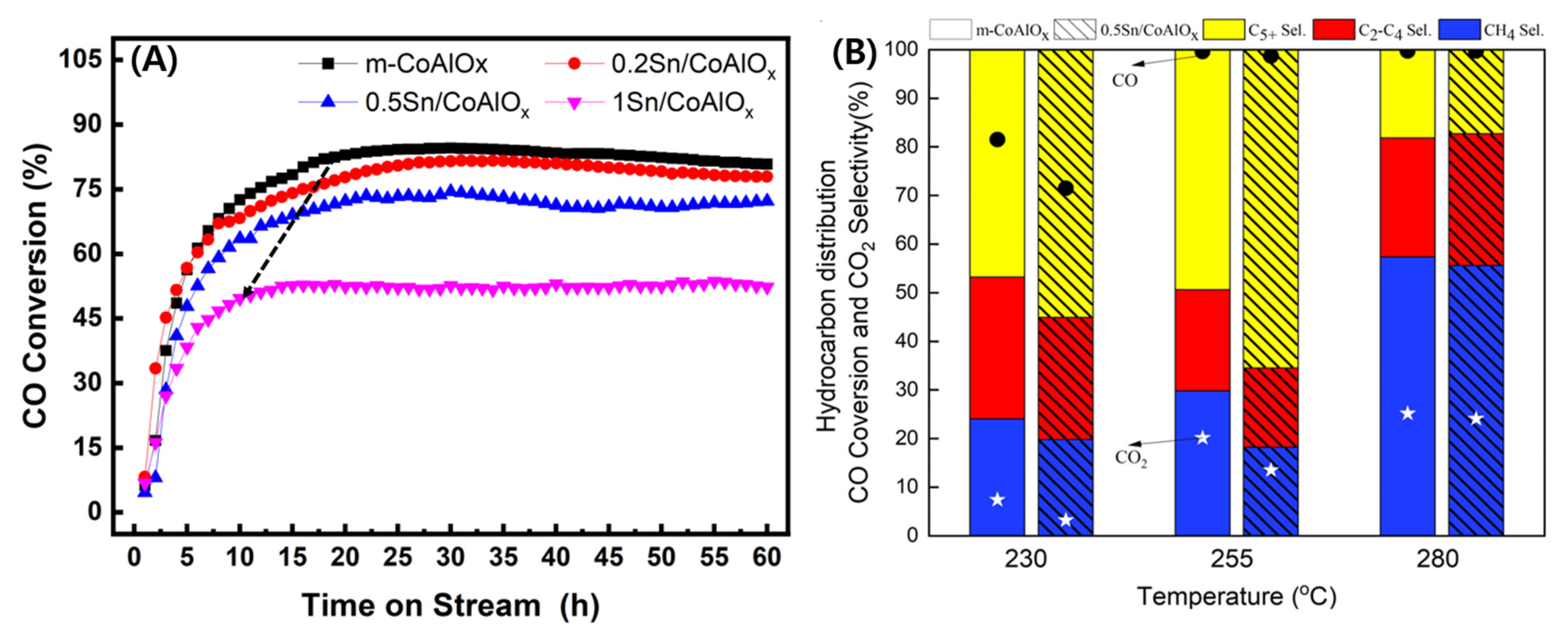
2.3. Catalytic Activity and Product Distribution on the Sn/m-CoAlOx Catalysts
3. Experimental Section
3.1. Catalyst Preparation and Activity Measurement
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khodakov, A.Y.; Wei, C.; Fongarland, P. Advances in the development of novel cobalt Fischer−Tropsch catalysts for synthesis of long-chainh and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.G.D.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Zhang, S.; Su, J.; Somorjai, G.A. A mini review of cobalt-based nanocatalyst in Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2020, 602, 117701–117710. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; Dillen, A.J.V.; Jong, K.P.D. Cobalt particle size effects in the Fischer−Tropsch reaction studied with carbon nanofiber Supported Catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Liu, B.; Cao, K.; Yang, P.; Zhao, Q.; Jiang, F.; Xu, Y.; Chen, R.; Liu, X. Particle-size-dependent methane selectivity evolution in Cobalt-based Fischer–Tropsch synthesis. ACS Catal. 2020, 10, 2799–2816. [Google Scholar] [CrossRef]
- Iglesia, E.; Soled, S.L.; Fiato, R. Fischer-Tropsch synthesis on cobalt and ruthenium. Metal dispersion and support effects on reaction rate and selectivity. J. Catal. 1992, 37, 212–224. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Deactivation and regeneration of commercial type Fischer-Tropsch Co-catalysts-A mini-review. Catalysts 2015, 5, 478–499. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.W.; Park, S.J.; Woo, M.H.; Cheon, J.Y.; Ha, K.S.; Jun, K.W.; Lee, D.H.; Jung, H.M. Enhanced catalytic performance by Zirconium Phosphate-modified SiO2-supported Ru-Co catalyst for Fischer-Tropsch synthesis. ChemCatChem 2011, 3, 1342–1347. [Google Scholar] [CrossRef]
- Kwack, S.H.; Bae, J.W.; Park, M.J.; Kim, S.M.; Ha, K.S.; Jun, K.W. Reaction modeling on the phosphorous-treated Ru/Co/Zr/SiO2 Fischer–Tropsch catalyst with the estimation of kinetic parameters and hydrocarbon distribution. Fuel 2011, 90, 1383–1394. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Orvat, J.; Wang, B.; Qiao, S.; Park, J.; Ahn, H. Highly ordered mesoporous cobalt oxide nanostructures: Synthesis, characterisation, magnetic properties, and applications for electrochemical energy devices. Chem. Eur. J. 2010, 16, 11020–11027. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, G.; Tang, Z.; Zhang, H. Preparation and application of ordered mesoporous metal oxide catalytic materials. Catal. Sur. Asia 2020, 24, 38–58. [Google Scholar] [CrossRef]
- Saad, A.; Cheng, Z.; Shen, H.; Thomas, T.; Yang, M. Recent advances in nanocasting cobalt-based mesoporous materials for energy storage and conversion. Electrocatalysis 2021, 11, 465–484. [Google Scholar] [CrossRef]
- Gupta, S.; Ciotonea, C.; Royer, S.; Acquin, J.; Vinod, C. Engineering pore morphology using silica template route over mesoporous cobalt oxide and its implications in atmospheric pressure carbon dioxide hydrogenation to olefins. Appl. Mater. Today 2020, 19, 100586. [Google Scholar] [CrossRef]
- Chinh, N.; Lee, J.; Seo, J.; Yang, E.; Lee, J.; Choi, K.; Lee, H.; Kim, J.; Lee, M.; Joo, S. Structure-dependent catalytic properties of mesoporous cobalt oxides in furfural hydrogenation. Appl. Catal. A Gen. 2019, 583, 117125. [Google Scholar]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, S.; Lu, J.; Luo, M. Effect of structural properties of mesoporous Co3O4 catalysts on methane combustion. Chem. Res. Chin. Univ. 2016, 32, 808–811. [Google Scholar] [CrossRef]
- Koo, H.M.; Ahn, C.I.; Lee, D.H.; Roh, H.S.; Shin, C.H.; Kye, H.; Bae, J.W. Roles of Al2O3 promoter for an enhanced structural stability of ordered-mesoporous Co3O4 catalyst during CO hydrogenation to hydrocarbons. Fuel 2018, 225, 460–471. [Google Scholar] [CrossRef]
- Ahn, C.I.; Koo, H.M.; Jo, J.M.; Roh, H.S.; Lee, J.B.; Lee, Y.J.; Jang, E.J.; Bae, J.W. Stabilized ordered-mesoporous Co3O4 structures using Al pillar for the superior CO hydrogenation activity to hydrocarbons. Appl. Catal. B Environ. 2016, 180, 139–149. [Google Scholar] [CrossRef]
- Ahn, C.I.; Park, Y.M.; Cho, J.M.; Lee, D.H.; Chung, C.H.; Cho, B.G.; Bae, J.W. Fischer-Trospch synthesis on ordered mesoporous Cobalt-based catalysts with compact multichannel fixed-bed reactor application: A review. Catal. Surv. Asia 2016, 20, 210–230. [Google Scholar] [CrossRef]
- Cho, J.M.; Lee, S.R.; Sun, J.; Tsubaki, N.; Jang, E.J.; Bae, J.W. Highly ordered mesoporous Fe2O3-ZrO2 bimetal oxides for an enhanced CO hydrogenation activity to hydrocarbons with their structural stability. ACS Catal. 2017, 7, 5955–5964. [Google Scholar] [CrossRef]
- Park, Y.M.; Le, D.H.; Lee, Y.J.; Roh, H.S.; Chung, C.H.; Bae, J.W. Ordered mesoporous Co3O4-Al2O3 binary metal oxides for CO Hydrogenation to hydrocarbons: Synergy effects of phosphorus modifier for an enhanced catalytic activity and stability. ChemCatChem 2019, 11, 1707–1721. [Google Scholar] [CrossRef]
- Ahn, C.I.; Bae, J.W. Fischer-Tropsch synthesis on the Al2O3-modified ordered mesoporous Co3O4 with an enhanced catalytic activity and stability. Catal. Today 2016, 265, 27–35. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2020, 63, 512–595. [Google Scholar] [CrossRef]
- Vosoughi, V.; Dalai, A.K.; Abatzoglou, N.; Hu, Y. Performances of promoted cobalt catalysts supported on mesoporous alumina for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 547, 155–163. [Google Scholar] [CrossRef]
- Shimura, K.; Miyazawa, T.; Hanaoka, T.; Hirata, S. Fischer-Tropsch synthesis over alumina supported cobalt catalyst: Effect of promoter addition. Appl. Catal. A Gen. 2015, 494, 1–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Yang, W.; Gu, S.; Yang, X.; Kheradmand, A.; Zhang, X.; Luo, Y.; Huang, J.; Jiang, Y. Promotional effect of Ni−Sn interaction over Ni supported on Sn-incorporated MCM-41 catalysts for CO2 reforming of CH4. Chemnanomat 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Zaffran, J.; Yang, B. Descriptor design in the computational screening of Ni-Based Catalysts with balanced activity and stability for dry reforming of methane reaction. ACS Catal. 2020, 10, 3074–3083. [Google Scholar] [CrossRef]
- Wang, J.; Chang, X.; Chen, S.; Sun, G.; Zhou, X.; Vovk, E.; Yang, Y.; Deng, W.; Zhao, Z.; Mu, R.; et al. On the role of Sn segregation of Pt-Sn catalysts for propane dehydrogenation. ACS Catal. 2021, 11, 4401–4410. [Google Scholar] [CrossRef]
- Hengne, A.M.; Samal, A.K.; Enakonda, L.D.; Harb, M.; Gevers, L.E.; Anjum, D.H.; Hedhili, M.N.; Saih, Y.; Huang, K.W.; Basset, J.M. Ni-Sn-supported ZrO2 catalysts modified by Indium for selective CO2 hydrogenation to methanol. ACS Omega 2018, 3, 3688–3701. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Subramanian, V.; Sushkevich, V.V.; Ordomsky, V.V.; Khodakov, A.Y. Effect of Sn additives on the CuZnAl–HZSM-5 hybrid catalysts for the direct DME synthesis from syngas. Appl. Catal. A Gen. 2015, 502, 370–379. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Z.; Sun, K.; Huang, W. Effects of Sn on the catalytic performance for one step syngas to DME in slurry reactor. New J. Chem. 2021, 45, 3783–3789. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramırez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Pisduangdaw, S.; Panpranot, J.; Methastidsook, C.; Chaisuk, C.; Faungnawakij, K.; Praserthdam, P.; Mekasuwandumrong, O. Characteristics and catalytic properties of Pt-Sn/Al2O3 nanoparticles synthesized by one-step flame spray pyrolysis in the dehydrogenation of propane. Appl. Catal. A Gen. 2009, 370, 1–6. [Google Scholar] [CrossRef]
- Rimaz, S.; Luwei, C.; Kawi, S.; Borgna, A. Promoting effect of Ge on Pt-based catalysts for dehydrogenation of propane to propylene. Appl. Catal. A Gen. 2019, 588, 117266. [Google Scholar] [CrossRef]
- Bratan, V.; Munteanu, C.; Hornoiu, C.; Vasile, A.; Papa, F.; State, R.; Preda, S.; Culita, D.; Ionescu, N.I. CO oxidation over Pd supported catalysts—In situ study of the electric and catalytic properties. Appl. Catal. B Environ. 2017, 2070, 166–173. [Google Scholar] [CrossRef]
- Sasikala, R.; Gupta, N.M.; Kulshreshtha, S.K. Temperature-programmed reduction and CO oxidation studies over Ce–Sn mixed oxides. Catal. Lett. 2001, 71, 69–73. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Wang, J.; Yan, J.; Fan, H.; Liu, Y.; Dai, H. Three-dimensional mesoporous manganese oxides and cobalt oxides: High-efficiency catalysts for the removal of toluene and carbon monoxide. Microporous Mesoporous Mater. 2012, 162, 199–206. [Google Scholar] [CrossRef]
- González-Prior, J.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; de Rivas, B. Catalytic removal of chlorinated compounds over ordered mesoporous cobalt oxides synthesised by hard-templating. Appl. Catal. B Environ. 2018, 222, 9–17. [Google Scholar] [CrossRef]


| Notation a | XRF b | N2-Sorption c | XRD d | XPS e | H2-TPR f | CO-Chem. g | O2 Titration g | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SnO2 (wt%) | Sg (m2/g) | PD (nm) | PV (cm3/g) | Crystallite Size of Co3O4/Co (nm) | ICo/ICon+ (Fresh/Used) | Uptake (mmol/g) | RD (%) | SCo (m2/gCo) | RDO2 (%) | ||
| LT | HT | ||||||||||
| m-CoAlOx | 0 | 109.7 | 3.8 | 0.14 | 17.2/12.9 | 0.99/2.51 | 2.64 | 8.67 | 70.5 | 6.6 | 76.1 |
| Sn(2)/m-CoAlOx | 0.25 | 102.7 | 4.0 | 0.14 | 15.7/11.8 | 0.92/2.54 | 2.24 | 7.46 | 66.3 | 6.3 | 72.3 |
| Sn(7)/m-CoAlOx | 0.65 | 105.4 | 4.0 | 0.14 | 14.7/11.0 | 0.92/2.78 | 2.42 | 6.83 | 62.4 | 5.5 | 64.2 |
| Sn(18)/m-CoAlOx | 1.82 | 92.2 | 4.1 | 0.11 | 14.3/10.7 | 0.98/3.03 | 1.52 | 3.82 | 42.2 | 4.0 | 50.2 |
| Catalyst | CO Conv. (C-mol%) | CO Conv. to CO2 (C-mol%) | Product Distribution (C-mol%) | Olefins in C2–C4 (%) | C5+ Yield (%) | α b | ||
|---|---|---|---|---|---|---|---|---|
| C1 | C2–C4 | C5+ | ||||||
| m-CoAlOx | 81.5 | 7.4 | 24.0 | 29.2 | 46.8 | 37.1 | 35.3 | 0.786 |
| Sn(2)/m-CoAlOx | 79.1 | 7.0 | 23.3 | 27.7 | 49.0 | 32.3 | 36.0 | 0.797 |
| Sn(7)/m-CoAlOx | 71.5 | 3.2 | 19.8 | 25.1 | 55.1 | 24.0 | 38.1 | 0.795 |
| Sn(18)/m-CoAlOx | 52.7 | 1.5 | 16.4 | 20.8 | 62.8 | 37.6 | 32.6 | 0.799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Han, S.B.; Wang, X.; Ali, M.; Bae, J.W. Effects of Sn Promoter on the Ordered Mesoporous Co3O4-Al2O3 Mixed Metal Oxide for Fischer–Tropsch Synthesis Reaction. Catalysts 2022, 12, 1447. https://doi.org/10.3390/catal12111447
Shen D, Han SB, Wang X, Ali M, Bae JW. Effects of Sn Promoter on the Ordered Mesoporous Co3O4-Al2O3 Mixed Metal Oxide for Fischer–Tropsch Synthesis Reaction. Catalysts. 2022; 12(11):1447. https://doi.org/10.3390/catal12111447
Chicago/Turabian StyleShen, Dongming, Sang Beom Han, Xu Wang, Mansoor Ali, and Jong Wook Bae. 2022. "Effects of Sn Promoter on the Ordered Mesoporous Co3O4-Al2O3 Mixed Metal Oxide for Fischer–Tropsch Synthesis Reaction" Catalysts 12, no. 11: 1447. https://doi.org/10.3390/catal12111447