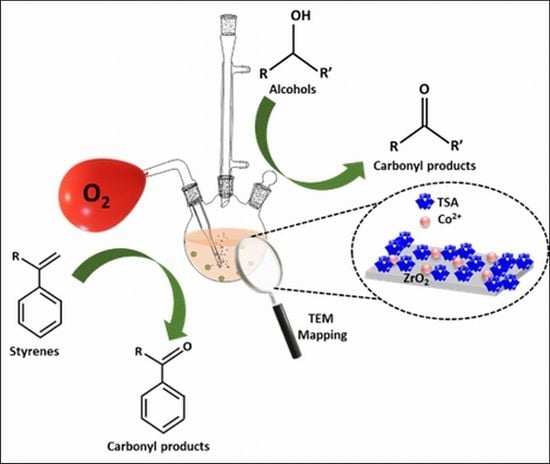
Selective Oxidation of Alcohols and Alkenes with Molecular Oxygen Catalyzed by Highly Dispersed Cobalt (II) Decorated 12-Tungstosilicic Acid-Modified Zirconia
Abstract
:1. Introduction
2. Results and Discussion
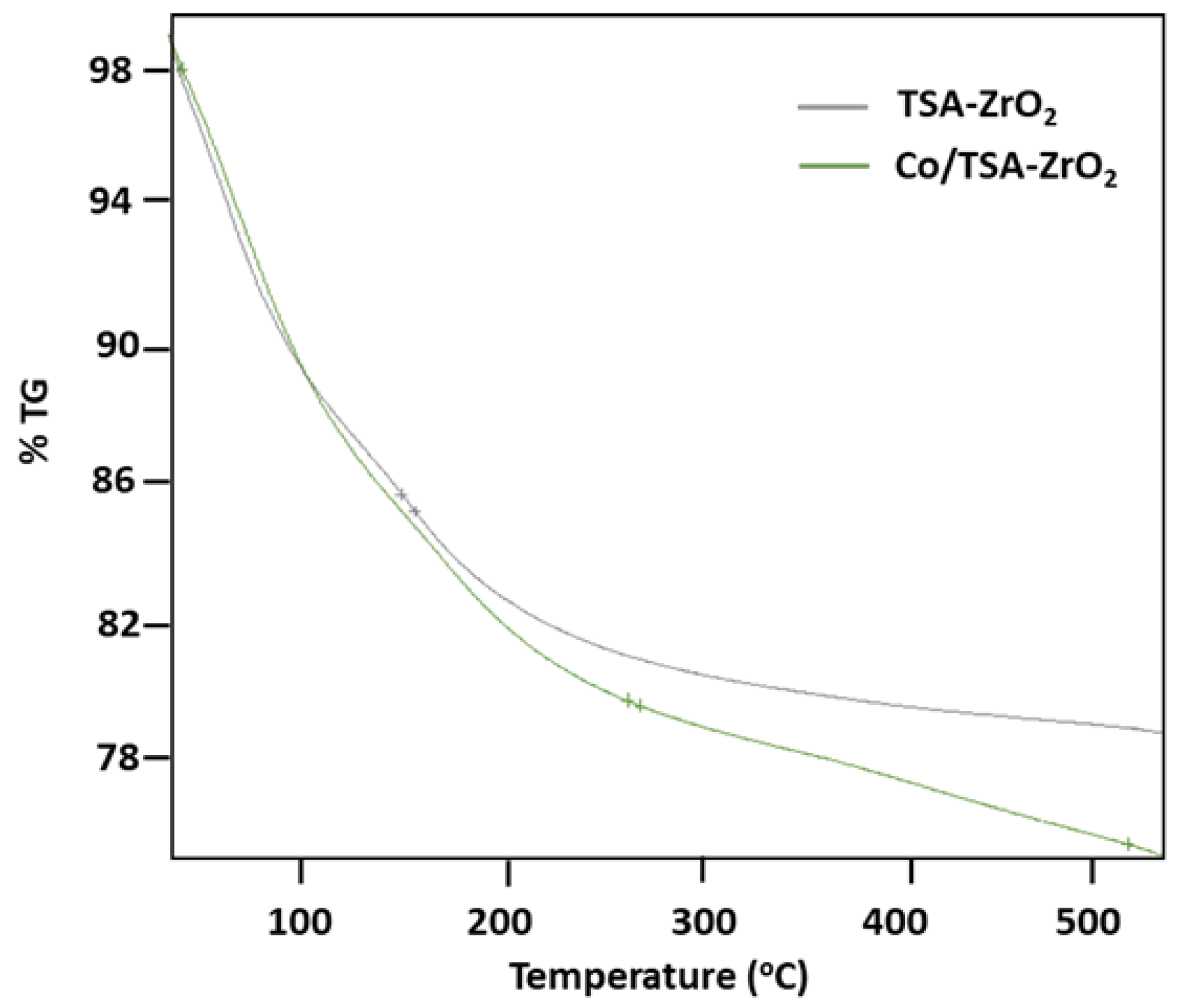
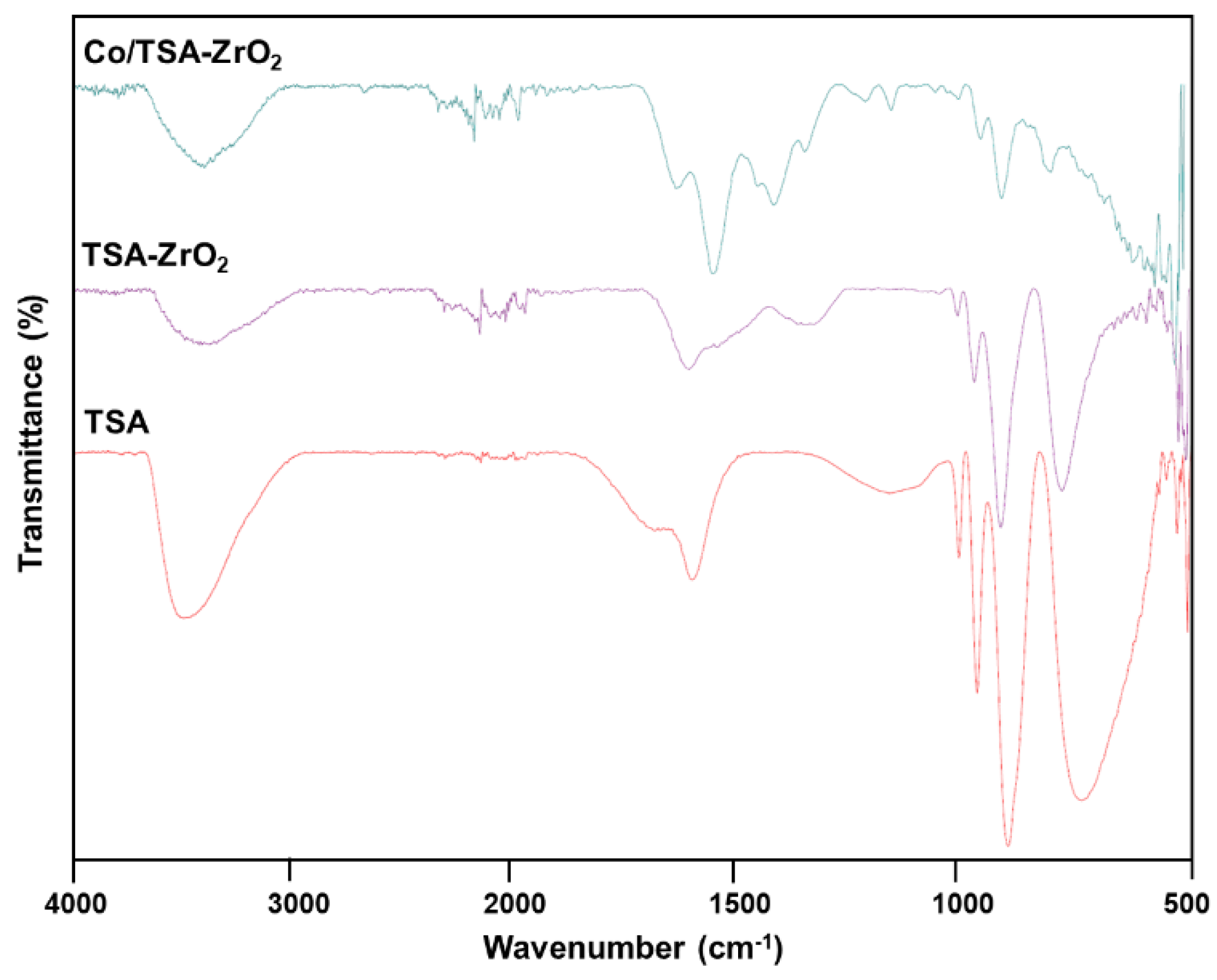
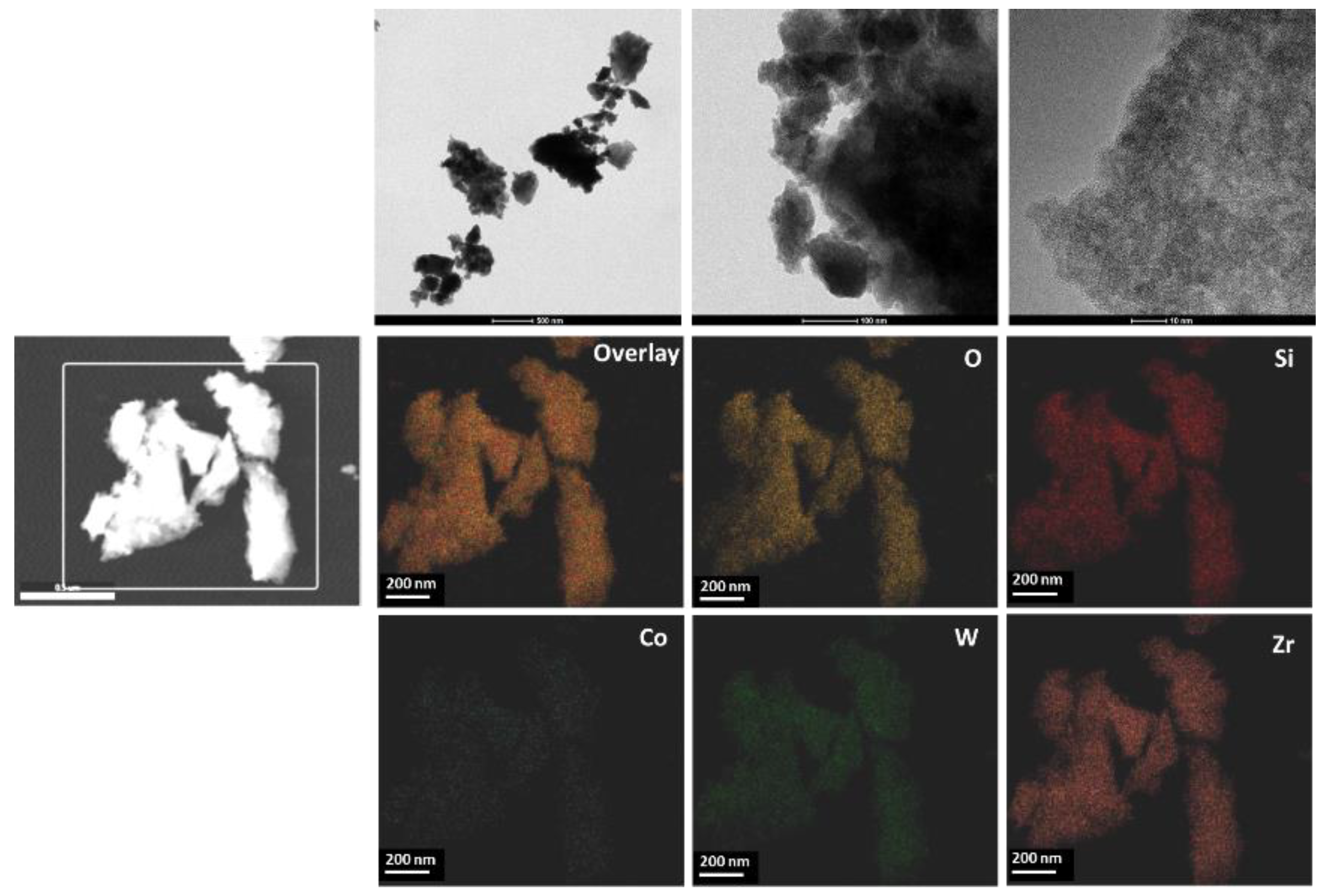
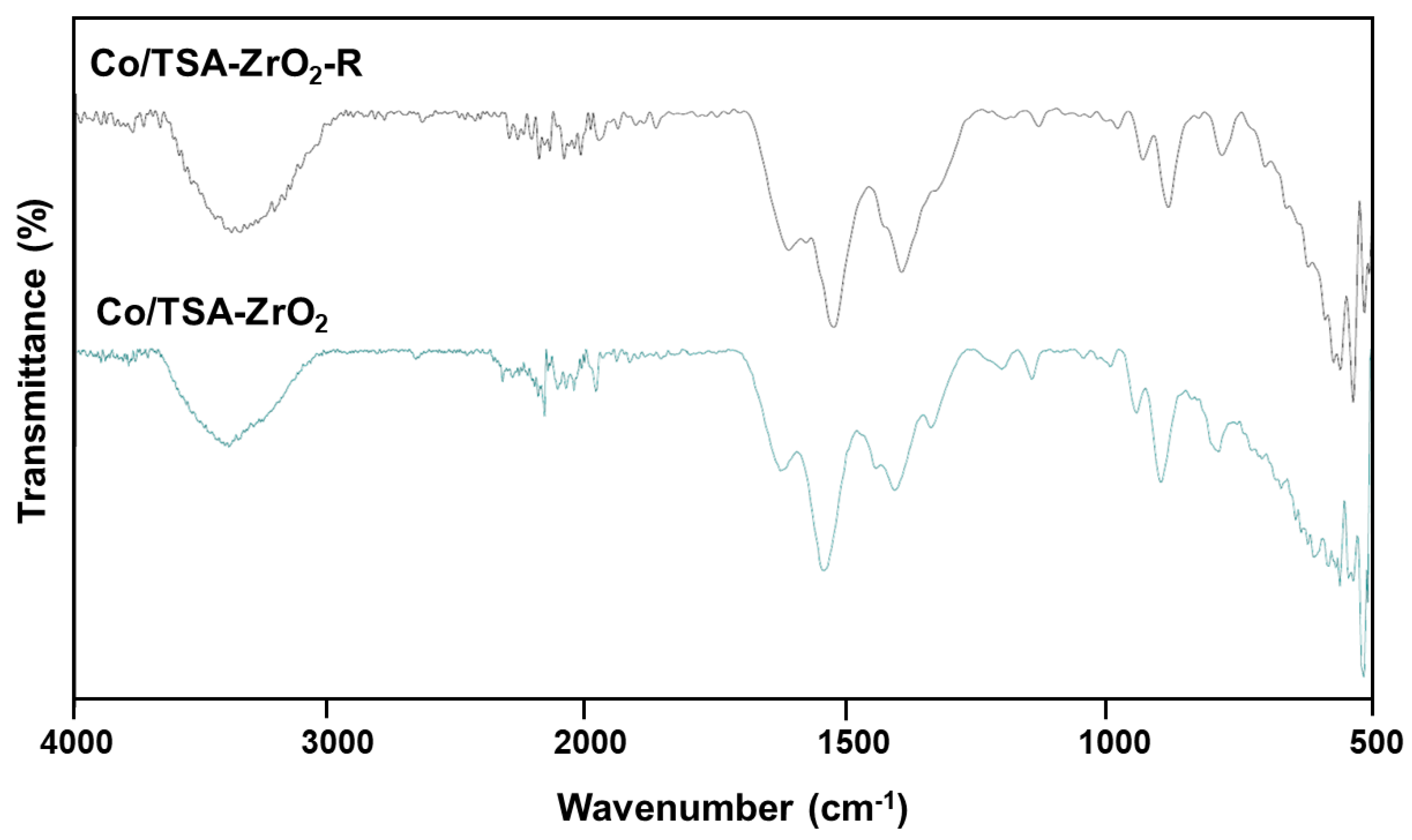
2.1. Characterization
2.2. Catalytic Activity
2.2.1. Oxidation of Alcohol
Oxidation of Benzyl Alcohol
- Effect of temperature
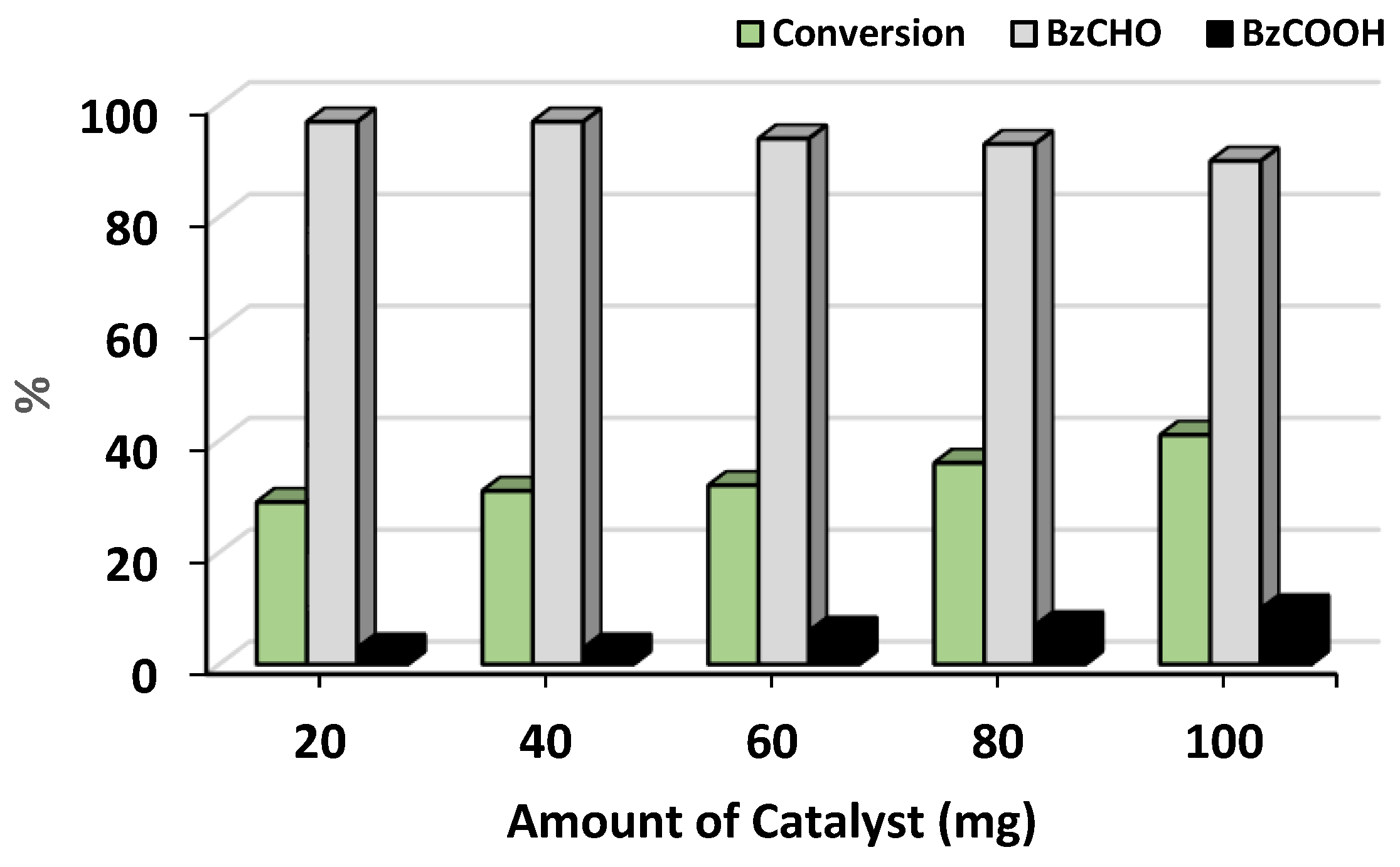
- Effect of catalyst amount
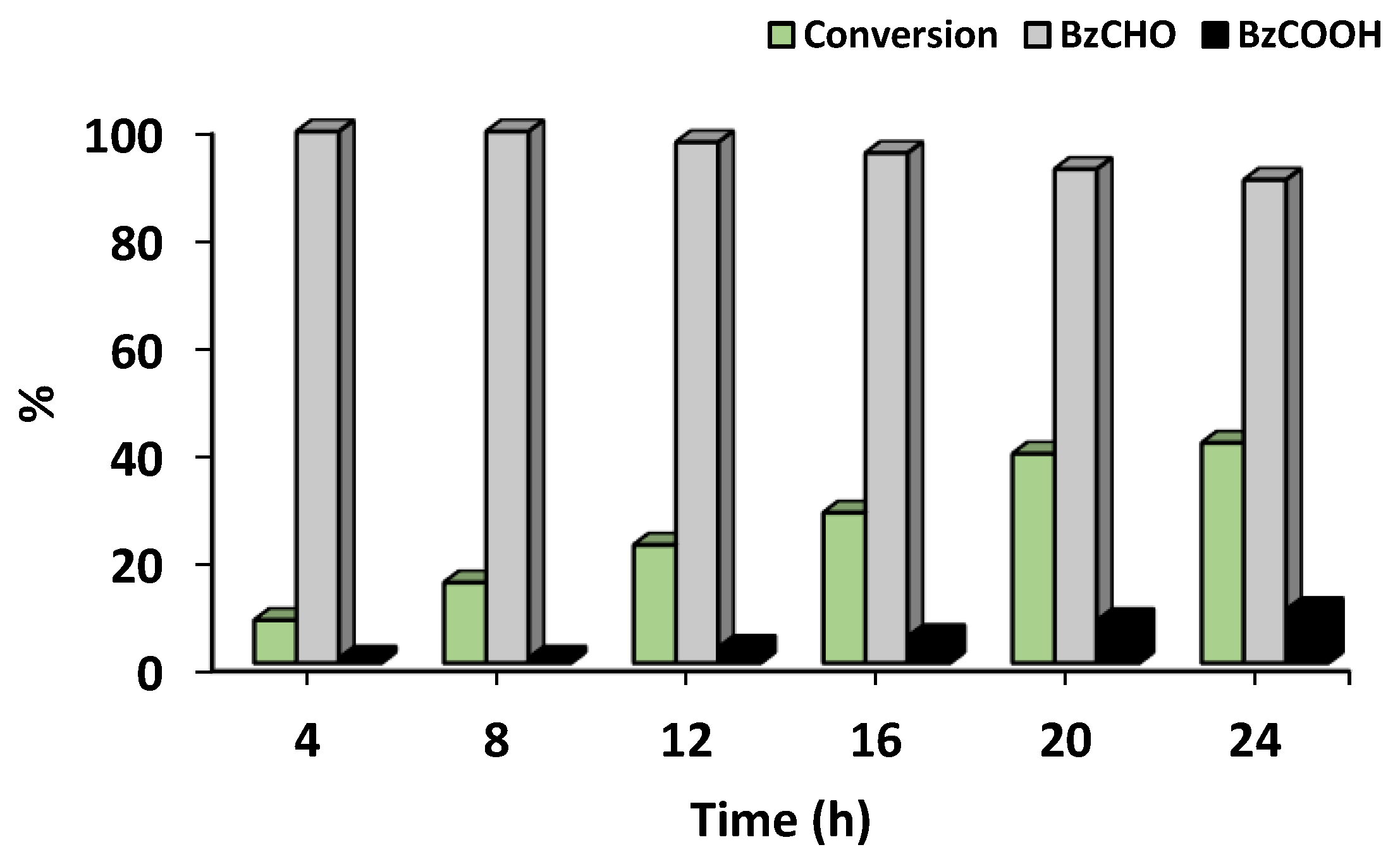
- Effect of reaction time
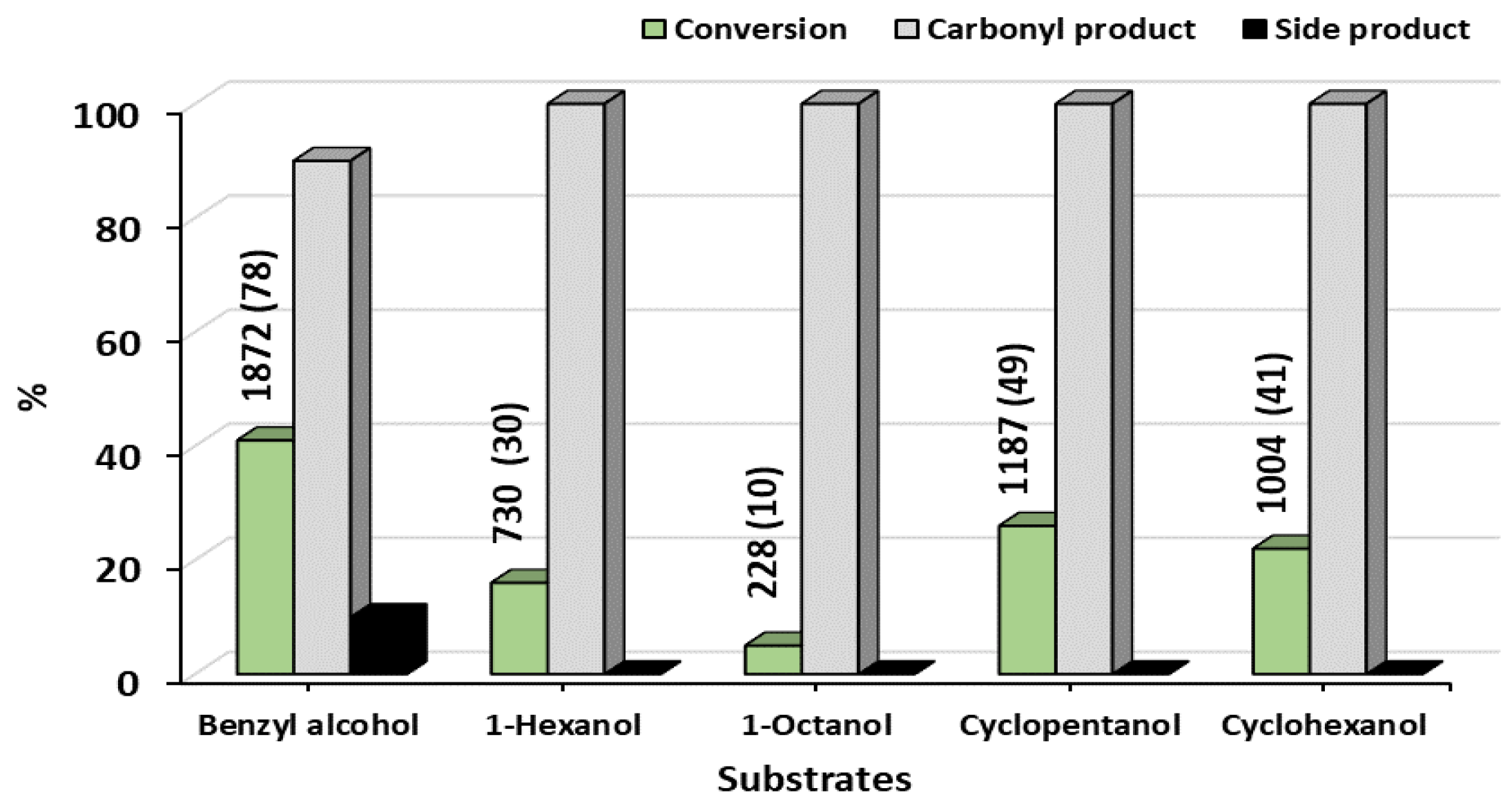
Scope and Limitations for Oxidation of Various Alcohols
2.2.2. Oxidation of Alkenes
2.2.3. Control Experiment
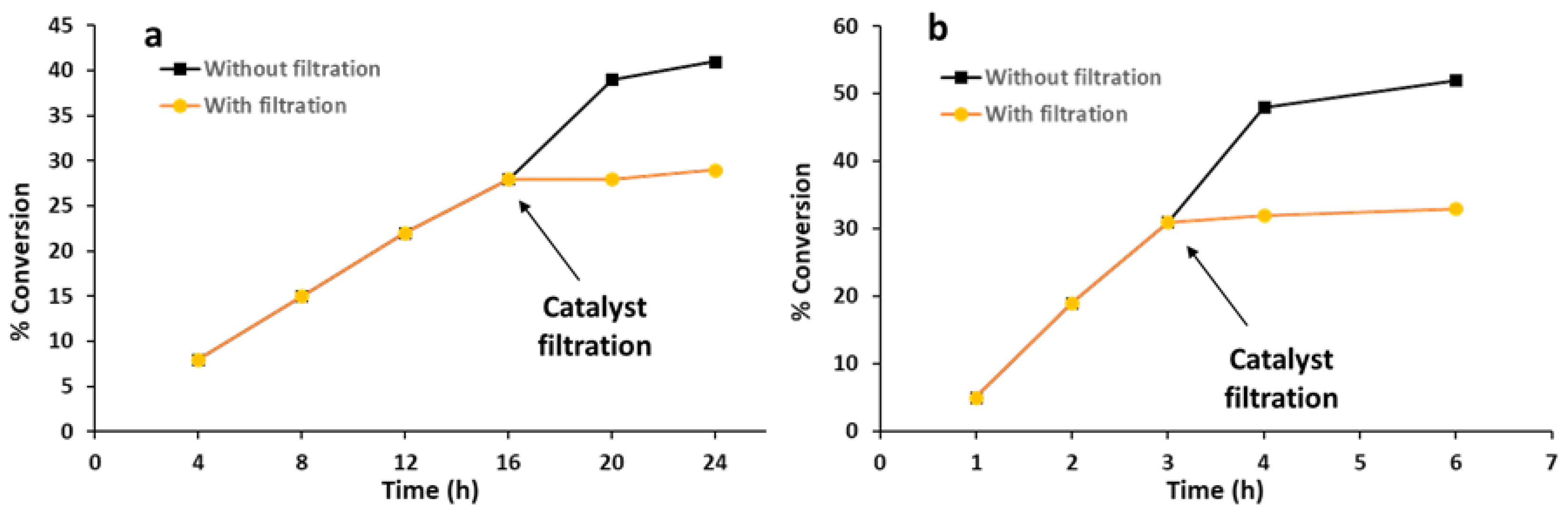
2.3. Heterogeneity Test
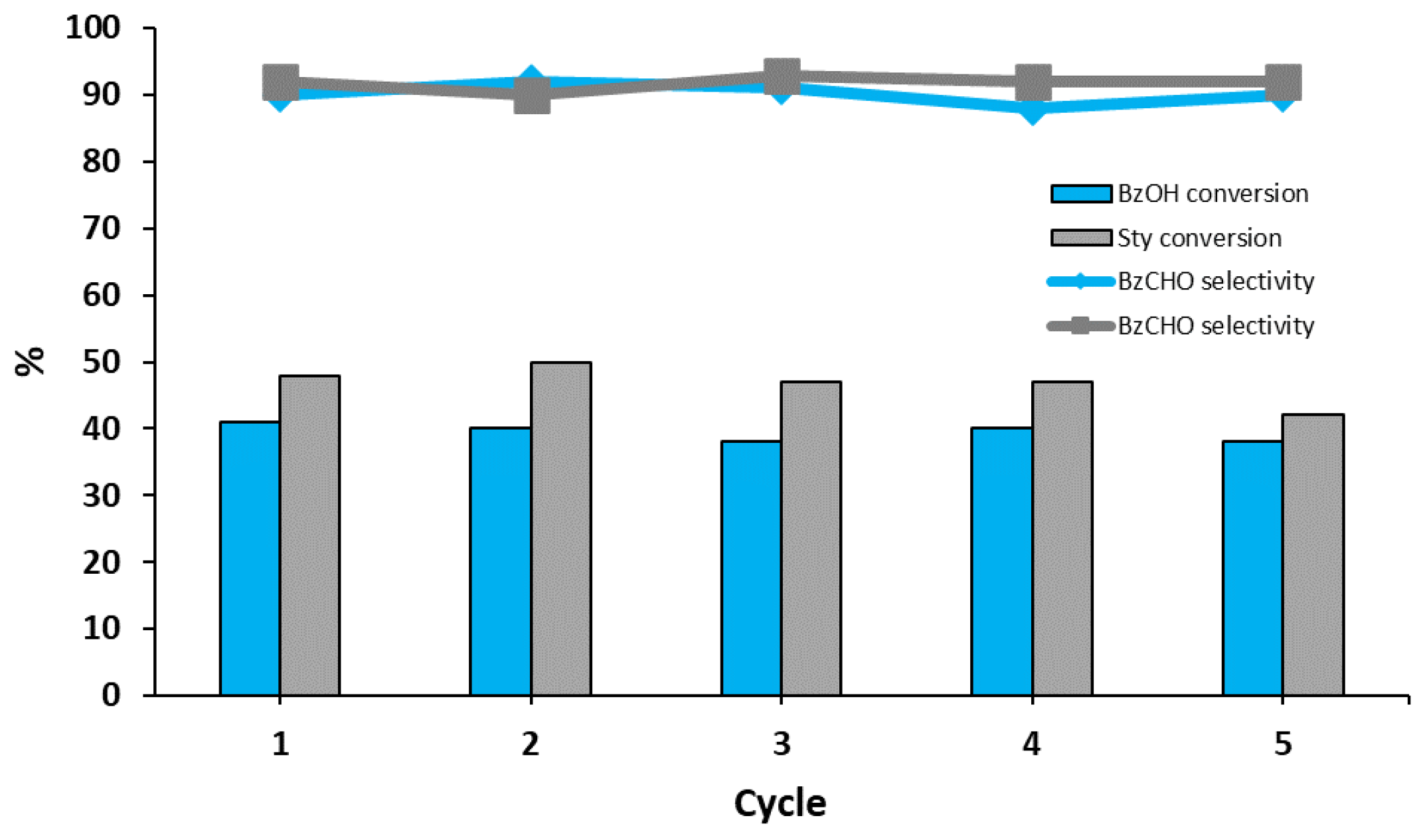
2.4. Recycling of the Catalyst
2.5. Comparison with Reported Catalyst
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Co(II) Supported TSA-ZrO2 (Co/TSA-ZrO2)
3.3. Analytical Techniques
3.4. Oxidation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, H.; Lin, Q.; Yu, A. Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies. Catalysts 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Misono, M. Chapter 4—Catalysis of Heteropoly Compounds (Polyoxometalates). In Studies in Surface Science and Catalysis; Misono, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 176, pp. 97–155. [Google Scholar]
- da Silva, M.J.; Rodrigues, A.A. Metal silicotungstate salts as catalysts in furfural oxidation reactions with hydrogen peroxide. Mol. Catal. 2020, 493, 111104. [Google Scholar] [CrossRef]
- Patil, U.; Saih, Y.; Abou-Hamad, E.; Hamieh, A.; Pelletier, J.D.A.; Basset, J.M. Low temperature activation of methane over a zinc-exchanged heteropolyacid as an entry to its selective oxidation to methanol and acetic acid. Chem. Commun. 2014, 50, 12348–12351. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Vilanculo, C.B.; Teixeira, M.G.; Julio, A.A. Catalysis of vegetable oil transesterification by Sn(II)-exchanged Keggin heteropolyacids: Bifunctional solid acid catalysts. React. Kinet. Mech. Catal. 2017, 122, 1011–1030. [Google Scholar] [CrossRef]
- Pedada, J.; Friedrich, H.B.; Singh, S. Synergistic role of Brønsted and Lewis acidity in alkali metal-exchanged heteropolyacid catalysts for esterification of acetic acid at room temperature. J. Iran. Chem. Soc. 2018, 15, 1411–1418. [Google Scholar] [CrossRef]
- Malkar, R.S.; Daly, H.; Hardacre, C.; Yadav, G.D. Novelty of iron-exchanged heteropolyacid encapsulated inside ZIF-8 as an active and superior catalyst in the esterification of furfuryl alcohol and acetic acid. React. Chem. Eng. 2019, 4, 1790–1802. [Google Scholar] [CrossRef]
- Desai, D.S.; Yadav, G.D. Solvent-free oxidative esterification of furfural to 2-methyl furoate using novel copper-exchanged tungstophosphoric acid supported on montmorillonite K-10 catalyst. Mol. Catal. 2022, 524, 112256. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, K.; Xiong, C.; Tang, X.; Chen, Q.; Wang, K.; Liu, L.-L.; Hung, C.-T.; Liu, S.-B. Transition-metal incorporated heteropolyacid-ionic liquid composite catalysts with tunable Brønsted/Lewis acidity for acetalization of benzaldehyde with ethylene glycol. Appl. Catal. A: Gen. 2017, 543, 115–124. [Google Scholar] [CrossRef]
- Desai, D.S.; Yadav, G.D. Friedel-crafts acylation of furan using chromium-exchanged dodecatungstophosphoric acid: Effect of support, mechanism and kinetic modelling. Clean Technol. Environ. Policy 2021, 23, 2429–2441. [Google Scholar] [CrossRef]
- da Silva, M.J.; de Andrade Leles, L.C.; Teixeira, M.G. Lewis acid metal cations exchanged heteropoly salts as catalysts in β-pinene etherification. React. Kinet. Mech. Catal. 2020, 131, 875–887. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Influence of alkaline metal on performance of supported silicotungstic acid catalysts in glycerol dehydration towards acrolein. Appl. Catal. A: Gen. 2011, 393, 331–339. [Google Scholar] [CrossRef]
- Koley, P.; Rao, B.S.; Sabri, Y.M.; Bhargava, S.K.; Tardio, J.; Lingaiah, N. Selective conversion of furfural into tetrahydrofurfuryl alcohol using a heteropoly acid-based material as a hydrogenation catalyst. Sustain. Energy Fuels 2020, 4, 4768–4779. [Google Scholar] [CrossRef]
- Shringarpure, P.; Patel, A. Cobalt (II) exchanged supported 12-tungstophosphoric acid: Synthesis, characterization and non-solvent liquid phase aerobic oxidation of alkenes. J. Mol. Catal. A: Chem. 2010, 321, 22–26. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Heck coupling catalyzed by Pd exchanged supported 12-tunstophosphoric acid—An efficient ligand free, low Pd-loading heterogeneous catalyst. RSC Adv. 2012, 2, 116–120. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Selective C=C Hydrogenation of Unsaturated Hydrocarbons in Neat Water Over Stabilized Palladium Nanoparticles Via Supported 12-Tungstophosphoric Acid. Catal. Lett. 2019, 149, 1476–1485. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Narkhede, N. Hydrogenation of Cyclohexene in Aqueous Solvent Mixture Over a Sustainable Recyclable Catalyst Comprising Palladium and Monolacunary Silicotungstate Anchored to MCM-41. Eur. J. Inorg. Chem. 2019, 2019, 423–429. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Nickel exchanged supported 12-tungstophosphoric acid: Synthesis, characterization and base free one-pot oxidative esterification of aldehyde and alcohol. RSC Adv. 2019, 9, 1460–1471. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Patel, J. Fe Exchanged Supported Phosphomolybdic Acid: Synthesis, Characterization and Low Temperature Water Mediated Hydrogenation of Cyclohexene. Catal. Lett. 2021, 152, 2716–2728. [Google Scholar] [CrossRef]
- Weerakkody, C.; Biswas, S.; Song, W.; He, J.; Wasalathanthri, N.; Dissanayake, S.; Kriz, D.A.; Dutta, B.; Suib, S.L. Controllable synthesis of mesoporous cobalt oxide for peroxide free catalytic epoxidation of alkenes under aerobic conditions. Appl. Catal. B Environ. 2018, 221, 681–690. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N.S.S.; Shafiei, N. Recent progresses in polymer supported cobalt complexes/nanoparticles for sustainable and selective oxidation reactions. Mol. Catal. 2020, 484, 110775. [Google Scholar] [CrossRef]
- Li, G.; Enache, D.I.; Edwards, J.; Carley, A.F.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of benzyl alcohol with oxygen using zeolite-supported Au and Au–Pd catalysts. Catal. Lett. 2006, 110, 7–13. [Google Scholar] [CrossRef]
- Xu, G.; Xia, Q.H.; Lu, X.H.; Zhang, Q.; Zhan, H.J. Selectively catalytic epoxidation of styrene with dry air over the composite catalysts of Co-ZSM-5 coordinated with ligands. J. Mol. Catal. A Chem. 2007, 266, 180–187. [Google Scholar] [CrossRef]
- Sebastian, J.; Jinka, K.M.; Jasra, R.V. Effect of alkali and alkaline earth metal ions on the catalytic epoxidation of styrene with molecular oxygen using cobalt(II)-exchanged zeolite X. J. Catal. 2006, 244, 208–218. [Google Scholar] [CrossRef]
- Tang, B.; Lu, X.H.; Zhou, D.; Lei, J.; Niu, Z.H.; Fan, J.; Xia, Q.H. Highly efficient epoxidation of styrene and α-pinene with air over Co2+-exchanged ZSM-5 and Beta zeolites. Catal. Commun. 2012, 21, 68–71. [Google Scholar] [CrossRef]
- Beier, M.J.; Kleist, W.; Wharmby, M.T.; Kissner, R.; Kimmerle, B.; Wright, P.A.; Grunwaldt, J.-D.; Baiker, A. Aerobic Epoxidation of Olefins Catalyzed by the Cobalt-Based Metal–Organic Framework STA-12(Co). Chem. A Eur. J. 2012, 18, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Hu, D.; Yang, X.; Zhang, H.; Zhang, W.; Li, J.; Jia, M.; Yu, J. Polyoxomolybdic Cobalt Encapsulated within Zr-Based Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Olefins Epoxidation. ACS Sustain. Chem. Eng. 2019, 7, 3624–3631. [Google Scholar] [CrossRef]
- Allahresani, A.; Naghdi, E.; Nasseri, M.A.; Hemmat, K. Selective oxidation of alcohols and sulfides via O2 using a Co(ii) salen complex catalyst immobilized on KCC-1: Synthesis and kinetic study. RSC Adv. 2020, 10, 37974–37981. [Google Scholar] [CrossRef]
- Hassan, S.; Kumar, R.; Tiwari, A.; Song, W.; van Haandel, L.; Pandey, J.K.; Hensen, E.; Chowdhury, B. Role of oxygen vacancy in cobalt doped ceria catalyst for styrene epoxidation using molecular oxygen. Mol. Catal. 2018, 451, 238–246. [Google Scholar] [CrossRef]
- Engel, R.V.; Alsaiari, R.; Nowicka, E.; Miedziak, P.J.; Kondrat, S.A.; Morgan, D.J.; Edwards, J.K.; Hutchings, G.J. Solvent-free aerobic epoxidation of 1-decene using supported cobalt catalysts. Catal. Today 2019, 333, 154–160. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, R.; Chang, J.; Hu, N.; Xu, C. Self-supporting Co3O4/Graphene Hybrid Films as Binder-free Anode Materials for Lithium Ion Batteries. Sci. Rep. 2018, 8, 3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Novel heterogeneous catalyst, supported undecamolybdophosphate: Synthesis, physico-chemical characterization and solvent-free oxidation of styrene. Dalton Trans. 2011, 40, 348–355. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mizuno, N. Heterogeneously catalyzed liquid-phase oxidation of alkanes and alcohols with molecular oxygen. New J. Chem. 2002, 26, 972–974. [Google Scholar] [CrossRef]
- Anjali Patel, S.P. (Ed.) Polyoxomolybdates as Green Catalysts for Aerobic Oxidation; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Pathan, S.; Patel, A. Keggin type transition metal substituted phosphomolybdates: Heterogeneous catalysts for selective aerobic oxidation of alcohols and alkenes under solvent free condition. Catal. Sci. Technol. 2014, 4, 648–656. [Google Scholar] [CrossRef]
- Zhu, J.; Faria, J.L.; Figueiredo, J.L.; Thomas, A. Reaction Mechanism of Aerobic Oxidation of Alcohols Conducted on Activated-Carbon-Supported Cobalt Oxide Catalysts. Chem. A Eur. J. 2011, 17, 7112–7117. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Guan, S.; Zhang, X.; Wang, B.; Cao, X.; Yu, Z.; He, Y.; Evans, D.G.; Feng, J.; et al. Ultrathin and Vacancy-Rich CoAl-Layered Double Hydroxide/Graphite Oxide Catalysts: Promotional Effect of Cobalt Vacancies and Oxygen Vacancies in Alcohol Oxidation. ACS Catal. 2018, 8, 3104–3115. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.G.; Jampaiah, D.; Chalkidis, A.; Sabri, Y.M.; Mayes, E.L.H.; Bhargava, S.K. Highly dispersed cobalt oxide nanoparticles on manganese oxide nanotubes for aerobic oxidation of benzyl alcohol. Catal. Commun. 2019, 130, 105763. [Google Scholar] [CrossRef]
- Bhatt, N.; Patel, A. Esterification of 1° and 2° alcohol using an ecofriendly solid acid catalyst comprising 12-tungstosilicic acid and hydrous zirconia. J. Mol. Catal. A: Chem. 2005, 238, 223–228. [Google Scholar] [CrossRef]
- Ranjan Sahu, H.; Ranga Rao, G. Characterization of combustion synthesized zirconia powder by UV-vis, IR and other techniques. Bull. Mater. Sci. 2000, 23, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Pathan, S.; Patel, A. Selective green oxidation of alcohols and alkenes with molecular oxygen using supported undecamolybdophosphate under solvent free condition. Chem. Eng. J. 2014, 243, 183–191. [Google Scholar] [CrossRef]



| Sr. | Catalysts | % Conversion | % Selectivity | TON | |
|---|---|---|---|---|---|
| Benzaldehyde | Other | ||||
| 1. | ZrO2 | -/- | - | - | - |
| 2. | TSA-ZrO2 | 8 a/7 b | >99 a/>99 b | - | - |
| 3 | Co-acetate c | 24 a/34 b | >86 a/>84 b | 14 a/16 b | - |
| 4. | Co/TSA-ZrO2 | 41 a/48 b | 90 a/92 b | 10 a/8 b | 1872 a/1461 b |
| 5. | Co/TSA-ZrO2 d | <2 a/3 b | >99 a/>99 b | - | - |
| 6. | Without catalyst e | -/- | - | - | - |
| Sr. | Catalysts | Conditions * | % Conversion | % Benzaldehyde | TON | Remarks | Refs. |
| Benzyl alcohol oxidation | |||||||
| 1 | Co(II) salen complex@KCC—1 | 1:60:0.5:60 | - | 95 ** | 1.8 | Isobutyraldehyde as co—substrate, HOAc—solvent | [29] |
| 2 | Co3O4/Activated carbon | 0.2:100:3:80 | 100 | - | - | Very high catalyst:substrate | [39] |
| 3 | CoAl-ELDH/GO | 1:100:4:120 | 92.2 | 99.3 | 5 | DMF—solvent | [40] |
| 4 | Co3O4/MnO2 | 1:25:12:100 | 93 | 99 | 16 | Air, 1,4—Dioxane– solvent | [41] |
| 5 | Co/TSA–ZrO2 | 100:100:24:90 | 41 | 90 | 1872 | TBHP—additive, solvent–free | Present work |
| Styrene oxidation | |||||||
| 6 | NaCoX96 | 10:200:4:100 | 100 | 67 | 16 | DMF—solvent | [25] |
| 7 | Co/TPA—ZrO2 | 100:100:4:80 | 76 | >99 | 623 | TBHP—additive, solvent-free | [15] |
| 8 | Co/TSA—ZrO2 | 100:150:4:80 | 48 | 92 | 1461 | TBHP—additive, solvent—free | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathan, S.; Patel, A.; Tilani, H. Selective Oxidation of Alcohols and Alkenes with Molecular Oxygen Catalyzed by Highly Dispersed Cobalt (II) Decorated 12-Tungstosilicic Acid-Modified Zirconia. Catalysts 2022, 12, 1622. https://doi.org/10.3390/catal12121622
Pathan S, Patel A, Tilani H. Selective Oxidation of Alcohols and Alkenes with Molecular Oxygen Catalyzed by Highly Dispersed Cobalt (II) Decorated 12-Tungstosilicic Acid-Modified Zirconia. Catalysts. 2022; 12(12):1622. https://doi.org/10.3390/catal12121622
Chicago/Turabian StylePathan, Soyeb, Anjali Patel, and Harshita Tilani. 2022. "Selective Oxidation of Alcohols and Alkenes with Molecular Oxygen Catalyzed by Highly Dispersed Cobalt (II) Decorated 12-Tungstosilicic Acid-Modified Zirconia" Catalysts 12, no. 12: 1622. https://doi.org/10.3390/catal12121622